Abstract
In this work, an assessment of groundwater quality and its compliance with Brazilian environmental protection standards was carried out. Ground waters from the Serra Geral aquifer are currently used for human consumption at the western region of the Brazilian state of Paraná. Ground water samples from 10 wells covering the entire Toledo municipality rural region were collected and analysed by two highly accurate and sensitive spectrometric techniques: inductively coupled plasma–optical emission spectrometry (ICP-OES) and total reflection X-ray spectrometry (TXRF). Among all detected elements, 18 elements (As, Ba, Br, Ca, Pb, Cl, Co, Cu, Cr, Fe, P, S, Mn, Ni, K, Ti, V and Zn) were measured by the TXRF technique while three elements (B, Mg and Na) were measured by ICP-OES. Trace element concentration levels were then compared with Brazilian environmental legislation (BEL). From the results obtained, concentrations of chromium, iron, arsenic, selenium, manganese and barium were detectable in some wells at slightly above the maximum limits allowed by the BEL.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Fresh water is being used in a great number of domestic and industrial anthropogenic activities and is in high demand due to the large increases in human populations in the last century. This heavy use also brings serious supply side problems and issues of environmental contamination around the world. As this natural resource is scarce and exhibits a non-uniform distribution on the Earth’s surface, exploration of the world’s groundwater reserves has become an actively pursued alternative to provide this essential resource to many populations lacking fresh water. An important characteristic of groundwater is its high quality, suitability for direct consumption by human beings, without the necessity of a potability process, and containing as it does just a few elements at very low concentrations derived from the decomposition of rocks forming the aquifer. Meanwhile, by increasing the superficial contamination of rivers and soils from various sources, frequently related to the infiltration of pesticides, fertilizers, domestic sewage and animal waste into aquifers or directly into tube wells, the groundwater might be compromised, introducing organic and inorganic pollutants (Justen et al. 2012; Zaporozec and Miller 2000) and becoming a major environmental concern.
Ground waters have shown vulnerability to contamination by heavy metals related to anthropogenic activities, as has already been found in two Brazilian states (Borba et al. 2003; Lopes et al. 2012). Among these pollutants, some metals exhibit great potential toxicity even at low concentrations, requiring the application of fast and sensitive multielemental analytical techniques to provide information on the composition of the groundwater (De la Calle et al. 2013). As a part of continuing efforts to monitor and control a series of metals, environmental regulatory agencies have established a few recommended maximum limits for metals in fresh water intended for human consumption, insisting on the application of atomic spectrometric techniques with high elementary sensitivity.
One well-established analytical technique is total reflection X-ray fluorescence spectrometry (TXRF), leading to the realization of limits of detection on the order of micrograms per gram or litre of sample due to decreased background noise and matrix effects (Obregón et al. 2014; West et al. 2011; Szoboszlai et al. 2009; Marcó and Hernández-Caraballo 2004; Klockenkamper and von Bohlen 1996). Additionally, the TXRF technique is currently used to determine concentrations of heavier elements (Rizzutto et al. 2006; Espinoza-Quiñones et al. 2010a, b), while lighter elements such as Na, Mg and B are difficult to quantify due to their poor detection limits. In environmental sample analysis, inductively coupled plasma–optical emission spectrometry (ICP-OES) and inductively coupled plasma-mass spectrometry (ICP-MS) have been widely used (Silliman et al. 2007; Ikem et al. 2002), but with high maintenance costs among other instrumental requirements (Antosz et al. 2012).
The aim of this work is to assess groundwater quality based on compliance with a series of physico-chemical parameters and element concentration values established by Brazilian protection standards. The determinations of major, minor and trace element concentrations in groundwater samples were made using the TXRF and ICP-OES techniques. A statistical multivariate method was applied to characterize the groundwater data in order to identify possible dependencies or correlations among all analysed parameters, looking for anthropogenic contaminations.
