Abstract
Messenger ribonucleic acid (mRNA) was discovered in 1961 as an intermediary for transferring genetic information from DNA to ribosomes for protein synthesis. The COVID-19 pandemic brought worldwide attention to mRNA vaccines. The emergency use authorization of two COVID-19 mRNA vaccines, BNT162b2 and mRNA-1273, were major achievements in the history of vaccine development. Lipid nanoparticles (LNPs), one of the most superior non-viral delivery vectors available, have made many exciting advances in clinical translation as part of the COVID-19 vaccine and therefore has the potential to accelerate the clinical translation of many gene drugs. In addition, due to these small size, biocompatibility and excellent biodegradability, LNPs can efficiently deliver nucleic acids into cells, which is particularly important for current mRNA therapeutic regimens. LNPs are composed cationic or pH-dependent ionizable lipid bilayer, polyethylene glycol (PEG), phospholipids, and cholesterol, represents an advanced system for the delivery of mRNA vaccines. Furthermore, optimization of these four components constituting the LNPs have demonstrated enhanced vaccine efficacy and diminished adverse effects. The incorporation of biodegradable lipids enhance the biocompatibility of LNPs, thereby improving its potential as an efficacious therapeutic approach for a wide range of challenging and intricate diseases, encompassing infectious diseases, liver disorders, cancer, cardiovascular diseases, cerebrovascular conditions, among others. Consequently, this review aims to furnish the scientific community with the most up-to-date information regarding mRNA vaccines and LNP delivery systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the emergence of SARS-CoV-2 in 2020, mRNA vaccines have garnered global attention. Leveraging years of research investigating mRNA vaccines as therapeutic strategies against cancer in clinical trials, COVID-19 vaccines have been swiftly developed and manufactured [1]. The US Food and Drug Administration (FDA) has granted approval to two mRNA vaccines, namely mRNA-1273 and BNT162b2, which have been administered to hundreds of millions of individuals [2]. These vaccines have demonstrated remarkable efficacy with consistent protection rates exceeding 90% [2]. In comparison to other vaccine types, mRNA vaccines possess several distinct advantages. (1) High safety: It is achieved by the cytoplasmic functionality of mRNA, eliminating the risk of integration into the host genome unlike DNA-based approaches [3, 4]. (2) Superior efficiency: The stability and translation efficiency of mRNA can be significantly improved through appropriate modification and sequence optimization [5, 6]. Additionally, an efficient delivery system has been developed to facilitate rapid uptake of mRNA and its expression in the cytoplasm. (3) Short development cycle: The mRNA encoding the antigenic protein can be designed as soon as the pathogen's genome sequence is determined [7, 8]. Subsequently, in vitro transcription (IVT) of the mRNA can be performed without cell expansion, thereby significantly enhancing production speed. (4) High flexibility: The original vaccine may become ineffective if a mutation occurs in the virus. In such cases, new mRNA vaccines can be rapidly redesigned and manufactured based on updated viral sequences. (5) The application scope is extensive: The mRNA technology, with its advanced principles and efficient development and production processes, has achieved significant breakthroughs in the treatment of infectious diseases, liver diseases, cancer, diabetes, among others.
The development of effective vaccines against the COVID-19 pandemic, namely BNT162b2 (Pfizer) and mRNA-1273 (Moderna), were rooted in a decade-long pioneering effort by those who recognized the therapeutic potential of mRNA. Since the initial report of mRNA by Francois Jacob et al. in 1961 [9], mRNA has been acknowledged as a pivotal intermediary between DNA coding sequences and the production of functional proteins. Subsequently, in 1990, Wolff et al. [10] established the groundwork for utilizing mRNA as a therapeutic agent through direct gene transfer in vivo by injecting naked mRNA containing reporter genes into the skeletal muscle of mice, thus providing proof of principle. Over the subsequent decades, substantial advancements have been made in comprehending the properties and potential applications of mRNA as a therapeutic agent [11].
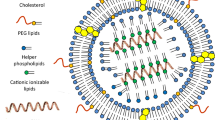
The components essential for the expression of the encoded antigen and induction of adaptive immunity in humans in a typical mRNA vaccine comprised two main elements: the mRNA and its delivery system. The mRNA encoding the desired protein or peptide serves as the molecular foundation for inducing specific immunity against the pathogen. Technically, mRNA vaccines are typically generated through in vitro transcription (IVT) from DNA templates containing T7 RNA polymerase [12]. Additionally, the delivery system plays a crucial role in mRNA vaccines [13]. The delivery system not only safeguards the mRNA against degradation in the extracellular environment but also facilitates its translocation into the cytoplasm of antigen presenting cells (APCs), potentially enhancing the expression of encoded antigens. The optimization of mRNA and its delivery systems has resulted in diverse clinical applications of mRNA vaccines. Various materials, such as lipids, lipid-like compounds, polymers, and protein derivatives, have been developed for mRNA delivery [14,15,16,17]. Among these advanced delivery systems, lipid nanoparticles (LNPs) represent a promising approach to enhance the efficacy and stability of mRNA vaccines [18, 19]. LNPs are composed of ionizable/cationic lipids, helper lipids, cholesterol, and polyethylene glycol (PEG)-coupled lipids. Ionizable/cationic lipids play a crucial role in determining the delivery and expression efficacy of mRNA vaccines. Both helper lipids and cholesterol contribute to the stabilization and promotion of membrane fusion in LNPs. Lastly, PEG-modified lipids are essential for enhancing stability in vivo applications due to stealth effects [20, 21].
While the SARS-CoV-2 mRNA vaccines have undoubtedly achieved remarkable success, there are still many challenges ahead. Despite individuals receiving vaccination against SARS-CoV-2 through mRNA vaccines, instances of confirmed COVID-19 cases have been reported [22]. Therefore, it is crucial to enhance the efficacy of mRNA vaccines by optimizing both the mRNA and its delivery system. Furthermore, when formulating future mRNA vaccines, considerations should be given to storage requirements and antigen mutation [23].
This review focuses on the advancements in mRNA delivery system, specifically highlighting the current status of LNPs and providing insights for future optimization. Additionally, we discussed the potential prospects of LNPs as a clinical treatment for mRNA delivery.
Lipid nanoparticles (LNPs)
The current FDA-approved LNPs formulations consist of four lipids: ionizable cationic lipids, helper lipids, cholesterol, and polyethylene glycol (PEG) [24, 25] (Fig. 1). These constituents facilitate the formation of monodisperse nanoparticles, enhance nanoparticle stability, promote efficient nucleic acid encapsulation, facilitate cellular uptake, and support nucleic acid release. Figure 2 illustrates three ionizable lipids, namely those employed in Onpattro®, Comirnaty®, and Spikevax® for clinical applications, all of which have received FDA approval.
