Abstract
The efficiency of phytoextraction is limited because of the low growth exhibited by plants under the stress of heavy metals. Impatiens (Impatiens walleriana) cuttings were grown in soils artificially contaminated with cadmium (Cd) and modified with chemical fertilizer to study the relationship among the leaf area, transpiration rate, and Cd accumulation. The subcellular distribution of Cd in various impatiens organs was also measured. Experimental results showed that there were positive, linear relationships between the leaf area and the transpiration rate. A similar relationship was found between the transpiration rate and the Cd accumulation in the shoots. Suitable management practices can be conducted to increase the transpiration rate and thus the plant’s phytoextraction efficiency. In the roots and leaves, Cd was mainly compartmentalized in the soluble fraction and the cell wall fraction, respectively. The varied subcellular distribution of Cd in the different organs was responsible for the high accumulation capacity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Phytoextraction of heavy metals (HMs) from contaminated soils is an emerging technique used in decontamination. This environmentally friendly technique is suitable in a large area of contaminated soil that has a low to moderate concentration of HMs (Salt et al. 1995; Ernst 2005). Compared with other traditional techniques, phytoextraction has low remediation costs. However, because of the limited low biomass and low phytoavailability of some HMs, the efficiency of phytoextraction is often limited (Luo et al. 2006), and a prolonged remediation period is required. In addition to the accumulated HM concentration, many indexes are used to adjust the phytoextraction potential, and the bioconcentration factor (BCF = shoot HM concentration/soil HM concentration) and the translocation factor (TF = shoot HM concentration/root HM concentration) are the most frequently used criteria (Sun et al. 2009).
Although more than 420 species of hyperaccumulators that can accumulate higher concentrations of HMs have been discovered (Baker et al. 2000), few are cadmium (Cd) hyperaccumulators. In Taiwan, many garden flowers accumulate high HM concentrations (Lai et al. 2010). Among these plants, the Cd accumulation capacity of impatiens (Impatiens walleriana) has been examined. Lin et al. (2010) reported that impatiens accumulated 49–100 mg/kg of Cd in the shoots after being grown for 35 days in Cd-contaminated soils that contained approximately 10–18 mg/kg of Cd. Furthermore, the BCF and the TF were 5.0–5.7 and 1.0–1.7, respectively. After growing in the artificially Cd-contaminated soils (40 mg/kg) for 60 days, 342 mg/kg of Cd accumulated in the shoots of the impatiens samples. Moreover, the BCF and the TF were 9.1 and 1.7, respectively (Wei et al. 2012). Although the Cd accumulation capacity was high, the total removal was still low because of the low biomass.
The plant cell wall is primarily composed of cellulose, hemicellulose, pectin, and proteins. The hydroxyl or carboxyl groups provide many binding sites for HMs and thus decrease their migration (Wójcik et al. 2005; Qiu et al. 2011). Experimental results in previous studies show that subcellular HM compartmentalization in plants is related to their tolerance and detoxification (Wang et al. 2008; He et al. 2013). For instance, a high concentration of Cd in a resistant genotype of barley was compartmentalized in the cell wall, and a lower concentration was found in the organelles compared with the sensitive genotype (Wu et al. 2005).
For the water-soluble fraction of Cd in the soils, the Cd accumulation in the plants was mainly driven by the transpiration rate (TR), which had a close relationship with the leaf area (LA) (Salt et al. 1995; Liu et al. 2010). We supposed that increasing the LA will increase the TR, the biomass, and the Cd accumulation, and thus the total removal of HMs in plants. Therefore, the amount of time needed for phytoextraction during decontamination will decrease accordingly. However, the relationship between Cd accumulation and LA/TR of impatiens is firstly needed to be clarified. Result of a preliminary study shows that the application of biosolid could increase the biomass, LA, and TR of rainbow pink (Dianthus chinensis). Nevertheless, the release of nutrients after mineralization was quite different for different sources of biosolid. To overcome the uncertainty during the mineralization of biosolid, a pot experiment using artificially Cd-contaminated soils was conducted and chemical fertilizer was applied to affect the soil properties and the growth of the impatiens plants. The objectives of this study are the following: (1) to assess the effects of chemical fertilizer (applied to change the LA) on the TR and the Cd accumulation in impatiens and (2) to understand the tolerance and detoxification levels in the various organs of impatiens through subcellular compartmental analysis.
