Abstract
DENV infection outcomes depend on the host’s variable expression of immune receptors and mediators, leading to either resolution or exacerbation. While the NS3 protein is known to induce robust immune responses, the specific impact of its protease region epitopes remains unclear. This study investigated the effect of recombinant NS3 protease region proteins from all four DENV serotypes on splenocyte activation in BALB/c mice (n = 5/group). Mice were immunized with each protein, and their splenocytes were subsequently stimulated with homologous antigens. We measured the expression of costimulatory molecules (CD28, CD80, CD86, CD152) by flow cytometry, along with IL-2 production, CD25 expression, and examined the antigen-specific activation of CD4 + and CD8 + T cells. Additionally, the expression of IL-1, IL-10, and TGF-β1 in splenocytes from immunized animals was assessed. Apoptosis was evaluated using Annexin V/PI staining and DNA fragmentation analysis. Stimulation of splenocytes from immunized mice triggered apoptosis (phosphatidylserine exposure and caspase 3/7 activation) and increased costimulatory molecule expression, particularly CD152. Low IL-2 production and low CD25 expression, as well as sustained expression of the IL-10 gene. These results suggest that these molecules might be involved in mechanisms by which the NS3 protein contributes to viral persistence and disease pathogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The dengue virus (DENV), the causative agent of dengue fever, consists of four antigenically distinct serotypes (DENV1-DENV4) that share structural similarities. This mosquito-borne flavivirus primarily transmits through female Aedes mosquitoes (Aedes aegypti and Aedes albopictus). Different epidemiological patterns are associated with each serotype, and simultaneous circulation of multiple serotypes is common in hyperendemic regions. Dengue fever is a febrile illness affecting individuals of all ages [1]. Dengue infection can range from asymptomatic to debilitating, manifesting with diverse symptoms. These may include mild fever, disabling fever, severe headache, retroorbital pain, muscle and joint aches, and rashes. In severe cases, it can progress to shock syndrome, respiratory distress, significant bleeding, and life-threatening organ complications [2]. A new classification system for dengue virus infection was adopted to improve clinical severity assessment, reporting, and surveillance. This system categorizes dengue into two main groups: non-severe dengue (D-W) and severe dengue (DFG). Non-severe dengue is further subdivided into dengue without warning signs (D−W) and dengue with warning signs (D + W) [3]. The most significant challenge remains the development of a vaccine that offers effective protection against infection by the diverse dengue virus serotypes (DENV). Furthermore, potent and effective therapeutic drugs are urgently needed [4].
The dengue virus (DENV) comprises three structural proteins (C, prM/M, and E) and seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5). While the structural proteins form the virion architecture, the non-structural proteins orchestrate viral replication and virion assembly [5]. Among DENV molecules, non-structural protein 3 (NS3) stands out due to its critical roles in viral replication. Acting as both a protease and an RNA helicase, NS3 facilitates polyprotein processing and ensures efficient viral RNA replication. Recent studies suggest its involvement in virion assembly and release, further highlighting its multifaceted contribution. Additionally, NS3 serves as a major immunological target during infection, underscoring its significance in the host’s response to DENV [6, 7]. During infection, the NS3 protein may be a target for T cells, harboring numerous potential epitopes within its sequence. This implies a possible role for T cells in controlling infection. However, the question of whether these epitopes elicit a beneficial or detrimental T-cell response remains unanswered [7, 8]. Despite their critical role in pathogenesis, few studies have investigated the immunomodulatory effects of the NS3 protein [9, 10, 11]. Consequently, the precise roles of various viral proteins in disease progression remain elusive, their functions only partially defined due to incomplete characterization of the immune responses they trigger. In this work we evaluated the effect of immunization with recombinant NS3 protein (protease region; GST-NS3pro) of the four DENV serotypes on splenocyte activation in BALB/c mice.
Materials and methods
Expression and purification of GST-NS3pro1-4
Escherichia coli DH5α cells transformed with either parental (pGEX-5X-1) or recombinant expression vectors (pGEX-DVNS3-1-4) were inoculated into LB media containing 100 mg/L ampicillin (Sigma, St. Louis, MO, USA), and incubated at 37 °C overnight. Fresh LB media was incubated at 37 °C with the overnight culture (1:100) and protein production was induced by addition of isopropyl β-thiogalactoside to a final concentration of 0.1 mM. After 2 h of incubation, the cells were harvested and purification of the expressed proteins was carried out [12, 13]. Briefly, pelleted cells were resuspended in phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, and 1.4 mM KH2PO4, pH 7.4) and lysed by sonication. Lysate was then centrifuged at 3046×g for 20 min and the supernatants containing solubilized fusion proteins were mixed with glutathione-agarose beads (sulfur linkage; Sigma). After adsorption for 30 min, beads were collected and washed by centrifugation. Either GST or fusion proteins were eluted by competition with free glutathione (15 mM glutathione in 50 mM Tris–HCl pH 8.0), then acetone-precipitated. Proteins were resolved on 10% SDS-PAGE and visualized by staining with Coomassie brilliant blue [12].
