Abstract
While the presence of fecal indicator bacteria such as Escherichia coli in urban stormwater has been widely documented, their occurrence and persistence in sediments are not as well understood. Recent investigations suggest that E. coli can accumulate in drainage basin sediments and act as a fecal bacterial reservoir within a watershed. We investigate the prevalence of E. coli populations in a tidal creek stormwater catchment and examine their interaction with overlying stormwater under wet and dry weather conditions. Two rain events are sampled more intensively with samples collected prior to, during, and after rainfall to profile bacteria in each matrix throughout a storm. Results of profile sampling and estimates of sediment resuspension provide evidence for E. coli accumulation during dry conditions and entrainment in overlying waters during storm conditions. Profile results suggest the occurrence of steady-state E. coli populations in drainage basin sediments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nonpoint source contamination of surface water is a topic of growing concern. Substantial nonpoint source pollution can be generated as stormwater accumulates within a drainage basin and moves towards a receiving waterbody (Makepeace et al. 1995). Unfortunately, in many regions, these receiving waters are riparian, estuarine, and coastal environments that have critical ecological and anthropogenic value (Barbier et al. 2011). Stormwater is an important component of the hydrologic cycle, aiding in recharge of lakes, streams, and aquifers (Winter et al. 1998). Stormwater not only provides essential fresh water for local flora and fauna but also acts as a transportation mechanism for nutrients (Wahl et al. 1997), organic matter, and detritus (Badin et al. 2008). However, in urban areas as well as lands adjacent to agricultural operations, stormwater may serve as a means of chemical and bacteriological pollutant collection and transport. For areas experiencing considerable runoff, the ecological implications are that stormwater may act as a regular source of pollutant input. Contaminants accumulated via overland flow are deposited into stormwater streams each time it rains, ultimately entering estuaries and the coastal ocean, degrading water quality (Ahn et al. 2005). Water quality impairment also has economic implications, as many coastal cities derive significant benefit from fisheries and recreational activities associated with these waters. Coastal areas are not only the recipient of upstream drainage but are also desirable places for human habitation. As a result, coastal urban environments seem to be disproportionately impacted by surface water runoff contamination (Mallin et al. 2000). These concerns for coastal areas may be exacerbated by expanding impervious cover associated with development and greater sources of pollutant input that have been shown to accompany an increasingly dense population (DiDonato et al. 2009).
Traditional investigations into bacterial contamination of surface waters have focused primarily on inputs from overland flow of waste materials (Athayde et al. 1983; Geldreich et al. 1968; Lord 1987). Agricultural runoff (US EPA 1998), pet waste (Ram et al. 2007), failing septic systems (Weiskel et al. 1996), and waterfowl (Lu et al. 2008) are among the growing list of identified sources contributing to the bacterial contamination of recipient waterbodies. Fecal indicator bacteria (FIB) are the conventional group of species that act as a proxy for the presence of more harmful pathogens in a waterbody. Epidemiological studies have shown these bacteria to be well correlated with increased risk of waterborne illness (Wade et al. 2003; Zmirou et al. 2003). As a result, FIB are used as a quality measure for the monitoring of recreational waters, fisheries, drinking water, and treated wastewater discharge. While overland transport of microbial contaminants is certainly a key component in the degradation of water quality, it is possible that there are other sources to consider.
Currently, there is a growing body of work suggesting that sediments and other matrices may play an important role in FIB prevalence and transport in a stormwater drainage system (Fries et al. 2006; Jamieson et al. 2005; Solo-Gabriele et al. 2000; Jeng et al. 2005; Ackerman and Weisberg 2003). These studies note bacterial persistence in a variety of substrates and vegetation types (Badgley et al. 2011) associated with streams (Jamieson et al. 2003), best management practice (BMP) detention ponds, lakes (Chandran et al. 2011), and beaches (Boehm et al. 2009). The underlying sediment environment has been particularly implicated as a potential reservoir for FIB. FIB including E. coli have been shown to adsorb to alluvial sediment particles (Jamieson et al. 2005; Friedlander et al. 2013) where they are able to survive more readily, accumulating in the banks of a stormwater collection basin. Because FIB such as E. coli are not well suited to life in the water column of a stream or estuary, adsorbing to sediment particles likely increases their chance for survival (Evison 1988; Winfield and Groisman 2003). Some of the suggested advantages of adsorbing to sediment particles include access to sediment-bound nutrients (Davies et al. 1995), protection from protozoan predation (Davies and Bavor 2000), and a potential shelter from UV inactivation (Fujioka et al. 1981). Adsorbing to sediment particles of larger mass also allows bacteria such as E. coli to settle out of suspension during times of decreased flow. The result of bacterial adsorption and settling could be the accumulation of substantial populations of FIB within the sediment environment, in addition to those found in overlying waters. These sediment-bound bacteria may then be resuspended during increased flow conditions, acting as an additional bacterial input during storms (Jamieson et al. 2005; Solo-Gabriele et al. 2000). The result of this sediment-driven response could influence the perception of water quality, which may appear to have been impacted by recent fecal inputs and associated pathogens but actually experiences elevated bacteria levels due to sediment resuspension. Thus, a better understanding of this bacterial reservoir and flushing phenomenon has important implications for the way water quality is monitored. Using FIB as a proxy for other pathogenic species is only reliable if these bacteria are associated with the same sources. If indicator bacteria such as E. coli have an extended persistence in sediment environments and a regular interchange with overlying water column, their efficacy as a proxy for fecal contamination may be problematic.
Here, we seek to further the understanding of drainage basin sediment as a possible source/sink for E. coli. Specifically, we aim to examine E. coli populations in sediment and water matrices across wet and dry conditions to determine if sediments in stormwater drainage basins act as a long-term bank and transport mechanism for E. coli. We employ a novel, more intensive sampling technique for rain events in an effort to profile E. coli populations within each matrix over the life of a storm event. We examine BMP bacterial removal efficiency as a means of investigating FIB transport via sediment particles. Additionally, we explore if physical characteristics of sediments such as grain size and organic content correlate with bacterial prevalence.