2 Material and Methods
2.1 Study Area
The area covered in this work is located in the western region of the Brazilian state of Paraná, where groundwaters are a secondary source of fresh water for human consumption. Ground waters are supplied from the Serra Geral Aquifer System which is one of the principal aquifers in the southern Brazilian states of Paraná, Santa Catarina and Rio Grande do Sul, covering an outcrop area of approximately 102,000 km2 with a subdivision into the North Serra Geral (about 64,000 km2) and South Serra Geral (about 38,000 km2). This aquifer is geological characterized by a basaltic to rhyolitic Mesozoic volcanic sequence belonging to the Serra Geral Formation with thickness varying from 50 to 1,500 m and an average of 550 m (Athayde et al. 2012). In general, the Serra Geral Formation consists mainly of volcanic rocks as basalts and basaltic andesites that are predominantly plagioclase, augite and pigeonite (Manasses et al. 2007), showing different tectonic structures and defining a fractured-type aquifer.
Geological studies (Mineropar 2005) of this region have identified rocks with high concentrations of iron oxide, arsenic and chromium in the region of Toledo municipality. Additionally, this geological formation mainly contains oxides of aluminium, calcium, magnesium, sodium, potassium and titanium as reported by Ruegg (1976). Hydrochemical characteristics of groundwater from the Serra Geral Aquifer System could be influenced by waters of other aquifers such as Guarani aquifer system that is a transnational aquifer as well as by tectonic structures and pumping of deep groundwater (Nanni et al. 2008). In the western region of the Brazilian state of Paraná, groundwater from the North Serra Geral aquifer is the main fresh water source used by the rural population of the municipality of Toledo. The groundwater quality could be also probably influenced by anthropic activities such as different agricultural crops, piggery farms, domestic septic tanks and a fish-processing factory located in the rural zone of the municipality of Toledo. Mining activities and metal-processing factories are not present in such area. This aquifer is constantly recharged with freshwater coming from rain and river waters, besides receiving waters coming from agricultural land use and the other anthropogenic activities.
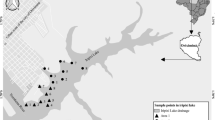
The city of Toledo (latitude 24°43′12″ S, longitude 53°44′36″ W, with an average altitude of 550 m) is located in the western part of the Brazilian state of Paraná in the basin of the São Francisco River. For an assessment of the effect of anthropogenic contamination in the aquifer water, 10 tube wells for domestic purposes were chosen covering the entire rural zone of the municipality of Toledo, as can be seen in Fig. 1. In particular, tube wells 6, 8 and 9 are surrounded by domestic septic tanks with distance less than 30 m. Approximately 150 m from tube well 1 there is a fish-processing factory. Beside tube wells 2, 3 and 4, piggery farms are installed, while tube wells 8 and 9 are located near poultry houses. Conversely, different agricultural crops surround tube wells 3 and 10.
2.2 Groundwater Sampling
Polyethylene bottles (1 L) used for sampling were subjected to a sterilization process according to the Standard Methods for the Examination of Water and Wastewater (APHA 2005). The collection and preservation of groundwater samples were carried out as recommended by the Environmental Sanitation Technology Agency of the Brazilian state of São Paulo (Brazil 1988). At each sampling site, groundwater samples were collected monthly in three cleaned 1-L polyethylene bottles from a tap installed at the outlet of the tube well for the different analyses proposed. Before each sampling, water from the tap was drained for about 15 min to dispose the remaining water in the pipe. An annual groundwater sampling procedure for monitoring a set of physico-chemical parameters and trace element concentrations was performed from November 2012 to October 2013.
2.3 Physico-Chemical Analysis
Measurements in loco of pH, dissolved oxygen (DO), electrical conductivity (EC) and temperature were performed simultaneously with four sensors installed at a multi-parameter water quality meter (YSI Pro plus). After collection, a portion of each sample was acidified to a pH less than 2, and thus was preserved and maintained under refrigeration for spectrometric analyses. Alkalinity, turbidity, chemical oxygen demand (COD), 5-day biochemical oxygen demand (DBO5), organic nitrogen, ammonia nitrogen, nitrate, nitrite and total phosphate were measured at the lab in accordance with the Standard Methods for the Examination of Water and Wastewater (APHA 2005).