Immunization of mice with GST-NS3pro1-4
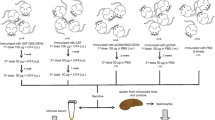
Mice were immunized according to the protocol described previously [13, 14]. Briefly, female BALB/c mice (6 to 8 weeks old) (n = 5) were immunized by intraperitoneal route. All mice were maintained according to the recommendations by our Institutional Animal Care and Use Committee. One group of mice was immunized with a dose of 100 μg of antigen and two more with 50 μg of the recombinant protein GST-NS3pro1-4. First immunizations were performed with the antigen emulsified in complete Freund’s adjuvant (CFA), and re-immunizations at one-week intervals were performed with incomplete Freund’s adjuvant (Gibco-BRL, Grand Island, NY, USA). A group that received three doses of 1 × PBS (200 μL) (vehicle control) was used, in addition, since the recombinant proteins are fused to GST, a group of mice (internal control) that received 3 doses of GST protein plus adjuvant was included in order to demonstrate that the GST protein does not develop immune activation (Fig. 1.).
Graphic representation of the immunization scheme, groups of BALB/c mice (n = 5) were immunized with three doses of GST-NS3pro protein corresponding to each of the recombinant proteins of each serotype (groups 1–4), group 5 was immunized with GST protein intraperitoneally (i.p.). Immunizations were performed with a 1 week interval between each dose. Spleens were obtained 7 days after the last immunization. Splenocytes were used for proliferation assays and determination of costimulatory and apoptotic molecules by flow cytometry. The same procedure was performed in parallel with splenocytes from mice receiving PBS only (group 6). CFA, complete Freund’s adjuvant; iCFA, incomplete Freund’s adjuvant; PBS, phosphate-buffered saline
T cell proliferation assay
Spleens from normal and immunized BALB/c mice were removed aseptically, dispersed into single-cell suspensions to obtain the splenocytes and erythrocytes removed by treating cell suspensions with ACK buffer (150 mM NH4Cl, 1 mM KHCO3, 0.1 mM Na2EDTA pH 7·4) according to the previously reported [15]. Briefly, splenocytes were resuspended in Dulbecco modified Eagle medium (DMEM) containing 10% heat inactivated FCS (Gibco, BRL), 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM l-glutamine, and 0.05 mM β-Mercaptoethanol. Spleen cells were cultured in 96 well flat bottom plates at 4 × 105/well and 37 °C under humidified atmosphere of 5% CO2. Cultures were stimulated in triplicate with 4 μg/mL of Con A (Con A, Sigma Chemical Co.) by 72 h, and with 10 μg/mL of the antigen (GST-NS3pro from the four serotypes individually and with a mixture of the four proteins) for 120 h in a total volume of 200 μl culture medium at the end of culture, cells were centrifuged at 120×g and resuspended in 100 μL of fresh medium, subsequently it was added 10 μL of the 12 mM MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] stock solution (Vybrant®, Molecular Probes) to each well. Finally, samples were mixed using a pipette and absorbance read at 570 nm in a microplate reader (Multiskan EX, Thermo Electron Corporation). Proliferation was reported as Stimulation Index. The Stimulation Index of each sample was calculated according to the following formula: SI = mean optical density (OD) of stimulated splenocytes / mean OD of unstimulated splenocytes. An SI greater than 3 was considered positive.
Flow cytometry assays
Following stimulation, cells were detached with cold 1% PBS-EDTA from each well, transferred to 1.5 ml tubes, and centrifuged at 189 ×g for 5 min. To the resulting cell suspension, 5 µl of SFB was added to block the Fc receptors for 30 min at 4 °C. At the end of incubation the cells were washed twice with 1X PBS and resuspended in a volume of 100 µl. Splenocytes were individually stained using the following antibodies: α-CD28-PE-Cy®5 (0.5 μg/100 μL), α-CD80 (B71)-FITC (0.25 μg/100 μL), α-CD86 (B72)-FITC (0.25 μg/100 μL), α-CD152 (CTLA-4)-PE (0.06 μg/100 μL), α-CD25-Pacific blue (25 μg/mL), and α-IL-2-Pacific blue (50 μg/mL). Bovine Serum Albumin (BSA) was used as a negative control of the expression of costimulation molecules, CD25 and Interleukin (IL)-2. An Internal control (GST) was used, as it is important to demonstrate that the GST portion is not involved in the development of the immune response induced by the recombinant GST-NS3pro proteins. A positive control and an isotype control were used for all determinations. For the analysis of proliferation of CD4 + and CD8 + subspopulations, the antibodies used were: α-CD4-PE (50 μg/mL), α-CD8-PE (25 μg/mL), and the CellTrace™ CFSE (Carboxyfluoresceinsuccinimidylester) proliferation kit was used as proliferation marker. The antibodies (Life Technologies) and proliferation marker were added to the cells and incubated for 40 min at 4 °C. Finally the cells were washed with PBS and each marker was read according to its fluorochrome in the flow cytometer (Attune Acoustic Cytometer, Applied Biosystems). The results were analyzed using FlowJo software (version 10, TreeStar, USA).