2 Methods
2.1 Site Description
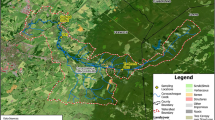
The study area was located in the upstream watershed of Withers Swash, a tidal estuary located in Myrtle Beach, South Carolina (Fig. 1). Myrtle Beach typifies the expanding urban coastal environment, which often experience problems with contaminated stormwater runoff due to increased impervious surface and development (Mallin et al. 2000). Withers Swash, with a surface area of 10.6 km2, accounts for the largest of 29 drainage basins within the Myrtle Beach area (Guimaraes 1995). As a result, Withers Swash receives stormwater runoff from a variety of land uses including commercial facilities, residential developments, amusement parks, and campgrounds, comprised of approximately 33 % impervious cover within the watershed (Tolleson et al. 1998). Currently, Withers Swash is on the South Carolina Department of Health and Environmental Control’s (SCDHEC) 303(d) list of impaired waterbodies due to elevated levels of FIB. As there is a regular tidal exchange, bacteria found in the estuary are frequently transported to the coastal environment, particularly during instances of heavy rainfall. The results of this communication with the ocean are health advisories for swimmers and permanent posting warning against swimming adjacent to the swash outfall, particularly after rainfall.
Fourteen study sites were identified in this investigation. Eleven of these were delineated to represent discrete subwatersheds within the Withers Swash drainage basin. All sites are located in upstream, open drainage ditches, excluding site 4 which is a stormwater pipe, and site 12 which is located in the tidally influenced body of Withers Swash. Four of these sites (3, 6, 7, and 9) are open streams located immediately downstream of a BMP stormwater pond outfall. Previous studies in this area classified the soils as Lakeland-Leon-Newhan, which are sandy and poorly drained and Brookman-Bladen with have a loamy surface layer and poorly drained clayey subsoil (Guimaraes 1995).
2.2 Sample Collection
Sampling was conducted from April through September 2012. Sediment and overlying stormwater samples were collected concurrently during each sampling event from all sites. In order to investigate bacterial activity in sediment and stormwater during instances of baseline and increased flow, samples were collected during two dry and three wet weather events. Criteria for a wet weather event were determined using hydrographs generated for each site prior to the sampling period. Based on these hydrographs, it was determined that at least 0.64 cm of accumulated rainfall would be required to generate significant overland flow and thus constitute a wet event. In accordance with EPA stormwater sampling guidance, both wet and dry sampling events also required 72-h antecedent dry conditions to be considered an independent event (US EPA 1992). Rainfall within the study area was monitored remotely using a weather station provided by the City of Myrtle Beach. In addition to these five independent sampling events, two storms were profiled in an effort to examine bacterial populations across the course of an entire rain event. Sediment and stormwater samples were collected at each site immediately prior to an anticipated rainfall (thus before the rising limb of the hydrograph) and considered “pre-rain” samples. Samples were collected successively, within approximately 2 h. Once the criteria for a wet weather event were met, sediment and stormwater were collected again from all sites, representing the “during rain” samples. “Post-rain” samples were then collected from each site after conditions had returned to approximately base flow levels, typically on the order of 24–48 h. Sampling at these three intervals was used to better understand bacterial persistence and fluctuations in population for each matrix during a storm event.
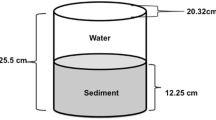
Sediment samples were collected from each site, midstream, approximately equidistant from each bank. All sites, excluding 10, maintain some water even during dry conditions. As a result, samples were collected at the approximate midpoint of the ditch in an effort to sample consistently saturated soils and avoid the potentially confounding effects of periodic wetting on bacterial prevalence. Samples were collected using a 2.5-cm plastic sediment core tube, sterilized with ethyl alcohol and triple field rinsed in stormwater prior to collection. At each location, the collection tube was pushed into the sediment to a depth of 5 cm, any water that entered the tube was decanted, and the sediment was placed in a sterilized polyethylene cup. Three samples were collected per site and composited into a single cup, representing the sediment sample for that site.
To confirm that three subsamples provided sufficient representation of the site, system variability testing was conducted. Development of this testing was based on the US Geologic Survey (USGS) Techniques in Water Resources Investigations Book 9 guidelines for number of required bottom-material samples (Radke 2005). Six discrete samples were collected at three sites to assess within-site variability. Formula 1 was employed for each site using the range of bacteria concentrations in the six samples to calculate the margin of error (d) associated with collecting three subsamples per site. Results demonstrated that three composited subsamples would adequately represent a site, with the margin of error for each site falling below ±28 most probable number (MPN) g−1 at a confidence interval of 95 %.
where
- n :
-
The number of required samples
- t :
-
Confidence interval from T table
- s :
-
The variance in prior samples, or if unknown, \( s=\frac{\mathrm{range}}{4} \)
- d :
-
Acceptable margin of error (±d MPN g−1)
At the time of sediment sample collection, grab samples of stormwater were collected in 100-ml sterile plastic bottles buffered by sodium thiosulfate to neutralize any residual chlorine present, which may kill bacteria. All samples were stored on ice and returned to the lab for bacterial analysis, conducted within 3 h of first sample collection.
2.3 Bacteria and Sediment Analyses
2.3.1 Bacterial Analysis
E. coli concentrations were determined for all sediment and stormwater samples using Colilert IDEXX defined substrate technology. Stormwater samples were analyzed and enumerated in accordance with manufacturer’s recommended methods and reported as MPN per 100 ml of sample.