The role of ionizable lipids
Ionizable lipids are amphiphilic compounds comprising three distinct domains: a polar head group, a hydrophobic tail region, and a linker connecting the two domains [26], as shown in Fig. 3. The head groups of ionizable lipids typically exhibit a positive charge. The size and charge density of the head group play crucial roles in nucleic acid wrapping, LNPs stabilization, cell membrane interaction, and endosome escape [27]. Common ionizable lipids typically possess a singular head group, although instances of multiple head groups can also be observed. Prominent examples of these head groups encompass amine, guanidine, and heterocyclic moieties [28] (Fig. 3). The ionizable lipids currently available for clinical use, namely DLin-MC3-DMA, ALC-0315, and SM-102, all feature tertiary amine head groups and represent the sole FDA-approved cationic lipids for RNA delivery [17, 29, 30] (Fig. 2).
The linker fragments establish a connection between the head and tail of LNPs, occasionally concealing themselves within the tail region, as observed in SM-102 and ALC-0315 (Fig. 1). This phenomenon significantly impacts the stability, biodegradability, cytotoxicity, and transfection efficiency of lipid nanoparticles [27, 28]. Ionizable lipids may contain one or more linker fragments (Fig. 3). However, the majority of ionizable lipids typically consist of a single type of linker fragment [31]. In general, linker fragments can be categorized as non-biodegradable (ethers and carbamates) or biodegradable (esters, amides, and thiols) [31]. The incorporation of biodegradable lipids enhance the tolerance of LNPs, facilitating rapid metabolism while maintaining mRNA delivery efficacy. The biodegradability of lipids can be enhanced by the incorporation of ester motifs. For instance, DLin-MC3-DMA, ALC-0315, and SM-102 encompass fragments bonded by esters [32, 33].
Ionizable lipids typically consist of 1 to 4 hydrophobic tails, each containing 8 to 20 carbon atoms [27, 28]. These tails can be saturated or unsaturated lipid chains, and the degree of unsaturation may impact nucleic acid delivery by modulating properties associated with membrane instability [28, 34] (Fig. 3). DLin-MC3-DMA features two linoleic acid tails, while ALC0315 and SM-102 have two branched saturated tails that are believed to possess a conical geometry and promote endosomal membrane destabilization for intracytoplasmic release of nucleic acid [35].
Currently, the primary constituents employed in LNPs formulation predominantly consist of ionizable lipids possessing adjustable pKa values, which can enhance nucleic acid loading efficiency and impact the stability and toxicity of LNPs, thereby further augmenting the efficacy of gene therapy [36]. Conventional cationic lipids with permanent charge, such as 1,2-dioxane-3-trimethylammonium-propane (DOTAP), previously employed for nucleic acid delivery, exhibit facile interaction with negatively charged serum proteins and tend to accumulate in the bloodstream [29, 36]. Consequently, the rapid clearance of LNPs by the monocyte-macrophage system ensues, resulting in a diminished half-life within the bloodstream [29]. Moreover, the relatively elevated hemolytic activity of cationic lipids amplifies the potential for toxic side effects, such as hemoglobin release resulting from impairment to the erythrocyte membrane [37]. To address these challenges, ionizable cationic lipids with pKa values (typically 6.0–7.0) have been developed to ensure efficient nucleic acid encapsulation under acidic conditions (e.g., pH 4.0, where the lipids are protonated) and reduced toxicity during circulation under physiological conditions (pH 7.4, where the lipids are nearly neutral) [38]. Upon entering the endosome (with a lower pH than the lipid pKa), the amine groups of ionizable lipids become protonated and interact with anionic groups on the endosomal membrane, facilitating nucleic acid release from the endosome [39]. Notably, LNPs with pKa values of 6.2–6.5 and 6.6–6.9 were found to be optimal for intrahepatic siRNA delivery and intramuscular mRNA vaccination, respectively [40, 41].
The role of PEGylated lipids
PEGylated lipids constitute a crucial component of LNPs, albeit accounting for the smallest molar percentage (typically 1.5 mol%). They exert significant influence on various key properties of LNPs, including particle size and dispersibility [42,43,44], as well as the stability of nanoparticles during preparation and storage. Moreover, polyvinyl alcohols also impact nucleic acid encapsulation efficiency [42], in vivo distribution [45], transfection efficiency [42], and immune response [46]. All these properties are intricately linked to the molar ratio of polyvinyl alcohols, as well as the structure and length of both the polyvinyl alcohol chain and lipid tail (alkyl/dialkyl chain).
PEGylated lipids comprise two distinct domains: PEGylated lipids forming the surface layer of the lipid particles, and lipid domains encapsulated within the core of the particles, with PEG domains extending outward from the surface. The spatial barrier effect of PEG polymers prevents plasma protein binding, thereby avoiding rapid clearance by the reticuloendothelial system (RES). Consequently, they have found extensive application in liposome systems to effectively prolong in vivo circulation time [26].
Polyvinyl alcohol enhances LNPs self-assembly by creating a hydrophilic spatial barrier on the surface of LNPs [43]. This spatial barrier also plays a crucial role in preventing nanoparticle aggregation, thereby contributing to their stability [42, 43]. Lokugamage et al. [47] demonstrated that formulations lacking polyvinyl alcohol resulted in unstable and polydisperse LNPs with diameters exceeding 200 nm. Conversely, studies have shown that incorporating just 0.5 mol% of polyethylene glycol lipid can produce stable and homogeneous LNPs with diameters below 80 nm [48]. The stability of mRNA-LNPs were maintained for up to three weeks at 4 °C through monitoring particle size, PDI, Zeta potential, and encapsulation efficiency during storage [42, 49]. Furthermore, these studies indicated that different compositions of polyvinyl alcohol chains can adequately provide the necessary repulsive forces for particle formation without compromising long-term stability [42].
The particle size of LNPs must be strictly controlled during the preparation process, as it plays a pivotal role in determining the pharmacokinetics and biodistribution of the nanoparticles [50]. Kulkarni et al. [48] investigated the impact of varying the fixed molar ratio on lipid structure using cryo-electron microscopy, assuming that PEGylated lipids were exclusively located at the surface of LNPs [45]. Consequently, increasing the molar percentage of PEGylated lipids led to an augmented specific surface area, thereby reducing nanoparticle size.
While PEG is valuable for enhancing the stability and bio-conjugation of LNPs, its desorption plays a pivotal role in facilitating cell transfection [51, 52]. In a previous study conducted by Mui et al. [44], the desorption rates of three PEGylated lipids in siRNA-LNPs were tested, which had dialkyl chains consisting of 14, 16 or 18 carbons. It was observed that shorter carbon chains in PEGylated lipids led to faster desorption rates and better effects. The efficiency of LNPs in delivering nucleic acids is enhanced by reducing the peg-lipid content, as they exhibit increased binding affinity towards ApoE [38, 53, 54]. Additionally, the length of the PEGylated lipids anchor plays a crucial role in determining desorption kinetics [26]. In addition to length, the molecular weight of the PEG fragment also influences the delivery efficiency of LNPs [55].