Cytokine determination by RT-PCR
RNA from spleen cells (3 × 106 cells/mL) stimulated and unstimulated with the antigens were incubated at 37 °C in 24-well plates (Costar) in a 5% CO2 atmosphere. After 72 h (SE, Con A) and 120 h (recombinant proteins and GST) of incubation, cells were collected by centrifugation at 511 ×g for 10 min at 4 °C, total RNA was purified using the TRIzol method (GIBCO BRL), according to the manufacturer's instructions. Approximately 1 µg of RNA was retrotranscribed to cDNA with 1 µL of poly (dT) oligonucleotide, and 8 µL of water-DEPC, using Super Script II reverse transcriptase enzyme (Invitrogen). Subsequently the mixture (12 µL) of oligo dT (Invitrogen) and RNA was heated for 10 min at 70 °C and placed on ice rapidly for 2 min. 4 µL of 5X first strand buffer, 2 µL of 0.1 M DTT and 1 µL of dNTP's mixture (10 mM) (Perkin-Elmer) were added. The sample was incubated for 2 min at 42 °C. Finally, 1 µL of the reverse transcriptase enzyme (Super Script II) was added and incubated for 50 min at 42 °C. The reaction was inactivated by incubating for 15 min at 70 °C. The newly synthesized cDNA was used as a template for the PCR reaction. The amplification reaction (PCR) was performed with 1 µL (5% of the total RT reaction) of the above reaction product and 20 pmol of each of the oligonucleotides specific for the mouse cytokines (Table 1), using Taq polymerase "Gold" (Perkin-Elmer); PCR conditions for IL-10 and transforming growth factor-β1 (TGF-β1) were as follows, each PCR cycle consisted of a denaturation step (94 °C for 45 s), an annealing step (60 °C for 1 min) and an extension step (72 °C for 90 s), these steps were repeated for 30 cycles. For IL-2, the conditions were as follows, each PCR cycle consisted of a denaturation step (94 °C for 30 s), an alignment step (55 °C for 30 s) and an extension step (72 °C for 2 min), and these steps were repeated for 35 cycles. As a housekeeping control, the β-actin gene was amplified; each PCR cycle consisted of a denaturation step (95 °C for 1 min), an alignment step (55 °C for 2 min) and an extension step (72 °C for 2 min), these steps were repeated for 30 cycles. PCR reaction products were analyzed on 1.8% agarose gels and visualized by ethidium bromide staining (BIO-RAD) at a concentration of 0.5 µg/mL and visualized on a UV light transilluminator (UVP).
Apoptosis assay
To analyze the translocation of phosphatidylserine (PS) it was used the kit Alexa Fluor® 488 annexin V/Dead Cell Apoptosis Kit with Alexa® Fluor 488 annexin V and propidium iodide (PI) (Molecular Probes) for Flow Cytometry and for the determination of caspases it was used the CellEvent® Caspase-3/7 Green Flow Cytometry Assay Kit (Molecular Probes). Apoptotic cells were then evaluated by gating the PI- and Annexin V-positive cells using a flow cytometer (Attune Acoustic Cytometer, Applied Biosystems). Data were subsequently analyzed by FlowJo software (version10, TreeStar, USA). For the qualitative analysis of DNA fragmentation, from the stimulated splenocytes, the DNA was obtained by the phenol–chloroform method, and then electrophoresed in 1% agarose gels in the presence of a TAE buffer (Tris–Acetate 40 mM, EDTA 1 mM, pH 8.0) applying an initial electric charge of 100 V, and then reducing it to 80 V. The DNA was visualized with UV light in a transilluminator (UVP). Stimulation with dexamethasone (20 μg/mL) was used as a positive control for apoptosis assays.
Data analysis
Statistical analysis was performed with GraphPad Prism (Version 9.3.1). The results are presented as mean ± standard deviation. Analysis of variance (ANOVA) followed by Tukey’s post-hoc test was performed to compare the mean values among various groups. A P value of < 0.05 was considered statistically significant. The mean fluorescence intensity index was calculated by dividing the MFI obtained from mouse splenocytes immunized with recombinant GST-NS3 protein and stimulated with the corresponding antigens by the MFI obtained from non-immunized and non-stimulated splenocytes, using the following formula:
MFI index = (MFI of stimulated splenocytes)/(MFI of unstimulated splenocytes).
A one-way ANOVA was performed using Dunnett’s test to compare the MFI Indices of each molecule of interest from splenocytes of non-immunized mice against the MFI Indices of splenocytes from GST-NS3-immunized mice. A statistically significant difference was considered with a p < 0.05.
Results
Immunization with recombinant GST-NS3pro protein does not induce splenocyte activation
Splenocytes from normal and individually immunized mice were stimulated in vitro with each of the four recombinant GST-NS3pro serotype proteins. It was observed that splenocytes from normal and immunized mice did not respond to stimulation with GST-NS3pro proteins of the 4 serotypes, assayed individually nor to stimulation using a mixture of the four proteins (GST-NS3pro1-4). However, when splenocytes were nonspecifically stimulated with the Con A mitogen, only splenocytes from normal animals responded to the stimulus (Fig. 2). All cells from both normal and immunized animals had viability greater than 94% (data not shown). Splenocytes from the control groups (GST and PBS) did not show any activation (data not shown). The possible contamination of recombinant proteins with Lipopolysaccharide (LPS) was also analyzed, obtaining values below 0.005 EU/mL (data not shown). Because splenocytes from mice immunized with each of the recombinant proteins behaved similarly, only a representative result from mice immunized with the recombinant proteins individually is shown in the following results.