For sediment bacterial analysis, samples were manually homogenized using a sterilized spatula. Two 10 g aliquots were removed from each sample. One aliquot was given a 24-h drying treatment in a 100 °C oven. The resulting dry mass was used in calculating bacterial concentration. Pore water content of sediment samples, determined from drying treatments, was compared against bacteria concentrations using basic regression and a Wilcoxon signed-rank test. Results showed a significant correlation using each test although the relationship was weak (r 2 = 0.047, p = 0.024). The second 10 g aliquot was added to a sterile 250-ml glass container. Two hundred milliliters of sterile Milli-Q high-purity water was added to the container. The container was then manually shaken for a period of 2 min to resuspend and desorb E. coli bacteria and sediment particles. The mixture was allowed to settle again for a period of 5 min so that larger fraction particles unlikely to harbor bacteria could again settle out. The supernate from this agitation process was then filtered through a sterile 30-μm nylon mesh filter to further remove the sediment fraction above this threshold. The resulting filtrate was then analyzed using Colilert for FIB enumeration and reported as MPN per gram of dry sediment. Bacteria dilution and resuspension methods were developed based on similar work by Solo-Gabriele et al. (2000). This method was adapted to accommodate varying sediment grain sizes in the study area, which required the addition of a 5-min settling period. Resuspension and enumeration techniques were verified prior to field sampling to ensure that bacteria were not lost due to resuspension methods. In brief, bacteria samples were processed in the described manner, testing a variety of shaking and settling times to determine the best combination for extracting and detecting the most bacteria possible (93.2 % recovery for selected method). Additionally, 30-μm filters were plated on Easygel ® (Micrology Laboratories, LLC) culture plates to ensure that bacteria associated with the larger sediment fraction were not lost due to the filtration process. Results of extraction testing showed minimal (<3 CFU) bacterial loss as a result of the filtration process.
2.3.2 Stream and Sediment Characterization
Grain size and percent organic content of sediment samples from each site were examined in order to investigate suggested correlations with their propensity to harbor FIB. Grain size analysis was conducted using a Beckman Coulter LS 13 320 Laser Diffractor. All samples were tested with and without a 48-h hydrogen peroxide treatment to determine if high organic content and aggregation produced a significant effect on the bacteria particle size relationship. A paired samples T test was used, and it was determined that samples analyzed for grain size using a peroxide treatment were not significantly different than those without (p = 0.689). As this study was interested in the effects of sediment particles on bacterial prevalence, regardless of aggregation, particle sizes determined without a hydrogen peroxide treatment were used for all statistical analyses.
Percent organic content of sediment samples was determined for each site using a loss on ignition (LOI) procedure. For this technique, the wet mass of a subsample of sediment from each site was determined using a mass balance. These subsamples were then given a 24-hr drying treatment at 100 °C to reach a constant mass once all interstitial water was evaporated. The dry mass of each subsample was recorded, and samples were then ignited in a muffle furnace at 500 °C for a period of 6 h (ASTM D2974-07a). Mass after ignition was determined, and this process of igniting and weighing was repeated until all organic content had been oxidized and samples again reached a stable mass. Organic content for sediments from each site was determined as a percentage of the total dry mass for all statistical analyses.
Estimates of flow (formula 2) and bed shear stress (formula 3) for dry and wet weather conditions were calculated in order to investigate the propensity for sediment resuspension within the system. Flow was determined using Manning’s equation describing flow as a function of stream geometry
where
- Q :
-
Flow (m3 s−1)
- n :
-
Manning’s roughness coefficient (using 0.035)
- R :
-
Hydraulic radius (m)
- S :
-
Slope (m m−1)
- A :
-
Cross-sectional area of flow (m2)
where
- y :
-
The specific weight of water (N m−3)
2.4 Sediment and Stormwater Normalization
To compare bacteria populations between sediment and stormwater matrices, a normalization calculation was employed to bring all bacteria measures to a common unit. A modified version of the calculation proposed by Badgley et al. (2011) was used to estimate FIB populations in terms of surface area (CFU m−2) based on stormwater and sediment concentrations and characteristics. The following formulas were used to determine bacterial population density for sediment (formula 4) and stormwater (formula 5) samples.
where
- SEDm 2 :
-
E. coli concentration (CFU m−2)
- SEDg :
-
E. coli concentration (CFU g−1 sediment)
- SEDde :
-
Depth of E. coli colonization (5 cm used for all calculations)
- SEDdn :
-
Sediment density (g cm−1)
where
- SWm 2 :
-
E. coli concentration (CFU m−2)
- SWml :
-
E. coli concentration (CFU 100 ml−1)
- SWde :
-
Stormwater depth (m)
2.5 Statistical Analyses
Sediment and stormwater were sampled during two dry and three wet events. Additionally, two storms were profiled with samples taken prior to, during, and after rain. For analysis, data from the two dry events as well as pre-rain data from the two profiled storms were used and considered dry weather data. Wet event data consisted of the three discrete wet events as well as the “during rain” data collected during the two profiled storms. Post-rain data were only used for within-site comparisons of sediment and stormwater concentrations throughout a rain event.
All statistical analyses were conducted using SPSS Statistical Software version 20. It was typically necessary to log transform E. coli and grain size data in order to meet normality assumptions of parametric statistical techniques. Percent organic content measurements were logit transformed, as they are percent, not measurement data. Where possible multiple techniques were employed to verify results. An a priori significance level of 0.05 is used for all tests unless otherwise stated.
3 Results
3.1 Bacterial Enumeration
Sediment and stormwater showed considerable variability according to weather condition and site with E. coli concentrations ranging from 1.5 to 794.6 MPN g−1 in sediment and 20.0 to >48,392.0 MPN 100 ml−1 in stormwater (Table 1). The data from the two profiled rain events (Table 2) showed a similar range in sediment (1.8 to 715.3 MPN g−1) and stormwater (22.0 to >48,392.0 MPN 100 ml−1) E. coli concentrations.
3.2 Physical Characteristics
Results of grain size analyses with and without hydrogen peroxide treatments and percent organic content determination can be found in Table 3. These data were used to examine the relationship between sediment particle grain size, percent organic content, and E. coli concentrations of the system as a whole via multiple linear regression. Sediment E. coli values for each site were averaged for all sampling events and regressed against the two physical components (grain size and percent organic content). Average E. coli values for wet and dry sampling events only were also analyzed individually for correlation with grain size and organic content. Grain size and organic content effects on E. coli were also examined using multiple analysis of variance (MANOVA) to note if changes in either component significantly influenced sediment E. coli concentrations.
Multiple regression analyses showed a significant positive correlation between grain size, organic content, and sediment E. coli concentrations during wet weather samples (adjusted r 2 = 0.396, p = 0.042). Of the two independent factors, grain size made the greatest unique contribution to explaining the overall variation in sediment E. coli concentrations (β = 0.709, p = 0.021).