2.4 Atomic Spectrometric Techniques
In order to assess seasonal and long-term variations in trace element behaviour in groundwater, highly sensitive spectrometric techniques such as ICP-OES and TXRF were used for multi-element determination. As such techniques lead to similar detection limits for heavier element determination, measurements of 18 elements: As, Ba, Br, Ca, Pb, Cl, Co, Cu, Cr, Fe, P, S, Mn, Ni, K, Ti, V and Zn, were mainly performed by the TXRF technique. Meanwhile, ICP-OES was mainly used to measure concentrations of light elements such B (Z = 5), Mg (Z = 12) and Na (Z = 11) that would be difficult to determine by the TXRF technique.
Quantitative TXRF analysis of the groundwater was performed by an internal standard procedure. Additionally, the use of small aliquots of an internal standard-doped sample and the formation of a thin layer on a TXRF sample support preclude matrix effects (Marguí et al. 2010), allowing detection limits and control sample contamination (Dhara and Misra 2011) to be improved comparatively. The detection of fluorescence radiation spectral lines (K and L X-ray series) emitted by irradiated samples has allowed the identification and determination of each element’s concentration in a rapid and simultaneous analysis of many elements from Na to U in the periodic table (Stosnach 2005).
2.5 TXRF Measurements
In the present study, a portable benchtop TXRF spectrometer (S2 PICOFOX, Bruker AXS Microanalysis GmbH) was used. An air-cooled low power X-ray metal-ceramic tube with a molybdenum target, working at a max power of 50 W, and a liquid nitrogen-free Silicon Drift Detector (SSD) featured the compact S2 PICOFOX TXRF instrument (Bruker 2007). This instrument contains a sample changer for a cassette of 25 samples.
Before preparation of TXRF sample carriers, a cleaning procedure of the TXRF quartz glass sample carriers (30 mm in diameter and 3 mm thickness) was done to avoid contamination. First, residues on the sample carrier surface were removed by mechanically wiping each sample carrier with a fluff-free tissue paper soaked in acetone. Using a 1-L borosilicate beaker, a set of sample carriers positioned in a washing cassette was submitted to a sequential removal of impurities by applying a 2-h heating (80 °C) process performed with three different cleaning solutions. A 10 % RBS 50 solution, a 10 % HNO3 solution, and Milli-Q water were used in that order as media of cleaning. A rinsing step with Milli-Q water was included after each cleaning process. Finally, the sample carriers were dried in an oven at 80 °C for 30 min.
To obtain a thin, small spot of dry residue for TXRF analysis, a 10 μL drop of silicon solution was pipetted in the centre of the TXRF quartz glass sample carrier and then dried at 80 °C for a few minutes. Using 2-mL Eppendorf-type microtubes, a 100-μL aliquot of polyvinyl alcohol was added to 890 μL of a groundwater sample in order to enhance the homogeneity of the samples and avoid any crystal formation after sample drying. Additionally, a 10-μL aliquot of a Gallium standard solution (1.0 g L−1, Sigma-Aldrich) was added as an internal standard. Then, a 5-μL aliquot of a Ga-doped groundwater sample was pipetted into the centre of a cleaned, siliconized TXRF quartz glass sample carrier and left to dry at room temperature in a laminar flow hood. For TXRF analysis on the same groundwater sample, at least three different sample carriers were prepared in this way. For TXRF analysis assessment, sample carriers of a multi-element certified, reference standard solution (Fluka Analytical, 90243 for ICP) containing diverse elements at 10 mg L−1 (Ag, Ba, Ca, Cd, Co, Cu, Fe, Mg, Mn, Sr and Zn), 50 mg L−1 (Al, B, Cr, Li, Mo, Na, Ni and Tl) and 100 mg L−1 (Bi, K and Pb) were prepared in quintuplicate. In each batch of 25 samples, procedural blanks were included.
As a slight drift of the spectroscopic amplification can occur for longer operating periods, an instrumental gain correction procedure was performed before each sample batch. By obtaining two 60-s TXRF spectra of a 1-μg Se standard sample, the spectroscopy amplification could be reset daily by the Gain Correction function of the PICOFOX spectrometer. The data acquisition time was 1,000 s per sample. The Spectra software facilitates calibration, evaluation and storage of the data. Ground water sample TXRF spectra were obtained in triplicate. The Spectra software provides the net peak area (after baseline subtraction), the detection limits (as three times the square root of the background area) and the concentration of each element.