2 Materials and Methods
2.1 Soil Collection and Analysis
The soil samples used in this study were collected from the surface layer (0–20 cm) of uncontaminated Inceptisol in central Taiwan. The soil samples were air dried, ground, and passed through 10-, 80-, or 100-mesh stainless steel sieves with various analytical procedures. The basic properties of the representative samples were then determined in accordance with Wei et al.’s (2012) method, which includes the water-holding capacity (WHC), pH (soil/water = 1/1; w/v), EC w (soil/water = 1/1; w/v), organic carbon content (OC), and total Cd concentration.
In accordance with the WHC, suitable volume solutions of Cd(NO3)·4H2O dissolved in deionized water (DI water) were sprayed on the sieved (5 mesh) and air-dried samples to achieve the target concentration (as an individual element) as follows: (i) Cd0 (control with no Cd spiked) and (ii) Cd40 (the final target total Cd concentration was 40 mg/kg). To simulate indigenous Cd-contaminated soils as closely as possible, these artificially Cd-contaminated soils were subjected to the incubation treatment reported by Blaylock et al. (1997) and Chen et al. (2010).
2.2 Pot Experiment
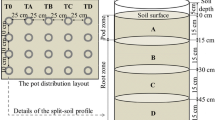
In addition to Cd treatments, two chemical fertilizer (CF) treatments were applied in this study with six replicates, including (i) CK—control with no CF and (ii) CF—N (as urea)/P2O5 (as KH2PO4)/K2O (as KCl) = 100:100:100 kg/ha. Approximately 1.0 kg of treated soil samples were placed in rectangle pots (L 15 cm, W 15 cm, H 10 cm). One seedling from a 15-day-old rooted impatiens (I. walleriana) cutting with a similar appearance was then transplanted in each pot. The pot experiment was conducted in the phytotron (110 μmol s−1 m−2; day/night = 12/12 h) at MingDao University with air temperature of 26.2 ± 1.5 °C and relative humidity of 80.7 ± 10.1 %. To control the soil moisture content at 60–80 % of the WHC, the pot was weighed and DI water was added every 2–3 days to compensate for loss through evapotranspiration. After the seedling grew for 54 days, the uncovered surface of each pot was sealed with aluminum foil and weighed every 24 h to determine the TR of the impatiens grown in the various treatments. During the TR measurement period, the soil moisture content was adjusted at 80 % of the WHC after each measurement.
2.3 LA Measurement
The impatiens shoots were harvested 60 days after transplantation and divided into stems and leaves. The soil samples were subsequently broken up, and the roots were then harvested. The organs were washed with tap water to remove adhered soil particles and then immersed in 20 mM Na2-EDTA for 1 h to remove exchangeable Cd. The leaves of each impatiens were placed on the scanner, and then the scanned images were printed on A4 paper. Two methods were used to determine the LA: the LA mass and the LA LiCor. The black parts of the A4 paper were cut and weighed, and the LA mass was calculated using the proportion of the mass between black and white. The black parts of another piece of A4 paper were also cut out, and the LA LiCor was measured with a portable area meter (LI-COR, LI-3000 portable area meter).