These findings were supported by the results of a MANOVA test for grain size or organic content effects, which suggested that these factors contribute significantly to E. coli variability. Both main effects (Wilks λ = 0.575 and 0.474, respectively) and between-subject effects were significant for grain size and organic content, each with a p value of 0.002.
Estimates of flow and bed shear stress (Table 4) showed an expected change according to weather conditions. Both flows (p = 0.05) and shear stress (p = 0.019) values were significantly greater during wet weather conditions. Overall, the average increase in flow for a given site during wet conditions was 0.30 m3 s−1 which resulted in an average shear stress increase of 1.25 N m−2.
3.3 Weather and Site Effects
Weather and site effects were examined using MANOVA to determine if either variable plays a significant role in determining bacteria concentration of sediment or stormwater. Main effects were considered at a significance level of 0.05, while between-subject effects required a Bonferroni adjustment, using a 0.025 significance level. To verify these results, a nonparametric Mann-Whitney U test was conducted. The Mann-Whitney technique was used to test the null hypothesis that E. coli populations in sediment and stormwater would not significantly differ according to weather conditions at the time of sample collection.
MANOVA results showed that sample location within the watershed plays a role in determining bacterial concentration of sediment and stormwater samples (Wilks λ = 0.335, p < 0.001). This test also suggests that weather condition has an overall effect on E. coli concentration (Wilks λ = 0.519, p < 0.001); however, examining between-subject effects shows that weather only significantly influenced stormwater bacteria levels (p < 0.001), not those found in sediment samples (p = 0.656). Stormwater E. coli concentrations were significantly higher for samples collected during wet weather while sediment concentrations did not differ significantly by weather condition when considering the entire system. This does not imply that at all sites, sediment E. coli concentrations did not vary according to weather but is an assessment of the system as a whole across weather conditions. These findings were corroborated by the Mann-Whitney results, which rejected the null hypothesis that weather has no effect on E. coli concentrations for stormwater but could not reject this same hypothesis for sediment, indicating a significant weather effect for stormwater only (Fig. 2).
3.4 Estimate of Sediment and Stormwater E. coli Population Distribution
Sediment and stormwater E. coli measures, normalized to CFU m−2, were examined during dry and wet weather conditions to determine the relative population size of bacteria in each matrix (Table 5). Determining approximate E. coli loading and population distribution between sediment and stormwater during both wet and dry weather conditions facilitated the testing of the bacterial reservoir, flushing hypothesis. Normalized data for each matrix were log transformed, and a student’s t test was used to compare sediment and stormwater E. coli concentrations during dry conditions and then repeated for E. coli levels during wet conditions. In order to verify these findings, a Wilcoxon signed-rank technique was used to test the null hypothesis that normalized E. coli populations in sediment and stormwater would not significantly differ according to weather conditions at the time of sample collection.
Results of the student’s t test showed that E. coli populations were significantly larger in sediment samples than in stormwater when collected during dry conditions (p = 0.001). During wet conditions, stormwater E. coli concentrations were about five times as high as those in sediment, which was also a significant difference (p = 0.003). These results were verified by the results of the Wilcoxon signed-rank test, which rejected the null hypothesis for both stormwater and sediment, indicating a significant difference in E. coli values between each matrix during both wet (p = 0.004) and dry (p = 0.003) conditions. A comparison of normalized E. coli concentrations for each matrix is provided in Fig. 3.
4 Discussion
4.1 E. coli Prevalence in Sediments
This study focused on the sediment environment as a potential sink and source of FIB within a watershed. The results of field sampling showed that the FIB E. coli persists in the benthic environment of drainage basin streams and the estuary into which these waters drain. The upper 5 cm of sediment showed dense E. coli concentrations (up to 103 MPN g−1) during dry and wet weather conditions (Table 1). High E. coli concentrations in sediments during dry conditions suggest that these bacteria are able to adsorb to sediment particles, allowing them to fall out of suspension during times of decreased flow. Previous studies by Chandran et al. (2011) and Craig et al. (2004) have reported prolonged bacterial survival in the sediment environment, resulting in a reservoir of sediments with dense bacterial concentrations. Our results support this hypothesis, as E. coli concentrations in sediment were often as high or higher during dry weather conditions as those sampled during rain events (Figs. 2 and 4). These data suggest that the sediment environment enhances bacterial survival for those FIB that settle out of suspension, leading to a bacterial reservoir within the drainage basin.
Normalizing E. coli measures to a common unit (CFU m−2) allowed for further examination of E. coli populations in watershed sediments and stormwater (Fig. 5). These data showed that for samples collected during dry conditions, sediment E. coli concentrations were significantly higher than those found in stormwater, while wet conditions were associated with higher stormwater E. coli concentrations (Fig. 3). Greater bacterial densities during dry conditions support the buildup of a bacterial reservoir via sediment deposition. The relative bacterial populations of each matrix were also compared using these normalized measures. When considering the entire system, results indicated that during dry weather, sediment populations account for approximately 68 % of the total E. coli load (Table 5). This finding suggests that despite variability in sediment composition and hydrologic conditions between sites, reservoirs of E. coli accounting for 2/3 of the total E. coli population for the system are evident. While this comparison does not consider additional populations of FIB beyond stormwater and sediment (e.g., submerged aquatic vegetation), it does suggest that in parts of the system, the sediment environment may account for a significant fraction of the overall E. coli population between rain events (Table 5).
Rain event profile results showing bacterial concentrations in sediment before (Pre), during (During), and after (Post) rainfall for sites which exhibit a reservoir, flushing, and rebound response (a) and those that do not (b). Site 10 has no pre-rain sample, as it does not retain water during dry conditions
Physical characteristics of sediments such as organic content and grain size were also examined, as they have been suggested to influence bacterial survival in the sediment environment. In the Withers Swash, watershed grain size and organic content had a significant effect on sediment E. coli concentrations (r 2 = 0.396, p = 0.042), as multiple regression results showed that each was positively correlated with E. coli density. Within this analysis, grain size makes the largest contribution to explaining E. coli variability (β = 0.709, p = 0.021), indicating that these bacteria adsorb more frequently to larger sediment particles. Based on the findings of Chandran et al. (2011) and Craig et al. (2004) who explain enhanced bacterial survival within a more organic-rich sediment environment, we believe that our results suggest greater E. coli adsorption not to large grain size mineral sediments but aggregations of smaller, organic-rich particles.. It is possible that these bacteria survive longer once adsorbed to sediments with a higher organic content as a result of access to nutrients, which may be limited in more mineral-rich soils and the water column (Craig et al. 2004). The findings of Korajkic et al. (2013) showed the importance of predation on FIB survival in freshwater sediments. Their results indicate that adsorption to sediment particles may also provide shelter from protozoan predation.