The role of cholesterol
Cholesterol, as a constituent of the cell membrane, plays a crucial role in enhancing the stability of LNPs and facilitating cell membrane fusion [17, 20]. Therefore, optimizing the structural characteristics of cholesterol can effectively enhance the delivery efficiency of LNPs and confer them with unique functionalities. Paunovska et al. [56] demonstrated that incorporating esterified cholesterol into LNPs significantly improved their delivery efficiency. Furthermore, cholesterol has been exploited to enhance drug delivery systems. Patel et al. [57] conducted a screening study on various natural cholesterol analogues and discovered that β-sitosterol LNPs exhibited remarkably enhanced transfection efficiency.
Among LNPs formulations containing nucleic acids, lipid preparations incorporating cholesterol yielded two key findings: (1) Cholesterol is a readily exchangeable molecule capable of accumulating within liposomes during circulation [58], and (2) incorporation of cholesterol significantly diminishes the presence of surface-restricted proteins while enhancing the circulating half-life [59].
Lipids, including cholesterol, play a crucial role in the encapsulation of nucleic acids. By increasing membrane stiffness, cholesterol effectively reduces liposome drug leakage [17]. A recent study [44] demonstrated that the presence of helper lipids above a certain threshold was essential for achieving stable encapsulation. Specifically, a minimum concentration of 40 mol% cholesterol (in the absence of any phospholipids) was required to achieve nearly complete siRNA encapsulation.
The role of phospholipids
Phospholipids serve as helper lipids facilitating the formation of LNPs and promoting endosomal escape [60]. Moreover, they possess the ability to enhance liposome stability and improve internal circulation, thereby exerting a significant impact on the efficacy of mRNA-LNPs [61]. In preclinical studies and clinical applications, the commonly employed phospholipids include DSPC (1,2-distearoyl-sn-glycero-3-phosphocholine) and DOPE (dioleoylphosphatidylethanolamine). Presently, commercial LNPs systems exclusively incorporate DSPC, likely owing to its stability within liposomes used in commercial settings as well as its efficient intima disruption facilitated by ionizable lipids. Kauffman et al. [48, 61] reported that their DSPC-based LNPs exhibited a higher mRNA encapsulation rate compared to those containing DOPE. However, the latter demonstrated superior protein expression. Regarding safety, Oberli et al. [55] observed inflammation at the injection site in 20% of mice 5–10 days after administration of LNPs containing either DSPC or DOPE.
The LNPs-induced immune response
The human clinical trials of the Pfizer/BioNTech and Moderna vaccines have reported side effects such as pain, swelling, fever, and sleepiness [62,63,64]. These are typical symptoms associated with inflammation induced by cytokines such as IL-1β and IL-6 [65, 66]. Some clinicians and public health advocates interpreted the observed acute side effects as indicative of the vaccine's efficacy in eliciting an adaptive immune response. The observed side effects, however, were more in line with the acute inflammatory response induced by the vaccine [67]. Many studies [64, 68, 69] have shown that LNPs used for many preclinical studies are highly inflammatory. This could explain their potent adjuvant activity and their superiority, compared with other adjuvants, in supporting the induction of adaptive immune responses. Ionizable lipids were developed to mitigate the inflammatory properties associated with permanently charged cationic lipids [25]. However, studies have revealed that the proprietary ionizable lipid components of these LNPs induce an inflammatory response. The inflammation triggered by LNPs or their complexes with mRNAs is independent of the route of administration and is primarily characterized by neutrophil infiltration. The specific inflammatory pathways activated by these LNPs or their ionizable lipid components are currently not well understood. In theory, LNPs could activate various pathways or only one pathway, thereby triggering an inflammatory cascade. Some cationic/ionizable lipids bind and activate TLRs [29, 70, 71].
Relevant clinical interventions for LNPs therapy
Therapeutic mechanism of mRNA-LNPs
Ionizable lipids play a pivotal role in the formation of LNPs and in vivo transfection. Initially, ionizable lipids form electrostatic complexes with negatively charged mRNA molecules, thereby enhancing the stability of mRNA molecules [60, 72]. The pH neutrality of LNPs, attributed to the presence of ionizable lipids and PEGylated lipids, effectively mitigates nonspecific interactions with serum proteins in vivo [20, 21, 73]. After PEG lipids dissociation, LNPs are taken up by cells through an ApoE-dependent pathway. Upon reaching the cell membrane, mRNA-LNPs trigger fusion with the negatively charged cell membrane via an ionizable lipid, leading to endocytosis and cellular absorption [52]. Subsequently, lysosomes containing various hydrolytic enzymes create an acidic environment causing a decrease in pH values. At low pH values, protonated ionizable lipids induce the formation of a hexagonal phase structure that disrupts the bilayer structure of LNPs. This disruption facilitates the release of mRNA into the cell where it binds to ribosomes responsible for protein production and undergoes translation into viral proteins [52]. Antigens are released extracellularly or degraded by proteasomes, thereby exposing antigenic epitopes. Peptides are subsequently loaded onto major histocompatibility complex (MHC) class I to activate antigen-specific CD8+ T cells [74]. Exogenous proteins that are released early on are presented on MHC class II to stimulate CD4+ T cells activation [75]. CD4+ T cells can also co-stimulate B cells specific to the protein, leading to B cell maturation and subsequent antibody secretion [76] (Fig. 4).
Delivery mechanism of mRNA-LNPs. ① Endocytosis of mRNA-LNPs. ② Protonation of ionizable lipid. ③ Endocytosis mediates the internalization of post-translational mRNA, which then escapes from the endosomes into the cytosol. ④ The host cell ribosomes facilitate the translation of mRNA into the desired antigen protein intracellularly. ⑤ The major histocompatibility complex (MHC) class I presents peptide antigens on the cell surface for antigen presentation. ⑥ MHC class I presents peptide epitopes to CD8+ T cells. ⑦ Endocytosis of exogeneous protein released early. ⑧ Exogenous protein is degraded and presented by MHC class II epitope. ⑨ Exogeneous protein is presented on MHC class II to activate CD4+ T cells. CD4+ T cells can co-activate protein-specific B cells, leading to the maturation of B cells and the release of antibodies
RNA therapy
Gene therapy has emerged as a prominent avenue of clinical investigation in recent years, with LNPs serving as nucleic acid carriers for various gene therapeutic modalities including antisense oligonucleotide (ASO), short interfering RNA (siRNA), microRNA (miRNA), and mRNA, each exerting distinct mechanisms of action [77]. The target mRNA is hybridized and cleaved by ASO through the action mediated by RNAse-H. SiRNA and miRNA inhibit mRNA translation through the RISC-mediated RNAi pathway, thereby preventing protein synthesis. In contrast, mRNA binds to ribosomes via capsids and poly(A)-binding proteins, leading to the translation of therapeutic proteins. This is the underlying mechanism of mRNA-LNPs vaccines [77].
mRNA vaccines could induce strong and robust immune responses in both preclinical models and clinical trials. Adjuvants are used to stimulate specific components of the immune system to enhance the immunogenicity of the vaccine. Huang et al. [78] found that combining mRNA-encoded genetic adjuvants with mRNA-encoded antigens and adding them to lipid nanoparticle vaccines can enhance immune responses against viral and tumor antigens. A research team immunized mice with.