2.4 Subcellular Distribution Analysis
The varying subcellular distribution of Cd in the roots, stems, and leaves of impatiens was analyzed in accordance with Wang et al.’s (2008) method with some modifications. After the six seedlings grew in the different treatments for 60 days, three seedlings were randomly selected for this analysis. Fresh plant organs were homogenized with pestles and motors, which contained 0.25 M sucrose, 50 mM Tris–HCl (pH = 7.5), and 1 mM C4H10O2S2. The homogenate was centrifuged at 3000 rpm for 15 min, and the precipitated residue was the cell wall fraction (F cw). The supernatant solution was then centrifuged at 12,000 rpm for 30 min. The supernatant solution and the residue were the soluble fraction (F s) and the organelles fraction (F co), respectively. All steps were performed at 4 °C. The supernatant solution was first evaporated to dryness and then digested with HNO3/HClO4 (v/v = 3/1). The same processes were performed for the two types of residue. The Cd concentrations in the various fractions were determined with ICP/OES (PerkinElmer Optima 2100 DV). The Cd concentrations of the three subcellular fractions were totaled, and the recovery rate was calculated with the ratio between F co + F cw + F s and the total Cd concentrations. The data was considered valid when the recovery rate was in the 90–110 % range.
2.5 Plant and Soil Analysis
The three other seedlings were used to analyze the accumulated Cd concentration in the various organs. The plant organs were washed again with DI water, oven dried at 70 °C for 72 h, weighed, and then ground with a grinder. After being digested with HNO3/HClO4 (v/v = 3/1), quantified with DI water to 25 mL, and filtered with filter papers (Whatman no. 42), the Cd concentrations in the filtered solutions were measured with a flame atomic absorption spectrometer (FAAS; PerkinElmer AAnalyst 200). The soil samples collected after the pot experiment were pretreated with the processes described in Sect. 2.1.
2.6 Statistical Analysis
A statistical analysis was performed using Statistical Package for Social Science (SPSS; Armonk, NY, USA). The differences in the DW, LA, TR, Cd accumulation, and total Cd removal among the mean values of the Cd and CF treatments were evaluated with one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test. Statistical significance was defined at the level of p = 0.05.
3 Results
3.1 Soil Properties
The study soil was neutral (pH 7.32) with low ECw (0.44 dS/m) and low OC content (0.84 %). The initial soil total Cd concentration was 0.63 ± 0.31 mg kg−1, which was below the control standard (5.0 mg/kg) for croplands set by the Soil and Groundwater Contamination Remediation Act of Taiwan. After artificially spiking, the final soil total Cd concentration in the Cd40-CK and Cd40-CF reached 37.90 ± 0.96 and 37.82 ± 1.26 mg/kg, respectively.
The CF application had drastic effects on the soil properties (Table 1). Relative to CK, the pH in the CF treatments decreased from 7.2–7.4 to 6.8–7.1. However, the increases in the soil ECw reached 1.6–2.1 dS/m in the CF treatments compared with CK, and the highest value was close to the threshold (ECw = 2.5 dS/m) of saline soil.
3.2 Growth Responses of and Cd Accumulation in Impatiens
The CF and Cd treatments decreased and significantly decreased (p < 0.05) the DW of various impatiens organs (Fig. 1). Compared with CK, the DW of the roots, stems, and leaves of the impatiens grown in the CF treatments decreased or significantly decreased (p < 0.05) by 72–75, 44–62, and 52–74 %, respectively. For the Cd treatments and compared with CK, the decreases in the DW of the roots, stems, and leaves decreased by 27–34, 40–60, and 36–66 %, respectively. Similar negative effects of CF and Cd were observed in the LA that was measured with two methods (Fig. 2). Compared with CK, the LA of impatiens grown in the CF and Cd treatments decreased or significantly decreased (p < 0.05) by 55–76 and 49–69 %, respectively. The TR also significantly decreased (p < 0.05) by 62–87 and 53–84 % in the CF and Cd treatments, respectively, compared with CK (Fig. 3).