2.6 ICP-OES Measurements
A dual-view inductively coupled plasma–optical emission spectrometer (Optima 7000 DV, Perkin-Elmer) was used to perform elemental concentration measurements as a complement to the TXRF analysis. As shown in Table 1, conditions recommended by the manufacturers were established for adequate excitation of a wide range of elements. The sample introduction, RF generator, plasma and spectrometer systems were controlled through Optima WinLab32™ software. The ICP-OES response was firstly calibrated against standards of known composition that contained the analytes sought (B, Mg and Na). The calibration blank and standards were prepared in 2 % nitric acid. The set of calibration standards was prepared from a single Boron stock solution and multi-element stock solutions containing Na and Mg analytes. The accuracy and precision of the analysis method were tested through the analysis of a certified reference material (Fluka Analytical, 90243 for ICP), containing 50 mg L−1 B, 50 mg L−1 Na and 10 mg L−1 Mg. Acidic groundwater samples were analysed directly.
2.7 Statistical Analysis
To determine whether the elemental concentration and physico-chemical data from the groundwater samples were normally distributed, the null hypothesis was tested for an alpha level of 0.05 on the basis of the Shapiro-Wilk test, in which if the p value is greater than 0.05 the sample data are assumed to come from a normally distributed population. A cluster analysis was performed in order to show the similarities among the groundwater composition data collected from the set of 10 tube wells. In order to avoid an influence of the measuring scale and number of variables as well as the existent correlations among them, the set of data were standardized by applying a linear transformation of each measured real value (X) to a relative-to-mean value dispersion (Z) normalized by the standard deviation (σ), as shown in Eq. 1. In the experimental situation where the parameter and concentration values were below the detection limits, a value of one half of the detection limit was considered as an attribution criterion (Farnham et al. 2002). After the linear transformation of the data, a cluster analysis was performed using the Statistica® software. A cophenetic correlation coefficient was calculated to test the quality of the cluster analysis.
3 Results and Discussion
3.1 Characteristics of the Ground Waters
The analyses of all TXRF spectra obtained from groundwater samples were performed using the Spectra software, providing the main X-ray line energy and net peak area of each element. The following elements: As, Ba, Br, Ca, Pb, Cl, Co, Cu, Cr, Fe, P, S, Mn, Ni, K, Ti, V and Zn, were identified as constituents of groundwater based mainly on their strong X-ray Kα lines. Based on the concentration of the internal standard (10 mg L−1 Ga) and the Spectra software-calculated elemental sensitivities for the S2 PICOFOX TXRF spectrometer, the concentration and detection limit of each element were determined. From the accuracy test performed using the certified reference material (Fluka 90243), it was verified that the TXRF results were in good agreement with the certified values, providing a certified elemental concentration recovery in the 92–107 % range (data not shown). Meanwhile, the concentration results for Na, B and Mg were obtained by the ICP-OES technique and a certified reference material (Fluka 90243) also gave results within the reported certified concentration ranges. The annual mean concentration values calculated from the elemental data obtained by the TXRF and ICP-OES techniques are summarized in Table 2, indicating the elemental characteristics of the groundwater in the 10 tube wells.
From the groundwater physico-chemical analysis, there is scant evidence of the presence of organic contamination in groundwater in the 10 tube wells, with no significant values of organic nitrogen, ammonia nitrogen, nitrite or total phosphate found related to the limit of detection of each parameter. A high content of bicarbonate and calcium was predominantly found in groundwaters with a 6.6–7.2 pH value range in the Serra Geral aquifer system, as reported by the Water Institute of Brazilian State of Paraná (Brazil 2010). In the western region of the Brazilian state of Paraná, bicarbonate and calcium content was confirmed in 336 the tube wells by Athayde et al. (2007), but with pH values inadequate for human consumption in 36 tube wells of them with values out of recommended pH range of 6.0–9.5 by the Brazilian Health Ministry (Brazil 2011). In Table 3, annual content values of temperature, pH, DO, EC and alkalinity were observed in groundwaters at the 10 tube wells, complying with the Brazilian norm for groundwaters (Brazil 2011). The alkaline pH (8.0–9.4) of Serra Geral aquifer groundwaters could be attributed to the strong influence of the groundwater characteristic of the Guarani aquifer.