4.2 BMP Sampling and Bacterial Transport
BMP efficiency testing provided an additional means of examining bacterial accumulation and transport associated with drainage basin sediment. As stormwater ponds retain water, the flow rate is decreased and sediment particles are allowed to settle out of suspension. Bacteria adsorbed to sediment particles are also deposited, resulting in a reduction in downstream FIB concentrations. A MANOVA was conducted to examine the impact that the presence (n = 4 site)/absence (n = 10 sites) of a BMP had on sediment and stormwater E. coli concentrations. Main effects results indicated a significant BMP effect on overall E. coli concentrations (Wilks λ = 0.887, p < 0.001). Between-subject effects verified that samples of both sediment (p = 0.004) and stormwater (p = 0.014) collected from BMP outfalls have significantly lower E. coli concentrations.
Results from our comparisons indicated that the four sites sampled at BMP outfalls had significantly lower E. coli concentrations than those without a BMP (Fig. 6). This suggests that bacteria are being transported via sediment adsorption versus freely floating cells and that increased residence times in BMP ponds are effective at removing bacteria from the water column. This finding is consistent with estimated settling times of E. coli cells not adsorbed to sediment particles of 0.0052–0.021 cm h−1 (McClaine and Ford 2002). Even using the most generous settling velocity estimates, freely floating E. coli cells would only settle 14.1 cm over the course of a month under no-flow conditions. Settling times for sediment particles and the associated bacteria are more complex and vary according to particle size; however, a study by Schillinger and Gannon (1985) reported 73–86 % of particles with a diameter greater than 5 μm settling in 5 h. While retention in a BMP may also allow for inactivation via UV exposure or predation, adsorption to sediment particles has been suggested to provide shelter and diminish the likelihood of these types of cell death (Fujioka et al. 1981; Davies and Bavor 2000). Therefore, while unassociated bacteria within a retention pond are likely still influenced by these factors, those attached to sediment particles may experience some level of protection and are more readily removed from the water column via deposition. It could be thus reasoned that this same settling of bacteria-laden particles occurring in the stormwater ponds would occur in the stormwater streams as flow rates slow and could account for the increases in bacteria following a storm event (Fig. 4a).
4.3 Evidence for Resuspension
Sampling sediment and stormwater throughout the course of a rain event provided greater insight into FIB activity. Dry weather sampling offered evidence of bacterial adsorption and deposition while wet weather sampling alone simply described bacteria levels in a given water sample. Profiling an entire rain event at all sites, however, allowed for comparison of populations in each matrix within the same rainfall event. This provided more direct evidence of bacterial accumulation, mobilization, and population rebound. Sixty-six percent (18 of 27) of profiles produced exhibited a trend consistent with bacterial reservoir and flushing activity (Fig. 4a). These sites contained elevated concentrations of E. coli in sediments immediately prior to rainfall and a subsequent decrease of bacteria during the stormwater event. A drop in sediment E. coli concentrations coinciding with elevated flows during rainfall was considered on a site-by-site basis and suggested that sediment-associated bacteria were resuspended into the water column. Comparing pre-rain and during rain bacteria concentrations using a student’s t test for sites exhibiting reservoir and flushing behavior (Fig. 4a) confirmed that pre-rain E. coli concentrations (mean = 149.7, SE = 41.7 MPN g−1) were significantly greater (p = 0.014) than during rain samples (mean = 39.1, SE = 7.8 MPN g−1). This sampling technique was beneficial, as it allowed for approximation of bacterial exchange between matrices using basic water quality monitoring tests.
Further evidence for the resuspension of sediment-associated bacteria was provided by comparing estimates of stormwater flow and bed shear stress. Table 4 shows the expected increases in flow and thus bed shear stress during wet weather conditions. When wet weather shear stress values are compared to the critical shear stress values required to mobilize noncohesive particles (based on Shields 1936), values at all sites in the study area exceed the critical threshold. Sites 7, 9, 13, and 14 have smaller mean grain size particles and may be considered cohesive. For these sites, estimates of critical shear stress are more complex and relate to organic content, water chemistry, and temperature (Mehta et al. 1989). However, as noted in Jamieson et al. (2005), flume studies conducted by Partheniades (1965) have shown critical shear stress values for cohesive sediments ranging from 0.5 to 1.5 N m−2. Wet shear stress values for three of the four sites containing cohesive sediments fall above the upper bound of this range, with the fourth falling well within (Table 4). Results of shear stress estimates thus support findings of bacterial sampling in demonstrating the capacity for regular resuspension of sediments and thus contribution of associated E. coli to overlying waters during storms.
Post-rain data were examined to investigate sediment bacterial population recovery and the time frame in which deposition may regenerate an FIB bank in streambeds. Interestingly, almost 60 % (16 of 27) of profiles showed sediment E. coli concentration rebound within 48 h to levels similar to those observed prior to rainfall (Fig. 4a). A student’s t test was employed and confirmed no significant difference between E. coli concentration in sediment collected pre- and post-rainfall (means of 149.7 and 170.5 MPN g−1, respectively, p = 0.76), suggesting a relatively rapid return to pre-rain concentrations. Samples were collected at the end of the falling limb of the hydrograph, suggesting upstream contributions of sediment associated FIB, which settle out as flows recede. While FIB enter the drainage system via overland flow and sediment resuspension, it is unlikely that unassociated bacteria could deposit into the benthic environment under typical conditions, due to their small mass. This emphasizes the importance of sediment-bound FIB between rain events. Adsorbing to sediment particles likely provides a competitive advantage for bacteria and is the primary driver of FIB accumulation within a watershed. These findings suggest that E. coli may persist in a relatively steady-state concentration between rain events based on some inherent quality of the drainage basin. Finding a rapid return to near pre-rain conditions was surprising based on the range of environmental factors, which could influence bacterial survival. As discussed earlier, the study sites vary considerably in both potential FIB sources and drainage basin characteristics. Thus, it should be expected that each site would respond differently to antecedent weather conditions, magnitude of a storm event, and hydrologic features of the site, particularly the presence of BMPs. However, despite this environmental variability, all of the 14 sites sampled exhibited a characteristic decline and rebound of sediment E. coli concentrations during at least one storm event. This implies that the sediment bacterial accumulation, flushing, and rebound phenomenon may be ubiquitous under a wide range of conditions.