Proliferation assay of splenocytes from normal mice and mice immunized with the recombinant GST-NS3 proteins of the four serotypes. Results are presented as the mean of the stimulation index ± the standard deviation. The result is representative of three independent experiments.***P < 0.0001. Ns, not stimulated; ConA, concanavaline A; H-Ag, homologous antigen; GST-NS3pro1-4 (protein mixture)
Identification of costimulation molecules
The expression of co-stimulatory molecules such as CD80, CD86, CD28 and CD152 was analyzed. 75% of splenocytes from normal animals stimulated with ConA expressed CD80 (FMII 15.8; 95%CI 17.7 ± 13.9), 76% CD86 (FMII 17; 95%CI 16.53 ± 17.47), 65% CD28 (FMII 18; 95%CI 15.54 ± 18.46), and 35% CD152 (FMII 17.6; 95%CI 16.88 ± 18.32). Stimulation with the individual recombinant proteins and with the GST-NS3pro1-4 mixture induced CD80 expression in 20% of the population (FMII 6.2, CI95% 5.7–6.7, and 6.7, CI95% 6.6–6.8, respectively), approximately 23 and 16% of the population were positive for CD28 expression (FMII 6. 3, 95%CI 5.8–6.8 and 8.9, 95%CI 7.5–10.3) and CD152 (FMII 5.7, 95%CI 4.8–6.9 and 6.4, 95%CI 5.2–7.6), respectively (Fig. 3A). Cells from GST-immunized mice only show the expression of co-stimulatory molecules under the Con A stimulus (Supplementary Fig. 1). On the other hand, the expression of costimulation molecules was analyzed in the splenocytes of animals immunized with the recombinant proteins of the 4 serotypes stimulated in vitro with their homologous antigen (H-Ag) and with the GST-NS3pro1-4 protein mixture. Stimulation with their H-Ag and GST-NS3pro1-4 induced in the cells of immunized animals a pattern of expression of CD80, CD86, CD28 and CD157 molecules similar to that observed by induction with ConA in these same cells, obtaining an FMII of 7.4, 95%CI 6.8–8. 0; 3.3, 95%CI 2.8–3.8; 6.6, 95%CI 6.4–6.7, and 13.2, 95%CI 12.0–14.3 in cells stimulated with their Ag-H, and an MFII of 10.1, 95%CI 9.9–10.3; 3.6; CI95% 3.5–3.6; 9.2, CI95% 9.0–9.4 and 14.9, CI95% 14.5–15.3 for cells stimulated with GST-NS3pro1-4, for CD80, CD86, CD28, and CD152, respectively (Fig. 3B). The expression of the costimulation molecules was similar in splenocytes from mice immunized with the recombinant proteins when stimulated with their H-Ag (Table 2, Supp Fig. 2). On average, 24% expression was observed for the CD80 molecule, 17% for CD86, 29% for CD28 and 35% for CD152. The results showed that splenocytes from immunized mice mostly expressed CD152 relative to the expression level of the other molecules analyzed (Fig. 3B) and a higher expression level of the costimulation molecules analyzed was observed in splenocytes from immunized mice compared to the expression levels observed in splenocytes from normal mice when stimulated with the viral proteins (Fig. 3A Vs Fig. 3B).
Expression of costimulatory molecules. A Splenocytes from normal animals. B Splenocytes from immunized animals. Splenocytes were stimulated with Con A, concanavalin A; BSA, bovine serine albumin; H-Ag, homologous antigen; GST-NS3pro1-4 (protein mixture) and non-stimulated (NS). The expression of the molecules was analyzed by flow cytometry, and the results are presented as the mean fluorescence intensity index (FMI index) ± standard deviation. Statistical analysis was performed using a Tukey test comparing non-immunized/non-stimulated vs. stimulated cells.***P < 0.0001. The result is representative of three independent experiments
IL-2 and CD25 expression analysis
In splenocytes from mice immunized with the recombinant proteins individually or in mixture, no difference in CD25 expression was observed when stimulated with its H-Ag and even under ConA-induced stimulation (7% Vs 8.7%; MFII 1.1, 95%CI 1.2–1.5 Vs 1.3, 95%CI 1.2–1.5)(P = 0.001). Similar behavior was observed when cells were stimulated with the recombinant protein mixture (GST-NS3pro1-4) (Fig. 4A). Only CD25 expression was observed in cells from non-immunized animals stimulated with ConA (Supp Fig. 3). A low IL-2 expression was observed when splenocytes from immunized mice were stimulated with their H-Ag and the recombinant protein mixture compared to IL-2 expression induced by ConA stimulation (6% Vs 32%; MFII 2.6, 95% CI 2.6–2.7 Vs 6.4, 95% CI 6.1–7.2) (P = 0.001) (Fig. 4B). A similar behavior was observed for all groups immunized with the recombinant proteins (Supp Fig. 4).