Analysing the annual values of EC and alkalinity, a wide variability in such parameters was observed among all tube wells, suggesting that rocks with different geological composition are present in the study area. The presence of nitrate was detected at all sampling sites, with annual values ranging from 0.5 to 0.9 mg L−1 with a standard deviation of 0.4 mg L−1. In addition, such constituents could cause deterioration of groundwater quality. It can be noticed that seasonal upper limits for nitrate content at the sampling sites were slightly below 10 mg L−1, the allowed limits recommended by the Brazilian norm for groundwaters, indicating that there is an anthropogenic influence resulting in a slight organic contamination of the groundwaters.
On the other hand, very small amounts of sulphur and phosphorous were detected only at sampling site 1, while such elements are expected to be present at concentrations below the detection limits for the other sampling sites. The major elements in the composition of groundwaters are Na, Ca, K, Mg and Fe. The elements Ca and Mg are well known to be currently present in the groundwater due to the hydrogeochemical removal of such elements from rocks rich in carbonates (Karavoltsos et al. 2008). In terms of the vanadium content in the groundwaters, sampling sites 2 and 4 showed mean concentration values of 0.21 and 0.30 mg L−1, respectively, in non-compliance with allowed limits recommended by the Brazilian norm for groundwater (Brazil 2008).
Although the natural presence of chromium is uncommon in groundwaters (Marcolan et al. 2008), sampling site 10 exhibited a 0.56-mg L−1 value for annual mean chromium concentration, slightly above the 0.55-mg L−1 allowed maximum limit recommended by the Brazilian norm for drinking water (Brazil 2011). As reported by Marcolan et al. (2008), industrial effluents from metal and wood processing, and tanneries, among others, could be the source of chromium contamination of groundwaters. However, such anthropogenic sources of chromium are absent in the rural area of the Toledo municipality. Conversely, geological studies (Mineropar 2005) of this region have identified rocks with chromium content, which could also act as a possible source for the chromium in the groundwaters. The presence of iron in the groundwater could also be attributed to the geological composition of the rocks in this region.
The arsenic content in groundwaters at almost all sampling sites was slightly above the 0.1-mg L−1 maximum limits recommended by the Brazilian norm (Brazil 2011). As a consequence of the strong influence of water-rock interactions and favourable geochemical and geophysical conditions for the mobility and accumulation of arsenic, a great variability in arsenic concentrations can be expected to occur in groundwaters (Smedley and Kinniburgh 2002). Conversely, further studies are necessary to elucidate the presence of arsenic in groundwater.
In comparison, the annual mean Se concentration of 0.l mg L−1 in groundwaters from sampling site 10 was above the allowed maximum limit allowed in Brazil. Selenium concentrations above the allowed limits for groundwaters have also been detected in India (Kumar and Riyazuddin 2011). Although small amounts of selenium are essential for life, a somewhat higher concentration may act as a toxin in human beings, indicating that the boundary between essentiality and toxicity is narrow.
The groundwater quality could be probably influenced by contamination sources that surrounded the tube wells, but evidences of the presence of organic contamination in groundwater in the 10 tube wells were scarce. Some metals detected in groundwaters could be more consistent for coming naturally from the aquifer geology.
3.2 Statistical Correlations
Performing the Shapiro-Wilk test, the elemental and physico-chemical parameter data did not show a normal distribution. Conversely, applying the non-parametric correlation method of Spearman to the data enabled similar behaviour to be found among the variables at a 95 % confidence level, as shown in Tables 4 and 5. According to the higher Spearman coefficient values in Table 4, the following elements: P, Cr, Mn, Ni, As and Br, exhibit the same tendency with variations in their concentrations. A possible explanation for the origin of this tendency could be the release of ions by the same type of rocks as a consequence of water-rock interactions (Mrazovac et al. 2013). In Table 5, alkalinity and EC are strongly correlated, due to the values of both parameters being related to the presence of ions that are released from the rocks and transported by groundwaters to the Earth’s surface.