Further analysis of normalized E. coli data was conducted in order to assess the amount of sediment required to account for bacterial loading into the water column via resuspension (Table 6). Amounts of sediment resuspension necessary for the sediment environment to account for 25, 50, and 100 % of the wet weather water column load were calculated for each site using formula 6.
where
- SEDres :
-
Amount of sediment resuspension (cm)
- SW ECwet :
-
Mean stormwater E. coli concentration (MPN m−2)
- SED ECdry :
-
Mean sediment E. coli concentration (MPN g−1)
- SEDdn :
-
Sediment density (g cm−3)
Results of this calculation demonstrate that, using measured sediment and stormwater E. coli concentrations, many sites require modest resuspension in order to experience significant loading from the sediment environment. It should be noted that required resuspension amounts are not intended to characterize the actual make up of the bacterial load for the stormwater at a given site. These estimates are provided to convey the ability of the sediment environment at many of the sites within our system to maintain and contribute substantial E. coli loads. Considering estimates of resuspension with rain event profiles and BMP effects provides multiple lines of evidence for the accumulation and resuspension of sediment-adsorbed E. coli and subsequent impact on water column concentrations.
4.4 Management Implications
The findings of this study add to the understanding of how populations of FIB enter and persist in a watershed. Results presented here provide evidence for the presence of a long-term reservoir of FIB in drainage basin sediments that are likely resuspended, enter the water column, and rapidly rebound to pre-rain levels during dry conditions. This suggests an additional source of FIB input that has been unaccounted for under conventional watershed management techniques. Many water quality monitoring programs require grabs of individual samples in an effort to characterize bacteriological health, with the understanding that during storm conditions, these samples represent only a snapshot of a dynamic system at the time of collection. Our findings suggest that sample results are likely also influenced by sediment inputs. Sites sampled from drainage pipes may show bacteria values lower than those observed within the same branch of the watershed that were collected from streams in which sediment can be resuspended. Streambed sediments may be eroded naturally, as bioturbation decreases the stability of upper layer sediments (Jones et al. 1994). Sediment disturbance may also come from anthropogenic origin, as expanding impervious cover leads to increased runoff velocity and greater erosion of benthic sediments (Paul and Meyer 2001). Erosion as a result of stormwater runoff could thus have further detrimental effects if it leads to the resuspension of sediments with dense bacterial populations. Localized areas of significant sediment input could therefore make identifying sources of impairment more difficult, as overland transport may be less important than long-term bacterial accumulation within streambeds.
These findings are epidemiologically important as E. coli serves as an indicator species, not the pathogenic species of concern. If E. coli posed the greatest health hazard, it would be less important where these bacteria originate and how long they persist. They are however only a proxy measure for the recent addition of waste material, which transmits pathogens such as Campylobacter and Salmonella shown to cause human illness. Any FIB persistence in the environment would thus impede the ability of typical bacteria tests to detect recent microbial impairment, as they are unable to distinguish between persisting and newly deposited FIB. The survival of FIB outside the host organism has been shown to correlate poorly with the survival of species for which they are an indicator Lemarchand and Lebaron 2003; Harwood et al. 2005; Noble and Fuhrman 2001). If FIB survive in the environment on time scales that differ from those of pathogenic species, they may not serve as a reliable indicator of their presence. These findings thus question the efficacy of conventional indicator species. A further concern is raised by the ubiquity and observed persistence of E. coli in sediments across the study area. These results may also suggest the possibility of an autochthonous population of FIB. Recent studies using genetic fingerprinting techniques have shown naturalized E. coli and enterococci populations present in lakes (Byappanahalli and Fujioka 2004), forest soils (Byappanahalli et al. 2006), watershed sands and sediments (Solo-Gabrielle et al. 2000; Jamieson et al. 2005), and submerged aquatic vegetation (Badgley et al. 2011; Byappanahalli et al. 2006). Evidence suggesting independent populations of these bacteria that are genetically different from those of recent enteric origin makes monitoring of bacteriological water quality more difficult. As a result, typical bacteria testing may be detecting a combination of recently deposited FIB, long survived FIB from sediments, and naturalized species of FIB with no way to determine the true public health hazard. Economic losses are also conceivable, as recreational beaches or fisheries may be unnecessarily closed due to inflated FIB numbers associated with sediment resuspension, not waste material in the watershed.
Findings of this study provide valuable information for watershed managers. Water resource monitoring and the development of total maximum daily loads (TMDL) for pollutants should be adapted given the evidence of bacterial persistence and resuspension within a drainage basin, as assessment of sustained FIB populations in sediment environments is likely required to investigate all potential sources of microbial impairment.
5 Conclusions
There are many factors that contribute to the bacteriological health of a waterbody. This study shows that E. coli persisting in the sediment environment could act as a significant source of bacteria to a watershed. A comparison of the population of E. coli contained in the water column and sediment indicates that sediment populations can account for the majority of the total E. coli on a per surface area basis, particularly during dry weather conditions. Observed decreases in sediment E. coli during stormwater events and estimates of required resuspension suggest a mobilization of sediment bacteria into the water column. Rapid recovery of sediment E. coli concentrations (~48 h) to near pre-storm conditions suggests that deposition and persistence can produce steady-state populations of FIB in sediments. The regulatory implications of this increased sediment bacteria load mean that stormwater FIB concentrations may not accurately reflect the inputs of new bacterial pollution and thus the actual risk to public health.
References
Ackerman, D., & Weisberg, S. B. (2003). Relationship between rainfall and beach bacterial concentrations on Santa Monica Bay beaches. Journal of Water and Health, 01, 85–89.