LNP(poly(I:C)) adjuvanted recombinant Spike protein from the SARS-CoV-2 virus yielded robust antigen-specific antibody titers [79]. Their study findings suggest that LNP formulation is a feasible and scalable approach that can greatly enhance the adjuvant properties of poly(I:C) by activating TLR3 and RLRs, and altering its pharmacokinetic and pharmacodynamic properties in vivo. Pamela T Wong’s group [80] developed a rationally designed IN adjuvant consisting of a combined nanoemulsion (NE)-based adjuvant and an RNA-based RIG-I agonist (IVT DI) to drive more robust, broadly protective antibody and T cell responses.
Given the remarkable safety and efficacy of mRNA vaccines employing LNPs as delivery vectors for COVID-19 prevention, the mRNA-LNPs delivery system has gained widespread utilization in the treatment of diverse ailments including cardiovascular diseases, cancers, and infectious diseases [81] (Fig. 5).
Application of mRNA-LNPs in infectious diseases
Since the first vaccine was used to treat cowpox in 1796 [82], researchers have developed vaccines to prevent and control many infectious diseases. Traditional vaccines, which consist mainly of inactivated pathogens, have had great success in preventing more than 30 infectious diseases worldwide, and even eradicated many of them. However, traditional vaccines against some of the more challenging infectious diseases have still failed to achieve high levels of protection. RNA vaccine technology is widely acknowledged as an advanced immunization approach capable of effectively inducing a robust humoral and cellular immune response. Given the rapid global dissemination of COVID-19, two mRNA vaccines targeting SARS-CoV-2, namely mRNA-1273 and BNT162b2, received emergency use authorization from the FDA in 2021 [83,84,85]. Furthermore, numerous vaccines designed for chronic or recurrent viral infections such as HIV were currently undergoing clinical trials [77]. These promising findings underscore the pivotal role of mRNA vaccines in shaping the future landscape of infectious disease prevention and control.
New or re-emerging infectious viruses are characterized by the emergence of novel pathogens or the resurgence of previously undetectable pathogens. These viruses pose a significant threat to human health due to their abrupt and uncontrollable transmission [86]. Firstly, traditional vaccines may not offer the expeditious progress required during a pandemic, owing to the imperative demand for efficacious vaccines in controlling novel or resurgent viruses [87]. Furthermore, the highly variable nature of re-emerging viruses like influenza presents significant challenges in developing a broadly effective vaccine [88]. mRNA vaccines offer immense potential for rapid, cost-effective, and scalable good manufacturing practices, making them an ideal platform for the development of highly efficacious and timely vaccines against emerging infections.
mRNA vaccines can be utilized for both prophylactic and therapeutic immunization purposes, rendering them highly versatile in their application. Moreover, their rapid production time enables swift response to emerging viral threats. Notably, the respiratory syncytial virus fusion glycoprotein (RSV-F) represents a conservative target for neutralizing antibodies and stands as the most promising antigen in RSV vaccine development. Recently, Espeseth et al. [89] have developed a lipid nanoparticle mRNA-LNPs vaccine by encapsulating chemically modified mRNA within LNPs, demonstrating robust immunogenicity and protective efficacy against respiratory syncytial virus (RSV). mRNA-LNPs vaccine elicited significant CD4 + and CD8 + T cell responses in mice compared to protein-based vaccines and triggered a strong cellular immune response against RSVF. This mRNA-LNPs vaccine expressing RSVF protein has the potential to be used safely and effectively to prevent RSV diseases [77]. Similarly, Moderna is also very active in the field of mRNA-LNPs vaccines for many infectious diseases.
An OspA-encoding mRNA-LNPs vaccine was designed and synthesized by Matthew et al. [90], which demonstrated superior immunogenicity and protective efficacy compared to an aluminum adjuvanted OspA protein subunit vaccine. Single immunization with OspA mRNA-LNPs induced potent humoral and cell-mediated immune responses in mice, resulting in protection against bacterial infection.
mRNA-LNPs vaccines can also be employed in veterinary medicine for the prevention of infectious diseases in animals. Saxena et al. [91] utilized a self-amplifying mRNA vaccine encoding the glycoprotein of rabies virus to elicit immune responses against canine rabies. Recently, VanBlargan et al. [92] have developed an mRNA-LNPs vaccine encoding the prM and E genes of deer Powassan virus and demonstrated its immunogenicity and efficacy in mice.
The mRNA vaccines (vaccine candidates) currently undergoing preclinical and clinical trials for infectious diseases are documented in Table 1.
Application of mRNA-LNPs in cardiovascular diseases
Heart failure is partially attributed to the activation of cardiac fibroblasts, which respond to myocardial injury and inflammation by excessively synthesizing fibrous material, leading to myocardial sclerosis and impairment of cardiac function [77]. Therefore, the scientists proposed the development of CAR-T cell therapy that targets activated cardiac fibroblasts. Typically, current CAR-T therapy involves isolating T cells from patients and modifying them in vitro to express CARs that recognize cancer cell surface antigens. These modified T cells are then amplified before being infused back into the patient to effectively eliminate cancer cells [77].
However, due to the pivotal role of fibroblasts in the process of wound healing, significant hurdles arise when employing CAR-T cell therapy for heart failure or other fibrotic disorders [77]. CAR-T cells can persist in patients for an extended duration, ranging from months to years, thereby exerting a prolonged inhibitory effect on fibroblast growth and potentially leading to complications in wound healing. The sustained presence of CAR-T cells is advantageous for cancer treatment as it ensures a durable therapeutic impact. However, in the case of patients with cardiac fibrosis who experience injuries subsequent to receiving conventional CAR-T therapy, the persistent existence of CAR-T cells may impede normal wound healing processes and pose safety concerns. To address this issue, researchers [93] engineered an mRNA that encodes a chimeric antigen receptor (CAR) capable of specifically binding to fibroblast activation protein expressed on the surface of fibroblasts. Subsequently, they encapsulated the mRNA within LNPs, which were further conjugated with an antibody targeting CD5 + T cells. This innovative approach enabled the generation of intact therapeutic CAR-T cells in vivo. Remarkably, experimental studies conducted in a murine model of heart failure demonstrated the efficacy of this treatment strategy in reducing cardiac fibrosis and restoring cardiac function [77].
Application of mRNA-LNPs in liver diseases
Rizvi et al. [94] developed a LNP-encapsulated nucleoside-modified mRNA that exhibited transient and sustained expression of hepatocyte growth factor (HGF) and epidermal growth factor (EGF) in murine hepatocytes. The liver-specific targeting of mRNA-LNPs were also demonstrated through intravenous administration of mRNA-LNPs encoding firefly luciferase, resulting in sustained protein expression for approximately 3 days. Furthermore, HGF mRNA-LNPs effectively induced hepatocyte proliferation. In murine models of chronic liver injury and nonalcoholic fatty liver disease, as well as in mice with acetaminophen-induced acute liver injury, administration of mRNA-LNPs containing hepatocyte growth factor (HGF) and epidermal growth factor (EGF) exhibited significant amelioration of steatosis, prompt activation of the hepatocyte regeneration pathway, and accelerated recovery of hepatic function following injury.