Effect of different treatments on the dry weight of impatiens. The same lowercase letters indicate no significant differences between treatments for the same organ. The same uppercase letters indicate no significant difference in the chemical fertilizer for the same Cd concentration and the same organ. Replicates (n) = 3
Effect of different treatments on the impatiens leaf area. The same lowercase letters indicate no significant differences between treatments for the same method. The same lowercase letters indicate no significant difference in the chemical fertilizer for the same Cd concentration and the same organ. Replicates (n) = 3
Because of the low Cd concentration in the Cd0, the Cd concentrations in the different organs of impatiens grown in Cd0 were not detectable regardless whether CF was applied or not (Fig. 4). The impatiens grown in the Cd40 accumulated higher concentrations of Cd compared with Cd0, and the roots accumulated higher concentrations of Cd compared with other organs. After growing in the Cd40-CK for 60 days, the Cd concentrations in the different organs of impatiens were in the decreasing order of 525 ± 197 (root), 102 ± 18 (stem), and 49 ± 8 mg/kg (leaf). Some of the Cd concentrations were beyond the threshold of a Cd hyperaccumulator (Cd > 100 mg/kg) (Baker et al. 2000). Except for the roots and compared with CK, the CF treatments significantly decreased (p < 0.05) the accumulated Cd concentration in the stems and leaves resulted from the decreasing in LA and TR (Figs. 2 and 3).
Effect of different treatments on the Cd accumulation of impatiens. The same lowercase letters indicate no significant differences between treatments for the same organ. The same capital letters indicate no significant difference in the chemical fertilizer for the same Cd concentration and the same organ. Replicates (n) = 3
After the seedlings grew in the Cd40-CK treatments for 60 days, the BCF of the stems and the leaves reached 2.7 and 1.3, respectively, which were all beyond the threshold (BCF > 1.0) of a hyperaccumulator (Sun et al. 2009). However, because of the high Cd concentrations in the roots, the TF of the stems and the leaves was approximately 0.2 and 0.1, respectively. At the end of the pot experiment and compared with CK, the soils had significantly higher ECw in the CF treatments (p < 0.05) (Table 1). The changes in the ECw not only affected growth exhibition, i.e., the DW, LA, and TR, but also had negative effects on the Cd accumulation in the impatiens shoots. For the roots, the Cd concentrations increased from 525 ± 197 to 705 ± 320 mg/kg (Fig. 4). However, the Cd concentration in the impatiens stems and leaves decreased significantly from 102 ± 18 and 49 ± 8 mg/kg to 65 ± 13 and 34 ± 9 mg/kg, respectively (p < 0.05). The CF treatments also decreased the BCF of the stems and the leaves to 1.7 and 0.9, respectively. The TF of the stems and leaves was less than 0.1 in the CF treatments.
As shown in Fig. 5, the total Cd removed (μg/plant) in the various organs of impatiens grown in Cd40-CK was in the decreasing order of roots (59.8 ± 40.8), stems (41.3 ± 30.3), and leaves (24.0 ± 14.3), while the roots accounted for 48 % of the total Cd in plants. Compared with CK, however, the Cd mass in the different organs of impatiens grown in the CF treatments decreased by 68 % (roots), 75 % (stems), and 79 % (leaves). This resulted from the decrease in the DW and the accumulated Cd concentrations in the CF treatments compared with CK.
Effect of different treatments on the total removal of Cd of impatiens. The same lowercase letters indicate no significant differences between treatments for the same organ. The same capital letters indicate no significant difference in the chemical fertilizer for the same Cd concentration and the same organ. Replicates (n) = 3
3.3 Subcellular Distribution
More than 76 % of the accumulated Cd in the roots of impatiens grown in Cd40 was compartmentalized in the F s. The CF application increased the percentages of F s in the stems and leaves of impatiens compared with CK (Fig. 6). Approximately 31–42 and 43–53 % of the accumulated Cd in the stems was compartmentalized in the F cw and F s, respectively. In contrast with the roots, Cd was mainly compartmentalized in the F cw in the leaves. Approximately 60–70 % of the Cd was compartmentalized in the Fcw in the leaves. Except for F co, the application of CF increased the Cd concentration in the F cw and F s.