Ahn, J. H., Grant, S. B., Surbeck, C. Q., DiGiacomo, P. M., Nezlin, N. P., & Jiang, S. (2005). Coastal water quality impacts of stormwater runoff from an urban watershed in Southern California. Environmental Science and Technology, 39(16), 5940–5953.
ASTM D2974-07a. Standard test methods for moisture, ash, and organic matter of peat and other organic soils. Method D 2974–00. West Conshohocken: American Society for Testing and Materials.
Athayde, D.N., Shelley, P.E., Driscoll, E.D., Gaboury, D., Boyd, G. (1983). Results of the Nationwide Urban Runoff Program, NTIS PB84-185545, US Environmental Protection Agency.
Badgley, B. D., Thomas, F. I. M., & Harwood, V. J. (2011). Quantifying environmental reservoirs of fecal indicator bacteria associated with sediment and submerged aquatic vegetation. Environmental Microbiology, 13(4), 932–942.
Badin, A., Faure, P., Bedell, J., & Delolme, C. (2008). Distribution of organic pollutants and natural organic matter in urban storm water sediments as a function of grain size. Science of the Total Environment, 403(1–3), 178–187.
Barbier, E. B., Hackler, S. D., Kennedy, C., Koch, W. E., Stier, A. C., & Silliman, B. R. (2011). The value of estuarine and coastal ecosystem services. Ecological Monographs, 81(2), 169–193.
Boehm, A. B., Griffith, J., McGee, C., Edge, T. A., Solo-Gabriele, H. M., Whitman, R., Cao, Y., Getrich, M., Jay, J. A., Ferguson, D., Goodwin, K. D., Lee, C. M., Madison, M., & Weisberg, S. B. (2009). Faecal indicator bacteria enumeration in beach sand: a comparison study of extraction methods in medium to coarse sands. Journal of Applied Microbiology, 107(5), 1740–1750.
Byappanahalli, M., & Fujioka, R. (2004). Indigenous soil bacteria and low moisture may limit but allow faecal bacteria to multiply and become a minor population in tropical soils. Water Science and Technology, 50(1), 27–32.
Byappanahalli, M.N., Whitman, R.L., Shively, D.A., Sadowsky, M.J., & Ishii, S. (2006). Population structure, persistencem and seasonality of autochthonous of Escherichia coli in temperate, coastal forest soils from a Great Lakes watershed. Environmental Microbiology, 8(3), 504–513.
Chandran, A., Varghese, S., Kandeler, E., Thomas, A., Hatha, M., & Mazumder, A. (2011). An assessment of potential public health risk associated with the extended survival of indicator and pathogenic bacteria in freshwater lake sediments. International Journal of Hygiene and Environmental Health, 214(3), 258–264.
Craig, D. L., Fallowfield, H. J., & Cromar, N. J. (2004). Use of microcosms to determine persistence of Escherichia coli in recreational coastal water and sediment and validation with in situ measurements. Journal of Applied Microbiology, 96(5), 922–930.
Davies, C. M., & Bavor, H. J. (2000). The fate of stormwater associated bacteria in constructed wetland and water pollution control pond systems. Journal of Applied Microbiology, 89(2), 349–360.
Davies, C. M., Long, J. A. H., Donald, M., & Ashbolt, N. J. (1995). Survival of fecal microorganisms in marine and freshwater sediments. Applied Environmental Microbiology, 61(5), 1888–1896.
DiDonato, G. T., Stewart, J. R., Sanger, D. M., Robinson, B. J., Thompson, B. C., Holland, A. F., & Van Dolah, R. F. (2009). Effects of changing land use on the microbial water quality of tidal creeks. Marine Pollution Bulletin, 58(1), 97–106.
Evison, L. M. (1988). Comparative studies on the survival of indicator organisms and pathogens in fresh and seawater. Water Science and Technology, 20(11–12), 309–315.
Friedlander, R. S., Vlamakis, H., Kim, P., Khan, M., Kolter, R., & Aizeberg, J. (2013). Bacterial flagella explore microscale hummocks and hollows to increase adhesion. Proceedings of the National Academy of Sciences, 110(14), 5624–5629.
Fries, J., Characklis, G., & Noble, R. (2006). Attachment of fecal indicator bacteria to particles in the Neuse River Estuary, N.C. Journal of Environmental Engineering, 134(10), 1338–1345.
Fujioka, R. S., Hashimoto, H. H., Siwak, E. B., & Young, R. (1981). Effect of sunlight on survival of indicator bacteria in seawater. Applied Environmental Microbiology, 41(3), 690–696.
Geldreich, E. E., Best, L. C., Kenner, B. A., & Van Donsel, D. J. (1968). The bacteriological aspects of stormwater pollution. Journal of the Water Pollution Control Federation, 40(11), 1861–1968.
Guimaraes, W.B. (1995). Water quality in the Withers Swash Basin, with emphasis on enteric bacteria, Myrtle Beach, South Carolina, 1991–1993. US Geological Survey Water Resources Investigations Report 95–4120.
Harwood, V. J., Levine, A. D., Scott, T. M., Chivukula, V., Lukasik, J., Farrah, S. R., & Rose, J. B. (2005). Validity of the indicator organism paradigm for pathogen reduction in reclaimed water and public health protection. Applied Environmental Microbiology, 71(6), 3163–3170.
Jamieson, R. C., Joy, D. M., Lee, H., Kostaschuck, R., & Gordon, R. J. (2003). Persistence of enteric bacteria in alluvial streams. Journal of Environmental Engineering and Science, 3(3), 203–212.
Jamieson, R. C., Joy, D. M., Lee, H., Kostaschuck, R., & Gorrdon, R. J. (2005). Transport and deposition of sediment-associated Escherichia coli in natural streams. Water Research, 39(12), 2665–2675.
Jeng, H., England, A. J., & Bradford, H. B. (2005). Indicator organisms associated with stormwater suspended particles and estuarine sediment. Journal of Environmental Science and Health Part A Toxic/Hazard Substances and Environmental Engineering, 40(40), 779–791.