Histograms of analysis of CD25 and IL-2 expression. Splenocytes from immunized animals were stimulated with Con A, concanavalin A; BSA, bovine serine albumin; H-Ag, homologous antigen; GST-NS3pro1-4 (protein mixture); GST and unstimulated (NS). A) CD25 expression. B) IL-2 expression. R1 corresponds to the cell population that was positive for staining, depending on the case. The upper right panel shows the mean of the FMI index ± the standard deviation corresponding to each histogram.***, **, *,P < 0.0001, > 0.001, < 0.5 versus unstimulated cells. The result is representative of three independent experiments
Expression of cytokines by RT-PCR
Cytokine gene fragments for IL-2, IL-10, and TGF-β1 were amplified. In cells from immunized animals, IL-10 expression was observed only when stimulated with both its homologous antigen and the mixture of recombinant proteins. IL-2 expression, on the other hand, was observed in cells from mice immunized with GST-NS3pro1 and GST-NS3pro2 when stimulated with the protein mixture, and additionally in cells from mice immunized with GST-NS3pro2 upon stimulation with its homologous antigen (Fig. 5).
Analysis of the proliferation of CD4 and CD8 subpopulations in splenocytes from immunized mice
To assess T cell activation following immunization with recombinant proteins, we analyzed the proliferation of CD4 + and CD8 + T lymphocyte populations in splenocytes from immunized mice. T-cell subpopulations present in splenocytes from nonimmunized animals responded similarly to Con A stimulation, showing a proliferation rate higher than 87%, but not to stimulation with GST and recombinant protein individually or in mixture (Fig. 6A). However, the T cell subpopulations present in the splenocytes of mice immunized with any of the recombinant proteins did not respond to stimulation with ConA (Fig. 6B), likewise, the CD4 + and CD8 + T cell populations of mice immunized with the different recombinant proteins were not able to respond to stimulation with their homologous antigen (Fig. 6C).
Determination of CD4 + and CD8 + lymphocyte proliferation in splenocytes. A Splenocytes from unimmunized, unstimulated and GST-stimulated mice (negative control), ConA (positive control) and GST-NS3pro1-4. B.Representative image of splenocytes from mice immunized with GST-NS3pro proteins, unstimulated and stimulated with Con A. C Mouse splenocytes immunized with each of the recombinant proteins and stimulated with their homologous antigen. Subsequently, the proliferation of CD4 and CD8 subpopulations was analyzed by flow cytometry
Analysis of phosphatidylserine (PS) exposure and determination of apoptosis
PS exposure was analyzed in the membrane of splenocytes from normal and immunized mice. Stimulation of the recombinant proteins individually as well as a mixture of them induced PS translocation in the same manner as observed by induction by dexamethasone in splenocytes from non-immunized animals compared to unstimulated splenocytes (P < 0.0001). Furthermore, it was observed that splenocytes from immunized mice show some degree of PS exposure and that this translocation increases when stimulated with their homologous antigen (H-Ag) and with the recombinant protein mixture (GST-NS3pro1-4) (P < 0.0001) (Fig. 7). The results suggest that prolonged antigen exposure favors PS translocation, as increased PS exposure was observed in splenocytes from immunized mice compared to splenocytes from normal mice (P < 0.0001) (Table 3, Supp Fig. 5). When the presence of caspases 3 and 7 was analyzed, it was observed that the antigenic stimulus generated by the recombinant proteins individually or that caused by the GST-NS3pro1-4 mixture induced the presence of caspases in splenocytes from normal mice and this increase was significant when compared with the presence of these enzymes in unstimulated splenocytes (P < 0.0001). Likewise, immunization with the recombinant proteins potentiates the expression of caspases 3 and 7 relative to that observed in splenocytes from non-immunized mice (P < 0.0001) (Table 3). Finally, by PI staining it was observed that splenocytes from both non-immunized and immunized mice showed no plasma membrane damage, ruling out the possible induction of necrosis by the stimulation of recombinant proteins (Fig. 8A), but a pattern of DNA fragmentation was observed in splenocytes from immunized mice that were stimulated with the recombinant proteins, suggesting that the proteins are capable of inducing an apoptotic state in splenocytes (Fig. 8B).
Phosphatidylserine (PS) staining with annexin V and expression of caspase 3 and 7 in splenocytes. Splenocytes from normal mice and mice immunized with the recombinant proteins were stimulated in vitro with their homologous antigen (H-Ag) and with the protein mixture (GST-NS3pro1-4). BSA (bovine serum albumin) is used as a negative control and dexamethasone as a positive control. R1 corresponds to the cell population that was positive for staining, depending on the case. NS, unstimulated. The result is representative of three independent experiments
Analysis of apoptosis in cells from immunized mice. A Propidium iodide staining in cells from mice immunized with recombinant proteins and stimulated in vitro with their H-Ag. B) DNA fragmentation in splenocytes from immunized mice stimulated with their Ag-H. (−) negative control, ( +) positive control, splenocytes from immunized mice stimulated in vitro with their Ag-H. (1) GST-NS3pro1, (2) GST-NS3pro2, (3) GST-NS3pro3, (4) GST-NS3pro4. NS, non-stimulated. Dexamethasone was used as positive control. BSA (bovine serum albumin) was used as negative control. R1 corresponds to the cell population that stained positive according to the case
Discussion
Reports indicate that both structural and non-structural DENV proteins harbor epitopes capable of eliciting T cell-mediated responses [16]. Among these, the non-structural protein 3 (NS3) stands out for its unique trifunctional nature, encompassing NTPase, helicase, and serine protease activities. This multifunctional protein plays a crucial role in the viral replication cycle. Interestingly, studies have revealed numerous immunodominant epitopes distributed throughout the NS3 protein, suggesting robust T cell responses against it [6, 17]. In this work, we focused on analyzing the effect of immunization with recombinant proteins of the protease region of the NS3 protein of DENV on splenocytes of BALB/c mice.