Application of mRNA-LNPs in cancer
Due to its rapid synthesis, mRNA has emerged as a suitable option for developing personalized vaccines, particularly in the context of highly heterogeneous diseases like cancer. The mRNA-LNPs vaccines have demonstrated the ability to elicit immune cell-mediated responses, thereby generating robust CD8 + T cell responses for effective eradication or reduction of tumor cells [77]. Consequently, they are currently regarded as highly promising therapeutic interventions for cancer [95,96,97,98,99]. Cancer mRNA vaccines can express tumor-associated antigens and elicit cell-mediated immune responses, thereby facilitating eradication or inhibition of cancer cells. Consequently, these vaccines are increasingly employed as therapeutic agents rather than prophylactic measures [100]. Recent studies have also demonstrated the development of mRNA vaccines with potent anticancer properties, eliciting robust and efficacious T cells and humoral immune responses. Lee et al. [101] employed tripalmitoyl-S-glycero-cysteine linked to a pentapeptide (PAM3CSK4, Pam3) as an adjuvant for encoding ovalbumin-bound mRNA in LNPs, thereby developing a Pam3-doped mRNA/Pam3-LNPs vaccine. Upon cellular internalization through endocytosis, the mRNA/Pam3-LNPs vaccine undergoes dissociation and release mRNA in acidic environments, which is subsequently recognized by TLR7/8 on the endosomal membrane. Through the synergistic effect of Pam3 and mRNA targeting different TLR subclasses, Pam-LNPs exhibit potent immune stimulation within cells and elicit robust antigen-specific CD8 + T cell responses, thus greatly augmenting the therapeutic efficacy of mRNA vaccines for cancer prevention [101].
Jamile et al. [102] compared three different mRNA vaccine modes in mice with HPV-16 infection-associated tumors. They prepared self-amplifying mRNA encapsulated in LNPs and unmodified and nucleoside-modified nonreplicating mRNA vaccines encoding a chimeric protein fused by HPV-16 E7 oncogene and herpes simplex virus type 1 (gDE7). The results showed that a single low-dose immunization with any of the gDE7 mRNA vaccines induced e7-specific CD8 + T cell activation and produced a memory T cell response that prevented tumor recurrence and eradicated subcutaneous tumors at different growth stages.
The development of therapeutic vaccines for cancer treatment has been a time-consuming process spanning several decades. Despite the approval of only one dendritic cell-based vaccine (sipuleucel-T) by the FDA for hormone-refractory prostate cancer, its effectiveness remains unsatisfactory [103]. The efficacy of cancer vaccines can be influenced by various factors, such as the limited specificity of tumor-associated antigens (TAAs), immune evasion mechanisms employed by cancer cells, and immunosuppressive conditions within the tumor microenvironment [104]. mRNA vaccines were demonstrated promising efficacy in clinical trials for anticancer treatment. However, neoantigen-based cancer vaccines face two primary challenges: limited neoantigen immunogenicity and high production costs [105]. mRNA vaccines have gained significant popularity as a promising approach for cancer treatment [106]. On one hand, mRNA vaccines offer advantages such as rapid development, cost-effective manufacturing, and safe administration, thereby alleviating the economic burden associated with neoantigen vaccines. On the other hand, mRNA vaccines capable of efficiently expressing multiple antigens in both in vivo and in vitro antigen-presenting cells (APCs) can elicit robust T cell responses against neoantigens.
In contrast to delivery systems employed for mRNA vaccines against pathogenic infections, therapeutic mRNA vaccines designed for cancer treatment necessitate a robust CD8 + and CD4 + T cell response [107]. Activation of type I interferon (IFN) has been demonstrated to play a crucial role in the development of cytotoxic T cell response [108]. Consequently, diverse strategies have been investigated to augment the activation of type I IFN. In addition to augmenting the immune response via delivery systems, injectable mRNA cancer vaccines have witnessed a transition from tumor-associated antigens (TAAs) to neoantigens. These neoantigens are characterized by their highly specific somatic mutations occurring randomly in individual tumor cells, which distinguishes them from normal cells. Consequently, they are regarded as ideal candidates for the development of cancer vaccines [109]. While traditional peptide-based vaccines are constrained by their limited immunogenicity and challenging physicochemical properties, mRNA vaccines offer greater design flexibility and have been demonstrated enhanced immune responses [106]. Sahin et al. [110] reported the first injectable mRNA cancer vaccine, which effectively elicited T-cell responses against multiple neoantigens in all patients with advanced melanoma through intranodal administration of patient-specific encoded neoantigens. Moreover, Moderna has successfully developed a range of mRNA cancer vaccines encoding neoantigens that specifically target multiple types of cancers. These innovative mRNA-based vaccines have demonstrated the ability to elicit T-cell responses against up to nine predicted neoantigens in a single patient. BioNTech has also developed a personalized mRNA cancer vaccine, known as RO7198457, which encodes a novel antigen specifically targeting advanced or metastatic solid tumors larger than 20 inches.
The rapid advancement in delivery systems and novel antigens has sparked a revolutionary transformation in mRNA cancer vaccines, with accumulating evidence substantiating the immense potential of individualized mRNA-encoded cancer vaccines. The mRNA vaccines (vaccine candidates) currently undergoing preclinical and clinical trials for cancer is documented in Table 2.
Future directions and prospects of mRNA-LNPs
The rapid development of mRNA COVID-19 vaccines, facilitated by advancements in mRNA technology and LNPs delivery systems, showcases the clinical potential of mRNA-LNPs formulations and provides a formidable tool to combat the SARS-CoV-2 pandemic. With the continuous exploration of the structural characteristics of ionizable lipids and auxiliary lipids, an increasing number of delivery systems with remarkable efficiency have been developed, leading to successive advancements in mRNA vaccines against various infectious diseases. Nevertheless, progress in clinical research on mRNA vaccines for cancer remains sluggish. Based on the insights gained from clinical studies, there is scope for further enhancement of mRNA-LNPs formulations.
mRNA-LNPs vaccines exhibit high instability and rapid degradation at room temperature, necessitating ultra-low temperature storage. Consequently, addressing the challenges associated with long-term preservation and long-distance transportation of mRNA-LNPs assumes paramount importance. Furthermore, the emergence of pathogen variants and immunosuppression of antigens pose persistent obstacles in the development of efficacious vaccines.
Encouragingly, the success of mRNA-LNPs vaccines in the treatment of COVID-19 has revealed their effectiveness. It is believed that mRNA-LNPs formulations will be more widely used and become a new breakthrough point in biopharmaceuticals.
Data availability
No datasets were generated or analyzed during the current study.