4 Discussion
The amount of N, P2O5, and K2O for garden flowers recommended by the Council of Agriculture of Taiwan was applied in this study. However, because the pot height was only 10 cm, approximately 50 % more CF was applied. The excess CF application raised the soil ECw (Table 1) and thus inhibited the growth and Cd accumulation in the impatiens plants. Nevertheless, the objective of applying CF is to increase impatiens growth and to enhance the TR and Cd accumulation; the experimental results also showed that there were significantly positive linear relationships among the LA, the TR, and the Cd accumulation. The phytoextraction efficiency of impatiens should be increased through the increase in the LA due to fertilization.
Because cuttings were used in this study, the DW of the shoots (the sum of the stems and leaves) was far lower than that of seedlings bought from flower markets even after the cuttings were grown for 60 days. This is why the DW of the shoots grown in Cd40-CK was only 31 % of that reported by Wei et al. (2012), who planted marketable impatiens seedlings in artificially Cd-contaminated soil with a total Cd concentration of 37.6 ± 1.2 mg/kg. The total Cd removed in the shoots was only 65.3 μg/plant, which was far below the value (approximate 1127 μg/plant) reported by Wei et al. (2012). Lai et al. (2010) indicated that extending the growing period from 1 to 2 months increases the HMs that accumulate in different garden flowers by the maximum 3.8-fold. Further studies are needed to assess the effects of extending the growing period on enhancing the phytoextraction efficiency of impatiens.
Two methods were used in this study to measure the LA of impatiens grown in soil with various treatments. As shown in Fig. 7, there was a good linear relationship between the LA mass and the LA LiCor (LA mass = 0.5928 LA LiCor + 26.1885; r 2 = 0.9922). The TR also had a good linear relationship with the LA mass and the LA LiCor (r 2 = 0.9919–0.9995; Fig. 8). Experimental results revealed that for the LA mass and the LA LiCor, the measured LA had a positive relationship with the TR. The experimental results of this study provide further evidence that phytoextraction efficiency is improved when the LA and the TR of impatiens are increased as shown in the next paragraph.
Compared with the impatiens grown in Cd40-CF, the impatiens grown in the Cd40-CK had a significantly larger LA and a higher TR (p < 0.05), and accumulated significantly higher concentrations of Cd in the stems and leaves (p < 0.05). Because a possible excessive amount of CF was applied and the soil ECw drastically increased compared with CK in the CF treatment (Table 1), the LA and the TR significantly decreased by 79–88 and 94 %, respectively (p < 0.05) (Figs. 2 and 3). The Cd concentrations in the leaves and stems of impatiens grown in Cd40-CF also decreased significantly by 77 and 83 % compared with Cd40-CK (p < 0.05). These results show that there was positive relationship between LA, TR, and the Cd accumulation. Impatiens with larger LA will have a higher TR, and the Cd accumulation in the shoots will thus increase. As mentioned by Liu et al. (2010), Cd accumulation in the shoots of plants was controlled by root uptake, radial transport, xylem loading, and translocation form root to shoot. All these processed was driven by transpiration. That is why many studies indicated the important role of TR on the translocation and accumulation of Cd. Salt et al. (1995) indicated that transpiration plays an important role in the mass flow and thus Cd translocation to the shoots of Brassica juncea. Liu et al. (2010) observed that there was a significantly positive relationship between shoot Cd concentration and leaf transpiration. Nevertheless, many studies have reported that Cd has a negative effect on the LA and chlorophyll content (Shi and Cao 2009; Xu et al. 2013). The total removal will decrease under Cd stress because of the decrease in LA and thus the TR.
In the roots of impatiens grown in Cd40-CK and Cd40-CF, approximately 76–85 % of the Cd was compartmentalized in the F s. However, approximately 60–69 % of the Cd in the leaves was compartmentalized in the F cw. The plant cell wall is primarily made up of cellulose, hemicellulose, lignin, mucilage, and proteins. The functional groups provided by these compounds could restrict the translocation of Cd and further protect the organelles from Cd toxicity (Qiu et al. 2011). Many studies have indicated that plants compartmentalize HMs in the cell wall to alleviate their toxicity (Lasat et al. 1996; Jarvis and Leung 2001; Wang et al. 2008) because the proteins and polysaccharides of the cell wall create a complex with the HMs and restrict their translocation (Allen and Jarrell 1989).