Previous studies suggest that the specific processing of DENV proteins influences the induction of protective immune responses. Intriguingly, some alterations in the immune response (specifically, T lymphocyte proliferation) may stem more from changes in antigen presentation by antigen-presenting cells rather than inherent defects within the T cells themselves [17]. A BALB/c mouse study utilizing the NS3 protein demonstrated that the antigen administration method significantly impacts splenocyte lymphoproliferative response. Surprisingly, the recombinant GST-NS3DENV protein failed to induce T cell proliferation in this model. However, DNA immunization triggered T cell expansion, suggesting distinct antigen processing and presentation pathways [14]. T cell activation requires two crucial signals: (1) binding of the T cell receptor (TCR) to the antigen-MHC-peptide complex displayed by antigen-presenting cells (APCs) and (2) engagement of costimulatory molecules. These combined signals promote T cell survival. However, the absence of costimulation or insufficiently strong initial signals can lead to a state known as anergy, where the T cell becomes unresponsive [18]. During an infection, inhibitory signals within the immune system can aid pathogen persistence. For T cells, two key mechanisms exist: anergy and exhaustion, distinct though potentially involving similar inhibitory molecules. Importantly, anergy induction often increases susceptibility to apoptosis, further contributing to T cell dysfunction [19,20,21].
The most well-studied T cell costimulatory pathway involves the CD28 receptor, interacting with two B7 family costimulatory molecules: B7-1 (CD80) and B7-2 (CD86). Initially discovered for their role in boosting T cell responses, these pathways now show versatility, with some costimulatory receptors even acting as T cell inhibitors. One such example is cytotoxic T lymphocyte-associated antigen 4 (CTLA-4/CD152), a homologue of CD28 that binds to B7-1 and B7-2 but with inducible expression following T cell activation [22].
Our study demonstrated that the recombinant NS3 protein (protease region) induces the expression of costimulatory molecules in splenocytes from both normal and immunized animals, with CD152 showing the most substantial increase. Notably, CD152 acts as a key regulator during early T cell activation. As a CD28 homologue with higher affinity for CD80/86 molecules, it can negatively regulate T cell activation through two mechanisms: (1) directly competing with CD28 for ligands, thus inhibiting positive activation signals, and (2) triggering a negative signaling pathway involving the TCR that weakens T cell-APC interaction [23]. T cell antiviral responses are influenced by the dynamic interplay of immunostimulatory and immunoinhibitory molecules on their surface. Notably, in other viral infections like hepatitis B, hepatitis C, and HIV, CD152 overexpression has been observed to deliver inhibitory signals that suppress T cell activation [24, 25]. Costimulation triggers T lymphocyte activation, leading to proliferation and differentiation into effector cells, but IL-2 is crucial for completing this process. IL-2 induces expression of the high-affinity IL-2 receptor (CD25) on T cell surfaces, amplifying the stimulatory signal and enabling clonal expansion. Both IL-2 and its receptor play key roles in immune tolerance and response mainly through their direct effects on T cells [26, 27].
Evaluating splenocyte staining with annexin V (binding to exposed phosphatidylserine on apoptotic cells) along with propidium iodide exclusion allowed us to identify early apoptosis induced by both immunization and stimulation with DENV recombinant proteins. Additionally, the presence of caspases 3 and 7 suggests the execution of an apoptotic event. These findings are consistent with previous reports showing DENV infection can induce apoptosis in various cell types, potentially mediated by prM/M, E and NS3 helicase proteins [27]. Despite numerous studies, the precise mechanisms of DENV-induced apoptosis remain incompletely understood. Available evidence suggests variations in apoptotic pathways across different cell types and DENV strains/serotypes [28].
Clonal anergy is linked not only to decreased expression of co-stimulatory molecules but also to cytokine-mediated regulation of IL-2 production. Regulatory T lymphocyte subpopulations producing IL-10 and TGF-β are known to suppress immune responses [29]. Our results showing IL-10 presence in splenocytes from mice immunized with recombinant GST-NS3 protein and stimulated with its homologous antigen suggest a potential regulation of the immune response by clonal anergy in T lymphocytes.
CD4 + T cells play a critical role in controlling DENV infection indirectly. They facilitate the activation and memory of B and CD8 + cells, and secrete inflammatory cytokines. In contrast, CD8 + T cells directly contribute to the antiviral response by killing infected cells and secreting IFN-γ and TNF-α [30]. However, a significant increase in Treg lymphocytes is observed during the acute phase of DENV infection. These Tregs suppress T cell proliferation by secreting IL-10 and inhibiting the production of vasoactive cytokines like TNF-α, IFN-γ, and IL-6 [31]. Furthermore, IL-10 appears to contribute to the pathogenesis of acute dengue infections by specifically suppressing T-lymphocyte immune responses against the dengue virus [32].