Jones, C. G., Lawton, J. H., & Schachak, M. (1994). Organisms as ecosystem engineers. Oikos, 69(3), 373–386.
Korajkic, A., Wanjugi, P., & Harwood, V. J. (2013). Indigenous microbiota and habitat influence Escherichia coli survival more than sunlight in simulated aquatic environments. Applied and Environmental Microbiology, 79(17), 5329–5337.
Lemarchand, K., & Lebaron, P. (2003). Occurrence of Salmonella spp. and Cryptosporidium spp. in a French coastal watershed: relationship with fecal indicators. FEMS Microbiology Letters, 218(1), 203–209.
Lord, B. N. (1987). Nonpoint source pollution from highway stormwater runoff. Science of the Total Environment, 59, 437–446.
Lu, J., Domingo, J. W. S., Lamendella, R., Edge, T., & Hill, S. (2008). Phylogenetic diversity and molecular detection of bacteria in gull feces. Applied and Environmental Microbiology, 75(13), 3969–3976.
Makepeace, D. K., Smith, D. W., & Stanley, S. J. (1995). Urban stormwater quality: summary of contaminant data. Critical Reviews in Environmental Science and Technology, 25(2), 93–139.
Mallin, M. A., Williams, K. E., Esham, E. C., & Lowe, R. P. (2000). Effect of human development on bacteriological water quality in coastal watersheds. Ecological Applications, 10(4), 1047–1056.
McClaine, J.W., & Ford, R.M. (2002). Characterizating the adhesion of motile and nonmontile Escherichia coli to a glass surface using a parallel-plate flow chamber. Biotechnology and Bioengineering, 78(2), 179–189.
Mehta, A., Hayter, E., Parker, W., Krone, R., & Teeter, A. (1989). Cohesive sediment transport. I: Process description. Journal of Hydraulic Engineering, 115, 1076–1093.
Noble, R. T., & Fuhrman, J. A. (2001). Enteroviruses detected by reverse transcriptase polymerase chain reaction from the coastal waters of Santa Monica Bay, California: low correlation to bacterial indicator levels. Hydrobiologia, 460(1–3), 175–184.
Partheniades, E. (1965). Erosion and deposition of cohesive soils. J Hydraul Eng-ASCE, 91, 105–139.
Paul, M. J., & Meyer, J. L. (2001). Streams in the urban landscape. Annual Review of Ecology and Systematics, 32, 333–65.
Radke D.B. (2005). Bottom-material samples. In: US Geological Survey TWRI book 9 (version 1.1), Handbooks for water-resource investigations (pp. 13–15).
Ram, J. L., Thompson, B., Turner, C., Nechvatal, J. M., Sheehan, H., & Bobrin, J. (2007). Identification of pets and raccoons as sources of bacterial contamination of urban storm sewers using a sequence-based bacterial source tracking method. Water Research, 41(16), 3605–3614.
Schillinger, J. E., & Gannon, J. J. (1985). Bacterial adsorption and suspended particles in urban stormwater. Journal Water Pollution Control Federation, 57(5), 384–389.
Shields, A. (1936). Application of similarity principles and turbulence research to bed-load movement. Hydrodynamics Laboratory Publ. No. 167, W. P. Ott, and J. C. van Uchelen, trans., US Dept. of Agr., Soil Conservation Service Cooperative Laboratory, California Institute of Technology, Pasadena, California
Solo-Gabriele, H. M., Wolfert, M. A., Desmarais, T. R., & Palmer, C. J. (2000). Sources of Escherichia coli in coastal subtropical environments. Applied Environmental Microbiology, 66(1), 230–237.
Tolleson, A. R., Wooten, J. M., Jenson, J. R., & Schill, S. (1998). Urban runoff analysis and evaluation in the coastal zone of Myrtle Beach. SC: Geometrics Inc.
US Environmental Protection Agency (1992). NPDES Stormwater Guidance Document. EPA 833-B-92-001.
US Environmental Protection Agency (1998). Water quality conditions in the United States: a profile from the 1998 National Water Quality Inventory Report to Congress, Office of Water, Washington, DC.
Wade, T. J., Pai, N., Eisenberg, J. N. S., & Colford, J. M. (2003). Do U.S. Environmental Protection Agency water quality guidelines for recreational waters prevent gastrointestinal illness? A systematic review and meta-analysis. Environmental Health Perspectives, 111(8), 1102–1109.
Wahl, M. H., McKellar, H. N., & Williams, T. M. (1997). Patterns of nutrient loading in forested and urbanized coastal streams. Journal of Experimental Marine Biology and Ecology, 213(1), 111–131.
Weiskel, P. K., Howes, B. L., & Heufelder, G. R. (1996). Coliform contamination of a coastal embayment: sources and transport pathways. Environmental Science and Technology, 30(6), 1872–1881.
Winfield, D. M., & Groisman, E. A. (2003). Role of nonhost environments in the lifestyles of Salmonella and Escherichia coli. Applied Environmental Microbiology, 69(7), 3687–3694.
Winter, T.C., Harvey, J.W., Franke, O.L., Alley, W.M. (1998) Ground water and surface water; a single resource. US Geologic Survey Circular 1139.
Zmirou, D., Pena, L., Ledrans, M., & Letertre, A. (2003). Risks associated with the microbiological quality of bodies of fresh and marine water used for recreational purposes: summary estimates based on published epidemiological studies. Archives of Environmental Health, 58(11), 703–711.
Acknowledgments
This research was funded by a US Army Corp of Engineers, Planning Assistance to the States grant (W912HN-10-2-0001), the M.K. Pentecost Ecology Fund, and the Coastal Carolina University Research Council. We would like to thank the City of Myrtle Beach Engineering and Stormwater departments for valuable information and maps regarding stormwater drainage, as well as providing access to weather station data. For guidance and assistance in sample collection and analysis, we thank the staff of the Coastal Carolina University Environmental Quality Lab.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 49 kb)
Rights and permissions
About this article
Cite this article
Curtis, K., Trapp, J.M. Evidence for the Accumulation and Steady-State Persistence of E. coli in Subtropical Drainage Basin Sediments. Water Air Soil Pollut 225, 2179 (2014). https://doi.org/10.1007/s11270-014-2179-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-2179-3