3.3 Clustering
A hierarchical clustering was applied to detect natural groupings in the element concentration data from the groundwaters of the 10 tube wells. As an appropriate metric, squared Euclidean distance was chosen, allowing wells to be grouped by similarities in their elemental compositions. A dendrogram is shown in Fig. 2, where a great similarity between sampling sites 2 and 4 is evidenced by their similar groundwater characteristics, forming a first group that is in contrast with groundwater characteristics for the other sampling sites. Another group could be comprised of sampling sites 7, 1 and 3 because they are geographically close to each other, raising the expectation that their similar groundwater characteristics originate from the rock formation. A statistical test based on the cophenetic correlation coefficient was applied to the elemental and physico-chemical parameter data for groundwater obtained for the sampling sites. A correlation value of 0.84 between the cophenetic and Euclidean distance matrices was found, confirming that the dendrogram is a good representation of the data set.
4 Conclusion
The determination of element concentrations by both TXRF and ICP-OES techniques yielded considerable information on the groundwaters, allowing us to assess the compliance to environmental norms as well as to associate a set of sampling sites in terms of similarities and dissimilarities.
A statistical analysis based on hierarchical clustering and Spearman correlation coefficients applied to the data on physico-chemical parameters and elemental concentrations revealed that sampling sites are vulnerable to anthropogenic contamination, as highlighted by the higher nitrate concentration values as well as other inorganic constituents of the groundwater. There was a mutual correlation among elements as well as among physico-chemical parameters.
A groundwater monitoring programme should be continued in the same wells and extended to others of the Serra Geral aquifer, looking for signs of anthropogenic contamination during long-term observation. Finally, further studies are necessary to assess the degree of vulnerability degree of the Serra Geral Aquifer to anthropogenic actions.
References
Antosz, F. J., Xiang, Y., Diaz, A. R., & Jensen, A. J. (2012). The use of total reflectance X-ray fluorescence (TXRF) for the determination of metals in the pharmaceutical industry. Journal of Pharmaceutical and Biomedical Analysis, 62, 17–22.
APHA. (2005). Standard methods for the examination of water and wastewater (21st ed.). Washington: American Public Health Association.
Athayde, G. B., Muller, C. V., Rosa Filho, E. R., & Hindi, E. C. (2007). Study on the types of the Serra Geral Aquifer water in the municipality of Rondon—Brazilian State of Paraná. Águas Subterrâneas, 21, 111–122. in Portuguese.
Athayde, G. B., Athayde, C. M. V., & Rosa Filho, E. F. (2012). Hidroestrutural partitioning and chemical capabilities of the Serra Geral Aquifer System in the Brazilian state of Paraná. Revista Brasileira de Geociencias, 42, 167–185. in Portuguese.
Borba, R. P., Figueiredo, B. R., & Matschullat, J. (2003). Geochemical distribution of arsenic in waters, sediments and weathered gold mineralized rocks from Iron Quadrangle, Brazil. Environmental Geology, 44, 39–52.
Brazil, Brazilian Council of Environmental Quality Regulation—CONAMA (2008). Norm 396/2008 (in Portuguese) published on the Union Official Newspaper of Brazil (7 April 2008), No 66, section I, pp. 64–68. http://www.mma.gov.br/port/conama/legiabre.cfm?codlegi=562. Accessed 28 Sept 2013.
Brazil (2010). Water Institute of Brazilian State of Paraná. Secretary of Environment and Hydric Resources. Diagnostic of ground hydric availabilities (in Portuguese). http://www.aguasparana.pr.gov.br/arquivos/File/PLERH/Produto1_2_ParteB_RevisaoFinal.pdf. Accessed 16 Dec 2014.
Brazil (2011). Health Ministry of Brazil. Norm 2914/2011 (in Portuguese) published on the Union Official Newspaper of Brazil (14 December 2011), No 66, section I, pp. 39–42. http://bvsms.saude.gov.br/bvs/saudelegis/gm/2011/prt2914_12_12_2011.html . Accessed 15 Oct 2012.