References
Karch CP, Burkhard P (2016) Vaccine technologies: From whole organisms to rationally designed protein assemblies. Biochem Pharmacol 120:1–14
Teo SP (2022) Review of COVID-19 mRNA Vaccines: BNT162b2 and mRNA-1273. J Pharm Pract 35(6):947–951
Maruggi G, Zhang C, Li J, Ulmer JB, Yu D (2019) mRNA as a transformative technology for vaccine development to control infectious diseases. Mol Ther 27(4):757–772
Iavarone C, O’Hagan DT, Yu D, Delahaye NF, Ulmer JB (2017) Mechanism of action of mRNA-based vaccines. Expert Rev Vaccines 16(9):871–881
Dunbar CE, High KA, Joung JK, Kohn DB, Ozawa K, Sadelain M (2018) Gene therapy comes of age. Science. https://doi.org/10.1126/science.aan4672
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337(6096):816–821
Hekele A, Bertholet S, Archer J, Gibson DG, Palladino G, Brito LA et al (2013) Rapidly produced SAM(®) vaccine against H7N9 influenza is immunogenic in mice. Emerg Microbes Infect 2(8):e52
Jackson NAC, Kester KE, Casimiro D, Gurunathan S, DeRosa F (2020) The promise of mRNA vaccines: a biotech and industrial perspective. NPJ Vaccines 5:11
Cobb M (2015) Who discovered messenger RNA? Curr Biol 25(13):R526–R532
Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A et al (1990) Direct gene transfer into mouse muscle in vivo. Science 247(4949 Pt 1):1465–1468
Phua KK, Leong KW, Nair SK (2013) Transfection efficiency and transgene expression kinetics of mRNA delivered in naked and nanoparticle format. J Control Release 166(3):227–233
Xu S, Yang K, Li R, Zhang L (2020) mRNA Vaccine Era-Mechanisms, Drug Platform and Clinical Prospection. Int J Mol Sci 21(18):6582
Aldosari BN, Alfagih IM, Almurshedi AS (2021) Lipid nanoparticles as delivery systems for RNA-based vaccines. Pharmaceutics 13(2):206
Zhao W, Hou X, Vick OG, Dong Y (2019) RNA delivery biomaterials for the treatment of genetic and rare diseases. Biomaterials 217:119291
Xiong Q, Lee GY, Ding J, Li W, Shi J (2018) Biomedical applications of mRNA nanomedicine. Nano Res 11(10):5281–5309
Gebre MS, Brito LA, Tostanoski LH, Edwards DK, Carfi A, Barouch DH (2021) Novel approaches for vaccine development. Cell 184(6):1589–1603
Kim J, Eygeris Y, Gupta M, Sahay G (2021) Self-assembled mRNA vaccines. Adv Drug Deliv Rev 170:83–112
Zhang C, Maruggi G, Shan H, Li J (2019) Advances in mRNA vaccines for infectious diseases. Front Immunol. https://doi.org/10.3389/fimmu.2019.00594
Verbeke R, Lentacker I, Smedt SCD, Dewitte H (2019) Three decades of messenger RNA vaccine development. Nano Today 28:100766
Hajj KAWK (2017) Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat Rev Mater 2:17056
Meng C, Chen Z, Li G, Welte T, Shen H (2021) Nanoplatforms for mRNA therapeutics. Advanced Therapeutics. 384:2212–22218
Hacisuleyman E, Hale C, Saito Y, Blachere NE, Bergh M, Conlon EG et al (2021) Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med 384(23):2212–2218
Crommelin DJA, Anchordoquy TJ, Volkin DB, Jiskoot W, Mastrobattista E (2021) Addressing the cold reality of mRNA vaccine stability. J Pharm Sci 110(3):997–1001
Schoenmaker L, Witzigmann D, Kulkarni JA, Verbeke R, Kersten G, Jiskoot W et al (2021) mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int J Pharm 601:120586
Kulkarni JA, Cullis PR, van der Meel R (2018) Lipid nanoparticles enabling gene therapies: from concepts to clinical utility. Nucleic Acid Ther 28(3):146–157
Hou X, Zaks T, Langer R, Dong Y (2021) Lipid nanoparticles for mRNA delivery. Nat Rev Mater 6(12):1078–1094
Ponti F, Campolungo M, Melchiori C, Bono N, Candiani G (2021) Cationic lipids for gene delivery: many players, one goal. Chem Phys Lipids 235:105032
Zhang Y, Sun C, Wang C, Jankovic KE, Dong Y (2021) Lipids and lipid derivatives for RNA delivery. Chem Rev 121(20):12181–12277
Samaridou E, Heyes J, Lutwyche P (2020) Lipid nanoparticles for nucleic acid delivery: current perspectives. Adv Drug Deliv Rev 154–155:37–63
Tanaka H, Takahashi T, Konishi M, Takata N, Akita H (2020) Self-Degradable lipid-like materials based on “hydrolysis accelerated by the intra-particle enrichment of reactant (hyper)” for messenger RNA delivery. Adv Funct Mater 30(34):1910575
Eygeris Y, Gupta M, Kim J, Sahay G (2022) Chemistry of lipid nanoparticles for RNA delivery. Acc Chem Res 55(1):2–12
Zhao X, Chen J, Qiu M, Li Y, Glass Z, Xu Q (2020) Imidazole-based synthetic LIPIDOIDS for in vivo MRNA delivery into primary t lymphocytes. Angew Chem Int Ed Engl 59(45):20083–20089
Zhao X, Glass Z, Chen J, Yang L, Kaplan DL, Xu Q (2021) mRNA delivery using BIOREDUCIBLE LIPIDOID nanoparticles facilitates neural differentiation of human mesenchymal stem cells. Adv Healthc Mater 10(4):e2000938
Heyes J, Palmer L, Bremner K, MacLachlan I (2005) Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. J Control Release 107(2):276–287
Buschmann MD, Carrasco MJ, Alishetty S, Paige M, Alameh MG, Weissman D (2021) Nanomaterial delivery systems for mRNA Vaccines. Vaccines (Basel). 9(1):65
Patel P, Ibrahim NM, Cheng K (2021) The importance of apparent pka in the development of nanoparticles encapsulating siRNA and mRNA. Trends Pharmacol Sci 42(6):448–460
Vhora I, Lalani R, Bhatt P, Patil S, Misra A (2019) Lipid-nucleic acid nanoparticles of novel ionizable lipids for systemic BMP-9 gene delivery to bone-marrow mesenchymal stem cells for osteoinduction. Int J Pharm 563:324–336
Kumar V, Qin J, Jiang Y, Duncan RG, Brigham B, Fishman S et al (2014) Shielding of lipid nanoparticles for siRNA delivery: impact on physicochemical properties, cytokine induction, and efficacy. Mol Ther Nucleic Acids 3(11):e210
Tam YY, Chen S, Cullis PR (2013) Advances in lipid nanoparticles for siRNA delivery. Pharmaceutics 5(3):498–507
Jayaraman M, Ansell SM, Mui BL, Tam YK, Chen J, Du X et al (2012) Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew Chem Int Ed Engl 51(34):8529–8533
Hassett KJ, Benenato KE, Jacquinet E, Lee A, Woods A, Yuzhakov O et al (2019) Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol Ther Nucleic Acids 15:1–11
Suzuki T, Suzuki Y, Hihara T, Kubara K, Kondo K, Hyodo K et al (2020) PEG shedding-rate-dependent blood clearance of PEGylated lipid nanoparticles in mice: faster PEG shedding attenuates anti-PEG IgM production. Int J Pharm 588:119792