In conclusion, the results of this work indicate that the protease region of the NS3 protein of DENV mainly induces CD152 expression on the surface of splenocytes of immunized mice, suggesting that this inhibitory signal provides a negative feedback by inhibiting proper T cell activation due to low CD25 expression and low IL-2 production, furthermore IL-10 expression could lead to an anergic state in splenocytes of immunized mice [33]. On the other hand, the induction of apoptosis in these cells may be one of the escape mechanisms of the virus, since by inducing apoptosis the T cell loses its proliferative effector functions and acquires the functions of memory T cells, allowing the virus to ensure its persistence and develop the pathology that characterizes this infection.
Although a myriad of T cell epitopes have been identified and a complete characterization of the responses to the different epitopes of the DENV proteins has not been performed, the results of this work show the need for further studies to better understand the events induced by the NS3 protein on T cell activation, either in the development of protection or in the immunopathology observed during the course of the disease.
Data availability
No datasets were generated or analysed during the current study.
References
World Health Organization. Dengue and severe dengue. 10 January 2022. Available from: https://www.who.int/en/news-room/fact-sheets/detail/dengue-and-severe-dengue
Harapan H, Michie A, Sasmono RT, Imrie A (2020) Dengue: a minireview. Viruses. https://doi.org/10.3390/v12080829
Ajlan BA, Alafif MM, Alawi MM, Akbar NA, Aldigs EK, Madani TA (2019) Assessment of the new World Health Organization’s dengue classification for predicting severity of illness and level of healthcare required. PLoS Negl Trop Dis 13(8):e0007144. https://doi.org/10.1371/journal.pntd.0007144
Kok BH, Lim HT, Lim CP, Lai NS, Leow CY, Leow CH (2023) Dengue virus infection: a review of pathogenesis, vaccines, diagnosis and therapy. Virus Res 15(324):199018. https://doi.org/10.1016/j.virusres.2022.199018
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI (2013) The global distribution and burden of dengue. Nature. https://doi.org/10.1038/nature12060
Gebhard LG, Iglesias NG, Byk LA, Filomatori CV, De Maio FA, Gamarnik AV (2016) A proline rich N-terminal region of the dengue virus NS3 is crucial for infectious particle production. J Virol 90:5451–5461. https://doi.org/10.1128/JVI.00206-16
Lescar J, Soh S, Lee LT, Vasudevan SG, Kang C, Lim SP (2018) The dengue virus replication complex: from RNA replication to protein-protein interactions to evasion of innate immunity. Adv Exp Med Biol 1062:115–129. https://doi.org/10.1007/978-981-10-8727-1_9
Weiskopf D, Angelo MA, de Azeredo EL, Sidney J, Greenbaum JA, Fernando AN, Broadwater A, Kolla RV, De Silva AD, de Silva AM, Mattia KA, Doranz BJ, Grey HM, Shresta S, Peters B, Sette A (2013) Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc Natl Acad Sci U S A 110(22):E2046–E2053. https://doi.org/10.1073/pnas.1305227110
Weiskopf D, Angelo MA, Bangs DJ, Sidney J, Paul S, Peters B, de Silva AD, Lindow JC, Diehl SA, Whitehead S, Durbin A, Kirkpatrick B, Sette A (2015) The human CD8+ T cell responses induced by a live attenuated tetravalent dengue vaccine are directed against highly conserved epitopes. J Virol 89(1):120–128. https://doi.org/10.1128/JVI.02129-14
Rothman AL (2011) Immunity to dengue virus: a tale of original antigenic in and tropical cytokine storms. Nat Rev Immunol 11:532–543
Bhatt P, Sabeena SP, Varma M, Arunkumar G (2021) Current understanding of the pathogenesis of dengue virus infection. Curr Microbiol 78(1):17–32. https://doi.org/10.1007/s00284-020-02284-w
López-Monteon A, Ramos-Ligonio A, Pérez-Castillo L, Talamás-Rohana P, Rosales-Encina JL (2003) Specific antibody immune response against the parasitic portion of a glutathione-S-transferase fusion protein. FASEB J 17(6):621–627. https://doi.org/10.1096/fj.02-0471com
Alvarez-Rodríguez LM, Ramos-Ligonio A, Rosales-Encina JL, Martínez-Cázares MT, Parissi-Crivelli A, López-Monteon A (2012) Expression, purification, and evaluation of diagnostic potential and immunogenicity of a recombinant NS3 protein from all serotypes of dengue virus. J Trop Med 2012:956875. https://doi.org/10.1155/2012/956875
Hurtado-Melgoza ML, Ramos-Ligonio A, Álvarez-Rodríguez LM, Meza-Menchaca T, López-Monteon A (2016) Differential humoral and cellular immunity induced by vaccination using plasmid DNA and protein recombinant expressing the NS3 protein of dengue virus type 3. J Biomed Sci 23(1):85. https://doi.org/10.1186/s12929-016-0302-z