Brazil (1988), Guide to Collection and Preservation of Groundwater Samples (in Portuguese). Brazilian state of Sao Paulo. http://www.cetesb.sp.gov.br/solo/areas_contaminadas/anexos/download/6410.pdf. Accessed 28 Sept 2011.
Bruker (2007), S2 PICOFOX total reflection X-ray fluorescence spectroscopy— working principles. Lab report. Berlin, Germany: Bruker AXS Microanalysis GmbH. [Report No.: XRF 426].
De La Calle, I., Cabaleiro, N., Romero, V., Lavilla, I., & Bendicho, C. (2013). Sample pretreatment strategies for total reflection X-ray fluorescence analysis: a tutorial review. Spectrochimica Acta Part B, 90, 23–54.
Dhara, S., & Misra, N. L. (2011). Application of total reflection X-ray fluorescence spectrometry for trace elemental analysis of rainwater. Pramana - Journal of Physics, 76(2), 361–366.
Espinoza-Quiñones, F. R., Módenes, A. N., Palácio, S. M., Szymanski, N., Welter, R. A., Rizzutto, M. A., Borba, C. E., & Kroumov, A. D. (2010a). Evaluation of trace element levels in muscles, liver and gonad of fish species from São Francisco River of the Paraná Brazilian state by using SR-TXRF technique. Applied Radiation and Isotopes, 68(12), 2202–2207.
Espinoza-Quiñones, F. R., Palácio, S. M., Módenes, A. N., Szymanski, N., Zacarkim, C. E., Zenatti, D. C., Fornari, M. M. T., Rizzutto, M. A., Tabacniks, M. H., Added, N., & Kroumov, A. D. (2010b). Water quality assessment of Toledo River and determination of metal concentrations by using SR-TXRF technique. Journal of Radioanalytical and Nuclear Chemistry, 283, 465–470.
Farnham, I. M., Singh, A. K., Stetzenbach, K. J., & Johannesson, K. H. (2002). Treatment of nondetects in multivariate analysis of groundwater geochemistry data. Chemometrics and Intelligent Laboratory Systems, 60, 265–281.
Ikem, A., Odueyungbo, S., Egiebor, N. O., & Nyavor, K. (2002). Chemical quality of bottled waters from three cities in eastern Alabama. The Science of the Total Environment, 285, 165–175.
Justen, G. C., Espinoza-Quiñones, F. R., Módenes, A. N., & Bergamasco, R. (2012). Elements concentration analysis in groundwater from the North Serra Geral aquifer in Santa Helena-Brazil using SR-TXRF spectrometer. Water Science & Technology, 66, 1029–1035.
Karavoltsos, S., Sakellari, A., Mihopoulos, N., Dassenakisa, M., & Scoullos, M. J. (2008). Evaluation of the quality of drinking water in regions of Greece. Desalination, 224, 317–329.
Klockenkamper, R., & Von Bohlen, A. (1996). Elemental analysis of environmental samples by total reflection X-ray fluorescence: a review. X-Ray Spectrometry, 25, 156–162.
Kumar, A. R., & Riyazuddin, P. (2011). Speciation of selenium in groundwater: seasonal variations and redox transformations. Journal of Hazardous Materials, 192, 263–269.
Lopes, D. D., Silva, S. M. C. P., Ferdandes, F., Teixeira, R. S., Celligoi, A., & Dall’antônia, L. H. (2012). Geophysical technique and groundwater monitoring to detect leachate contamination in the surrounding area of a landfill–Londrina (PR-Brazil). Journal of Environmental Management, 113, 481–487.
Manasses, F., Rosa Filho, E. F., & Bittencourt, A. V. L. (2007). Hydrogeochemical study of the Serra Geral Formation in the southwestern of the Brazilian State of Paraná. Águas Subterrâneas, 21, 49–58. in Portuguese.
Marcó, L. M., & Hernández-Caraballo, E. A. (2004). Direct analysis of biological samples by total reflection X-ray fluorescence. Spectrochimica Acta Part B, 59, 1077–1090.