Holland JW, Hui C, Cullis PR, Madden TD (1996) Poly(ethylene glycol)–lipid conjugates regulate the calcium-induced fusion of liposomes composed of phosphatidylethanolamine and phosphatidylserine. Biochemistry 35(8):2618–2624
Mui BL, Tam YK, Jayaraman M, Ansell SM, Du X, Tam YY et al (2013) Influence of polyethylene glycol lipid desorption rates on pharmacokinetics and pharmacodynamics of sirna lipid nanoparticles. Mol Ther Nucleic Acids 2(12):e139
Sebastiani F, Yanez Arteta M, Lerche M, Porcar L, Lang C, Bragg RA et al (2021) Apolipoprotein E binding drives structural and compositional rearrangement of mrna-containing lipid nanoparticles. ACS Nano 15(4):6709–6722
Judge A, McClintock K, Phelps JR, Maclachlan I (2006) Hypersensitivity and loss of disease site targeting caused by antibody responses to PEGylated liposomes. Mol Ther 13(2):328–337
Lokugamage MP, Vanover D, Beyersdorf J, Hatit MZC, Rotolo L, Echeverri ES et al (2021) Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat Biomed Eng 5(9):1059–1068
Kulkarni JA, Witzigmann D, Leung J, Tam YYC, Cullis PR (2019) On the role of helper lipids in lipid nanoparticle formulations of siRNA. Nanoscale 11(45):21733–21739
Zhang H, Leal J, Soto MR, Smyth HDC, Ghosh D (2020) Aerosolizable lipid nanoparticles for pulmonary delivery of mrna through design of experiments. Pharmaceutics 12(11):1042
Li SD, Huang L (2008) Pharmacokinetics and biodistribution of nanoparticles. Mol Pharm 5(4):496–504
Akinc A, Querbes W, De S, Qin J, Frank-Kamenetsky M, Jayaprakash KN et al (2010) Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol Ther 18(7):1357–1364
Miao L, Lin J, Huang Y, Li L, Delcassian D, Ge Y et al (2020) Synergistic lipid compositions for albumin receptor mediated delivery of mRNA to the liver. Nat Commun 11(1):2424
Ryals RC, Patel S, Acosta C, McKinney M, Pennesi ME, Sahay G (2020) The effects of PEGylation on LNP based mRNA delivery to the eye. PLoS ONE 15(10):e0241006
Chen S, Tam YYC, Lin PJC, Sung MMH, Tam YK, Cullis PR (2016) Influence of particle size on the in vivo potency of lipid nanoparticle formulations of siRNA. J Control Release 235:236–244
Oberli MA, Reichmuth AM, Dorkin JR, Mitchell MJ, Fenton OS, Jaklenec A et al (2017) Lipid nanoparticle assisted mrna delivery for potent cancer immunotherapy. Nano Lett 17(3):1326–1335
Paunovska K, Gil CJ, Lokugamage MP, Sago CD, Sato M, Lando GN et al (2018) Analyzing 2000 in vivo drug delivery data points reveals cholesterol structure impacts nanoparticle delivery. ACS Nano 12(8):8341–8349
Patel S, Ashwanikumar N, Robinson E, Xia Y, Mihai C, Griffith JP 3rd et al (2020) Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat Commun 11(1):983
Rodrigueza WV, Pritchard PH, Hope MJ (1993) The influence of size and composition on the cholesterol mobilizing properties of liposomes in vivo. Biochim Biophys Acta 1153(1):9–19
Semple SC, Chonn A, Cullis PR (1996) Influence of cholesterol on the association of plasma proteins with liposomes. Biochemistry 35(8):2521–2525
Koltover I, Salditt T, Rädler JO, Safinya CR (1998) An inverted hexagonal phase of cationic liposome-DNA complexes related to DNA release and delivery. Science 281(5373):78–81
Kauffman KJ, Dorkin JR, Yang JH, Heartlein MW, DeRosa F, Mir FF et al (2015) Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. Nano Lett 15(11):7300–7306
Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN et al (2020) An mRNA vaccine against sars-cov-2 - preliminary report. N Engl J Med 383(20):1920–1931
Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M et al (2020) COVID-19 vaccine BNT162b1 elicits human antibody and T(H)1 T cell responses. Nature 586(7830):594–599
Walsh EE, Frenck RW Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A et al (2020) Safety and immunogenicity of two rna-based covid-19 vaccine candidates. N Engl J Med 383(25):2439–2450
Dinarello CA (2018) Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev 281(1):8–27
Tanaka T, Narazaki M, Kishimoto T (2014) IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 6(10):a016295
Ndeupen S, Qin Z, Jacobsen S, Bouteau A, Estanbouli H, Igyártó BZ (2021) The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 24(12):103479
Laczkó D, Hogan MJ, Toulmin SA, Hicks P, Lederer K, Gaudette BT et al (2020) A single immunization with nucleoside-modified mrna vaccines elicits strong cellular and humoral immune responses against sars-cov-2 in mice. Immunity 53(4):724–32.e7
Lederer K, Castaño D, Gómez Atria D, Oguin TH 3rd, Wang S, Manzoni TB et al (2020) SARS-CoV-2 mRNA vaccines foster potent antigen-specific germinal center responses associated with neutralizing antibody generation. Immunity 53(6):1281–95.e5
Lonez C, Vandenbranden M, Ruysschaert JM (2012) Cationic lipids activate intracellular signaling pathways. Adv Drug Deliv Rev 64(15):1749–1758
Tanaka T, Legat A, Adam E, Steuve J, Gatot JS, Vandenbranden M et al (2008) DiC14-amidine cationic liposomes stimulate myeloid dendritic cells through toll-like receptor 4. Eur J Immunol 38(5):1351–1357
Blakney AK, McKay PF, Yus BI, Aldon Y, Shattock RJ (2019) Inside out: optimization of lipid nanoparticle formulations for exterior complexation and in vivo delivery of saRNA. Gene Ther 26(9):363–372
Kowalski PS, Rudra A, Miao L, Anderson DG (2019) Delivering the messenger: advances in technologies for therapeutic mrna delivery. Mol Ther 27(4):710–728
Alameh MG, Tombácz I, Bettini E, Lederer K, Sittplangkoon C, Wilmore JR et al (2021) Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 54(12):2877–92.e7
Lin PJ, Tam YY, Hafez I, Sandhu A, Chen S, Ciufolini MA et al (2013) Influence of cationic lipid composition on uptake and intracellular processing of lipid nanoparticle formulations of siRNA. Nanomedicine 9(2):233–246
Cui S, Wang Y, Gong Y, Lin X, Zhao Y, Zhi D et al (2018) Correlation of the cytotoxic effects of cationic lipids with their headgroups. Toxicol Res (Camb) 7(3):473–479
Yang L, Gong L, Wang P, Zhao X, Zhao F, Zhang Z et al (2022) Recent advances in lipid nanoparticles for delivery of mRNA. Pharmaceutics 14(12):2682
Tse SW, McKinney K, Walker W, Nguyen M, Iacovelli J, Small C et al (2021) mRNA-encoded, constitutively active STING(V155M) is a potent genetic adjuvant of antigen-specific CD8(+) T cell response. Mol Ther 29(7):2227–2238