Previous studies observed that HMs in the F s have a higher migration capacity than the other two fractions and the cell wall played an important role in the detoxification of HMs (Zhu et al. 2013). The CF treatment affected the growth of impatiens and thus the subcellular distribution of Cd, especially in the leaves. Compared with Cd40-CF, the impatiens grown in Cd40-CK had a better growth performance, and more percentage of Cd in the root was compartmentalized in the F s (Fig. 6). This means that the Cd accumulated in the roots of impatiens will transfer to stems or leaves easily because of their high mobility. Once transferred to the shoots, the Cd will compartmentalize in the cell wall to avoid Cd toxicity. For the leaves and compared with Cd40-CF, more Cd was compartmentalized in the F cw. However, the TF of the stems and leaves was less than 0.2 in the Cd40-CK and Cd40-CF treatments. The experimental result of this study was different from Wei et al. (2012), who used seedlings bought at a market and indicated that the TF of impatiens grown in Cd40 was 1.74. This phenomenon revealed that further study with a longer growing period is needed because cuttings instead of seedlings were used in this study.
5 Conclusions
There were positive, linear relationships between the LA, TR, and Cd accumulation of impatiens. Most of the Cd accumulated in the roots and leaves of impatiens was compartmentalized in the F s and F cw, respectively. The difference in the subcellular distribution in the various organs of impatiens is responsible for the accumulation and detoxification of Cd. In the roots of impatiens, the uptake Cd was compartmentalized mainly in the F s, which with high migration and can further translocate to shoots by the mass flow driven by transpiration. However, many previous studies indicated that the TR decreased under Cd stress. How to increase the LA is thus a key point in enhancing the phytoextraction efficiency of impatiens in future studies.
References
Allen, D. L., & Jarrell, W. M. (1989). Proton and copper adsorption to maize and soybean root cell walls. Plant Physiology, 89, 823–832.
Baker, A. J. M., McGrath, S. P., Reeves, R. D., & Smith, J. A. C. (2000). Metal hyperaccumulator plants: a review of the ecology and physiology of a biological resource for phytoremediation of metal-polluted soils. In N. Terry & G. Bañuelos (Eds.), Phytoremediation of contaminated soil and water (pp. 85–107). Boca Raton, FL: CRC Press, LLC.
Blaylock, M. J., Salt, D. E., Dushenkov, S., Zakharova, O., Gussman, C., Kapulnik, Y., Ensley, B. D., & Raskin, I. (1997). Enhanced accumulation of Pb in Indian mustard by soil-applied chelating agents. Environmental Science and Technology, 31, 860–865.
Chen, H. S., Huang, Q. Y., Liu, L. N., Cai, P., Liang, W., & Li, M. (2010). Poultry manure compost alleviates the phytotoxicity of soil cadmium: influence on growth of pakchoi (Brassica chinensis L.). Pedosphere, 20, 63–70.
Ernst, W. H. O. (2005). Phytoextraction of mine wastes—options and impossibilities. Chemie der Erde-Geochemistry, 65, 29–42.
He, S. Y., Wu, Q. L., & He, Z. L. (2013). Effect of DA-6 and EDTA alone or in combination on uptake, subcellular distribution and chemical form of Pb in Lolium perenne. Chemosphere, 93, 2782–2788.
Jarvis, M. D., & Leung, D. W. M. (2001). Chelated lead transport in Chamaecytisus proliferus (L.f.) link ssp. Proliferus var. palmensis (H. Christ): an ultrastructural study. Plant Science, 161, 433–441.
Lai, H. Y., Juang, K. W., & Chen, Z. S. (2010). Large-area experiment on uptake of metals by twelve plants growing in soils contaminated with multiple metals. International Journal of Phytoremediation, 12, 785–797.