Marcolan, L., Bourotte, C., & Bertolo, R. (2008). Estratificação das concentrações de cromo hexavalente nas águas subterrâneas do Aquífero Adamantina. In XV Brazilian congress of ground waters. SP: município de Urânia.
Marguí, E., Floor, G. H., Hidalgo, M., Kregsamer, P., Román-Ross, G., Streli, C., et al. (2010). Analytical possibilities of total reflection X-ray spectrometry (TXRF) for trace selenium determination in soils. Analytical Chemistry, 82, 7744–7751.
Mineropar – Minerais do Paraná S. A. (2005). Geoquímica de solo—Horizonte B: Relatório final de projeto, v. 2. Curitiba.
Mrazovac, S., Vojinovic-Mimloradov, M., Matic, I., & Maric, N. (2013). Multivariate statistical analyzing of chemical parameters of groundwater in Vojvodina. Chemie der Erde, 73, 217–225.
Nanni, A., Roisenberg, A., Fachel, J. M. G., Mesquita, G., & Danieli, C. (2008). Fluoride characterization by principal component analysis in the hydrochemical facies of Serra Geral Aquifer System in Southern Brazil. Annals of the Brazilian Academy of Sciences, 80, 693–701.
Obregón, P. L., Espinoza-Quiñones, F. R., & Módenes, A. N. (2014). Water quality monitoring of the Bezerra River (Cascavel, Brazil) using SR-TXRF. Journal of Chemistry and Chemical Engineering, 8, 587–595.
Rizzutto, M. A., Added, N., Tabacniks, M. H., Espinoza-Quiñones, F. R., Palácio, S. M., Galante, R. M., Rossi, N., Zenatti, D. C., Rossi, F. L., Welter, R. A., & Módenes, A. N. (2006). Trace element concetrations from São Francisco River-PR analyzed with PIXE technique. Journal of Radioanalytical and Nuclear Chemistry, 269, 727–731.
Ruegg, N. R. (1976). Distribution characteristics and content of main elements in basaltic rocks of the Paraná Basin. Instituto de Geociências, 7, 81–106. in Portuguese.
Silliman, S. E., Boukari, M., Crane, P., Azonsi, F., & Neal, C. R. (2007). Observations on elemental concentrations of groundwater in central Benin. Journal of Hydrology, 335, 374–388.
Smedley, P. L., & Kinniburgh, D. G. (2002). A review of the source, behavior and distribution of arsenic in natural waters. Applied Geochemistry, 17, 517–568.
Stosnach, H. (2005). Environmental trace-element analysis using a benchtop total reflection X-ray fluorescence spectrometer. Analytical Sciences : The International Journal of the Japan Society for Analytical Chemistry, 21, 873–876.
Szoboszlai, N., Polgari, Z., Mihucz, V. G., & Zaray, G. (2009). Recent trends in total reflection X-ray fluorescence spectrometry for biological applications. Analytica Chimica Acta, 633, 1–18.
West, M., Ellis, A. T., Potts, P. J., Streli, C., Vanhoof, C., Wegrzynek, D., & Wobrauschek, P. (2011). Atomic spectrometry update—X-ray fluorescence spectrometry. Journal of Analytical Atomic Spectrometry, 26, 1919–1963.
Zaporozec, A., & Miller, J. C. (2000). Ground-water pollution. Paris: UNESCO.
Acknowledgments
The authors would like to thank CNPq and CAPES for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• Ground water quality in the Serra Geral Brazilian aquifer is assessed.
• Monitoring shows that groundwaters are vulnerable to anthropogenic contamination.
• Sampling sites in terms of similarities and dissimilarities are grouped.
• A few metals are above the maximum limits allowed by the Brazilian norms.
Rights and permissions
About this article
Cite this article
Espinoza-Quiñones, F.R., Módenes, A.N., de Pauli, A.R. et al. Analysis of Trace Elements in Groundwater Using ICP-OES and TXRF Techniques and Its Compliance with Brazilian Protection Standards. Water Air Soil Pollut 226, 32 (2015). https://doi.org/10.1007/s11270-015-2315-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2315-8