Lamoot A, Jangra S, Laghlali G, Warang P, Singh G, Chang LA et al (2024) Lipid nanoparticle encapsulation empowers poly(I:C) to activate cytoplasmic RLRs and thereby increases its adjuvanticity. Small 20(10):e2306892
Jangra S, Landers JJ, Laghlali G, Rathnasinghe R, Warang P, Park SC et al (2023) Multicomponent intranasal adjuvant for mucosal and durable systemic SARS-CoV-2 immunity in young and aged mice. NPJ Vaccines 8(1):96
Del Pozo-Rodríguez A, Solinís M, Rodríguez-Gascón A (2016) Applications of lipid nanoparticles in gene therapy. Eur J Pharm Biopharm 109:184–193
Rusnock A (2009) Catching cowpox: the early spread of smallpox vaccination, 1798–1810. Bull Hist Med 83(1):17–36
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R et al (2021) Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384(5):403–416
Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M et al (2020) Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 383(25):2427–2438
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S et al (2020) Safety and efficacy of the BNT162B2 mRNA covid-19 vaccine. N Engl J Med 383(27):2603–2615
Feldmann H, Czub M, Jones S, Dick D, Garbutt M, Grolla A et al (2002) Emerging and re-emerging infectious diseases. Med Microbiol Immunol 191(2):63–74
Ura T, Yamashita A, Mizuki N, Okuda K, Shimada M (2021) New vaccine production platforms used in developing SARS-CoV-2 vaccine candidates. Vaccine 39(2):197–201
Yang W, Elankumaran S, Marr LC (2012) Relationship between humidity and influenza A viability in droplets and implications for influenza’s seasonality. PLoS ONE 7(10):e46789
Espeseth AS, Cejas PJ, Citron MP, Wang D, DiStefano DJ, Callahan C et al (2020) Modified mRNA/lipid nanoparticle-based vaccines expressing respiratory syncytial virus F protein variants are immunogenic and protective in rodent models of RSV infection. NPJ Vaccines 5(1):16
Pine M, Arora G, Hart TM, Bettini E, Gaudette BT, Muramatsu H et al (2023) Development of an mRNA-lipid nanoparticle vaccine against Lyme disease. Mol Ther 31(9):2702–2714
Saxena S, Sonwane AA, Dahiya SS, Patel CL, Saini M, Rai A et al (2009) Induction of immune responses and protection in mice against rabies using a self-replicating RNA vaccine encoding rabies virus glycoprotein. Vet Microbiol 136(1–2):36–44
VanBlargan LA, Himansu S, Foreman BM, Ebel GD, Pierson TC, Diamond MS (2018) An mRNA vaccine protects mice against multiple tick-transmitted flavivirus infections. Cell Rep 25(12):3382–92.e3
Rurik JG, Tombácz I, Yadegari A, Méndez Fernández PO, Shewale SV, Li L et al (2022) CAR T cells produced in vivo to treat cardiac injury. Science 375(6576):91–96
Rizvi F, Everton E, Smith AR, Liu H, Osota E, Beattie M et al (2021) Murine liver repair via transient activation of regenerative pathways in hepatocytes using lipid nanoparticle-complexed nucleoside-modified mRNA. Nat Commun 12(1):613
Grunwitz C, Kranz LM (2017) mRNA cancer vaccines-messages that prevail. Curr Top Microbiol Immunol 405:145–164
Yang J, Arya S, Lung P, Lin Q, Huang J, Li Q (2019) Hybrid nanovaccine for the co-delivery of the mRNA antigen and adjuvant. Nanoscale 11(45):21782–21789
Lei S, Zhang X, Men K, Gao Y, Yang X, Wu S et al (2020) Efficient colorectal cancer gene therapy with IL-15 mRNA nanoformulation. Mol Pharm 17(9):3378–3391
Mai Y, Guo J, Zhao Y, Ma S, Hou Y, Yang J (2020) Intranasal delivery of cationic liposome-protamine complex mRNA vaccine elicits effective anti-tumor immunity. Cell Immunol 354:104143
Wang Y, Su HH, Yang Y, Hu Y, Zhang L, Blancafort P et al (2013) Systemic delivery of modified mRNA encoding herpes simplex virus 1 thymidine kinase for targeted cancer gene therapy. Mol Ther 21(2):358–367
Zhang R, Billingsley MM, Mitchell MJ (2018) Biomaterials for vaccine-based cancer immunotherapy. J Control Release 292:256–276
Lee K, Kim SY, Seo Y, Kim MH, Chang J, Lee H (2020) Adjuvant incorporated lipid nanoparticles for enhanced mRNA-mediated cancer immunotherapy. Biomater Sci 8(4):1101–1105
Ramos da Silva J, Bitencourt Rodrigues K, Formoso Pelegrin G, Silva Sales N, Muramatsu H, de Oliveira SM et al (2023) Single immunizations of self-amplifying or non-replicating mRNA-LNP vaccines control HPV-associated tumors in mice. Sci Transl Med. 15(686):3464
Handy CE, Antonarakis ES (2018) Sipuleucel-T for the treatment of prostate cancer: novel insights and future directions. Future Oncol 14(10):907–917
Song Q, Zhang CD, Wu XH (2018) Therapeutic cancer vaccines: From initial findings to prospects. Immunol Lett 196:11–21
Zhang H, You X, Wang X, Cui L, Wang Z, Xu F et al (2021) Delivery of mRNA vaccine with a lipid-like material potentiates antitumor efficacy through Toll-like receptor 4 signaling. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.2005191118
Miao L, Zhang Y, Huang L (2021) mRNA vaccine for cancer immunotherapy. Mol Cancer 20(1):41
Tanyi JL, Bobisse S, Ophir E, Tuyaerts S, Roberti A, Genolet R et al (2018) Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aao5931
Snell LM, McGaha TL, Brooks DG (2017) Type I interferon in chronic virus infection and cancer. Trends Immunol 38(8):542–557
Yarchoan M, Johnson BA 3rd, Lutz ER, Laheru DA, Jaffee EM (2017) Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer 17(4):209–222
Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Löwer M et al (2017) Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547(7662):222–226
Acknowledgements
This study was supported by the National Key Research and Development Program of China (No. 2023YFB3210300).
Funding
This study was supported by the National Key Research and Development Program of China (No. 2023YFB3210300).
Author information
Authors and Affiliations
Contributions
Conceptualization: Hongqiang Shen, Bili Wang; Investigation: Biao Shen; Writing-original draft: Bili Wang, Biao Shen; Writing-review and editing: Hongqiang Shen, Wenqing Xiang.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no financial/commercial conflicts of interest.
Ethical approval
Not applicable.
Additional information
Edited by Juergen Richt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, B., Shen, B., Xiang, W. et al. Advances in the study of LNPs for mRNA delivery and clinical applications. Virus Genes (2024). https://doi.org/10.1007/s11262-024-02102-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11262-024-02102-6