Lasat, M. M., Baker, A. J. M., & Kochian, L. V. (1996). Physiological characterization of root Zn2+ absorption and translocation to shoots in Zn hyperaccumulator and nonaccumulator species of Thlaspi. Plant Physiology, 112, 1715–1722.
Lin, C. C., Lai, H. Y., & Chen, Z. S. (2010). Bioavailability assessment and accumulation by five garden flower species grown in artificially cadmium-contaminated soils. International Journal of Phytoremediation, 12, 454–467.
Liu, X. Q., Peng, K. J., Wang, A. G., Lian, C. L., & Shen, Z. G. (2010). Cadmium accumulation and distribution in population of Phytolacca americana L. and the role of transpiration. Chemosphere, 78, 1136–1141.
Luo, C., Shen, Z., Li, X., & Baker, A. J. M. (2006). Enhanced phytoextraction of Pb and other metals from artificially contaminated soil through the combined application of EDTA and EDDS. Chemosphere, 63, 1773–1784.
Qiu, Q., Wang, Y., Yang, Z., & Yuan, J. (2011). Effects of phosphorus supplied in soil on subcellular distribution and chemical forms in two Chinese flowering cabbage (Brassica parachinensis L.) cultivars differing in cadmium accumulation. Food and Chemical Toxicology, 49, 2260–2267.
Salt, D. E., Prince, R. C., Pickering, I. J., & Raskin, I. (1995). Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiology, 109, 1427–1433.
Shi, G. G., & Cao, Q. S. (2009). Leaf plasticity in peanut (Arachis hypogaea L.) in response to heavy metal stress. Environmental and Experimental Botany, 67, 112–117.
Sun, Y. B., Zhou, Q. X., Wang, L., & Liu, W. T. (2009). Cadmium tolerance and accumulation characteristics of Bidens pilosa L. as a potential Cd-hyperaccumulator. Journal of Hazardous Materials, 161, 808–814.
Wang, X., Liu, Y. G., Zeng, G. M., Chai, L. Y., Song, X. C., Min, Z. Y., & Xiao, X. (2008). Subcellular distribution and chemical forms of cadmium in Bechmeria nivea (L.) Gaud. Environmental and Experimental Botany, 62, 389–395.
Wei, J. L., Lai, H. Y., & Chen, Z. S. (2012). Chelator effects on bioconcentration and translocation of cadmium by hyperaccumulators Tagetes patula and Impatiens walleriana. Ecotoxicology and Environmental Safety, 84, 173–178.
Wójcik, M., Vangronsveld, J., D’Haen, J., & Tukiendorf, A. (2005). Cadmium tolerance in Thlaspi caerulescens: II. Localization of cadmium in Thlaspi caerulescens. Environmental and Experimental Botany, 53, 163–171.
Wu, F. B., Dong, J., Qian, Q. Q., & Zhang, G. P. (2005). Subcellular distribution and chemical form of Cd and Cd-Zn interaction in different barley genotypes. Chemosphere, 60, 1437–1446.
Xu, D., Chen, Z., Sun, K., Yan, D., Kang, M., & Zhao, Y. (2013). Effect of cadmium on the physiological parameters and the subcellular cadmium localization in the potato (Solanum tuberosum L.). Ecotoxicology and Environmental Safety, 97, 147–153.
Zhu, Q. H., Huang, D. Y., Liu, S. L., Luo, Z. C., Rao, Z. X., Cao, X. L., & Ren, X. F. (2013). Accumulation and subcellular distribution of cadmium in remie (Boehmeria nivea L. Gaud.) planted on elevated soil cadmium contents. Plant, Soil and Environment, 59, 57–61.
Acknowledgment
This research was sponsored by the National Science Council of Taiwan under grant no. NSC 102-2313-B-451-003.
Conflict of Interest
The author declares that there is no conflict of interest regarding the publication of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lai, HY. Effects of Leaf Area and Transpiration Rate on Accumulation and Compartmentalization of Cadmium in Impatiens walleriana . Water Air Soil Pollut 226, 2246 (2015). https://doi.org/10.1007/s11270-014-2246-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-2246-9