Ramos-Ligonio A, López-Monteon A, Talamás-Rohana P, Rosales-Encina JL (2004) Recombinant SSP4 protein from Trypanosoma cruzi amastigotes regulates nitric oxide production by macrophages. Parasite Immunol 26(10):409–418. https://doi.org/10.1111/j.0141-9838.2004.00729.x
Mathew A, Townsley E, Ennis FA (2014) Elucidating the role of T cells in protection against and pathogenesis of dengue virus infections. Future Microbiol 9(3):411–425. https://doi.org/10.2217/fmb.13.171
Fuentes-Miranda CJ, Sánchez-García FJ, Coker AR, Rojas-Espinosa O, Salinas-Tobón R, Moreno-Altamirano MM (2014) Dengue virus serotype-2 impairs proliferation of healthy donors’ T lymphocytes. Intervirology 57(2):83–92
Crepeau RL, Ford ML (2017) Challenges and opportunities in targeting the CD28/CTLA-4 pathway in transplantation and autoimmunity. Expert Opin Biol Ther 17(8):1001–1012. https://doi.org/10.1080/14712598.2017.1333595
Fathman CG, Lineberry NB (2007) Molecular mechanisms of CD4+ T-cell anergy. Nat Rev Immunol 7(8):599–609. https://doi.org/10.1038/nri2131
Valdor R, Macian F (2013) Induction and stability of the anergic phenotype in T cells. Semin Immunol 25(4):313–320. https://doi.org/10.1016/j.smim.2013.10.010
Srikanth S, Woo JS, Sun Z, Gwack Y (2017) Immunological disorders: regulation of Ca2+ Signaling in T Lymphocytes. Adv Exp Med Biol 993:397–424. https://doi.org/10.1007/978-3-319-57732-6_21
Greenwald RJ, Freeman GJ, Sharpe AH (2005) The B7 family revisited. Annu Rev Immunol 23:515–548. https://doi.org/10.1146/annurev.immunol.23.021704.115611
Attanasio J, Wherry EJ (2016) Costimulatory and coinhibitory receptor pathways in infectious disease. Immunity 44(5):1052–1068. https://doi.org/10.1016/j.immuni.2016.04.022
Cho H, Kang H, Lee HH, Kim CW (2017) Programmed cell death 1 (PD-1) and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) in viral hepatitis. Int J Mol Sci 18(7):1517. https://doi.org/10.3390/ijms18071517
Fahey LM, Brooks DG (2010) Opposing positive and negative regulation of T cell activity during viral persistence. Curr Opin Immunol 22(3):348–354. https://doi.org/10.1016/j.coi.2010.03.004
Létourneau S, Krieg C, Pantaleo G, Boyman O (2009) IL-2- and CD25-dependent immunoregulatory mechanisms in the homeostasis of T-cell subsets. J Allergy Clin Immunol 123(4):758–762. https://doi.org/10.1016/j.jaci.2009.02.011
Duarte dos Santos CN, Frenkiel MP, Courageot MP (2000) Determinants in the envelope E protein and viral RNA helicase NS3 that influence the induction of apoptosis in response to infection with dengue type 1 virus. Virology 274(2):292–308. https://doi.org/10.1006/viro.2000.0457
de Martins S, Silveira GF, Alves LR, Dos Santos CN, Bordignon J (2012) Dendritic cell apoptosis and the pathogenesis of dengue. Viruses 4(11):2736–53. https://doi.org/10.3390/v4112736
Jaramillo FJ, Gómez LM, Anaya JM (2006) Regulatory T cells. Infection and Autoimmunity Therapeutic implications Infectio 10(3):178–185
Castro-Mussot ME, Machain-Williams C, Loroño-Pino MA, Salazar MI (2013) Respuesta inmune e inmunopatogénesis en las infecciones con el virus del dengue [Immune responses and immunopathogenesis in infections with dengue virus]. Gac Med Mex 149(5):531–40
Katzelnick LC, Coloma J, Harris E (2017) Dengue: knowledge gaps, unmet needs, and research priorities. Lancet Infect Dis 17(3):e88–e100. https://doi.org/10.1016/S1473-3099(16)30473-X
Malavige GN, Jeewandara C, Alles KM, Salimi M, Gomes L, Kamaladasa A, Jayaratne SD, Ogg GS (2013) Suppression of virus specific immune responses by IL-10 in acute dengue infection. PLoS Negl Trop Dis 7(9):e2409. https://doi.org/10.1371/journal.pntd.0002409
Tai X, Van Laethem F, Pobezinsky L, Guinter T, Sharrow SO, Adams A, Granger L, Kruhlak M, Lindsten T, Thompson CB, Feigenbaum L, Singer A (2012) Basis of CTLA-4 function in regulatory and conventional CD4(+) T cells. Blood 119(22):5155–5163. https://doi.org/10.1182/blood-2011-11-388918
Acknowledgements
VARC received a doctoral scholarship (No. 289379), KGA and MCG (No. 288057/894260) received a scholarship for master's studies from CONAHCYT, Mexico.
Author information
Authors and Affiliations
Contributions
VARC Formal analysis; Investigation; Methodology Writing—original draft. ARL Validation Writing—original draft; Writing—review & editing KGA Formal analysis; Investigation; Methodology MCG Formal analysis; Investigation; Methodology MSLC Formal analysis; Investigation; Methodology ALM Conceptualization, Project administration, Validation, Writing—original draft; Writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Edited by Erik Karlsson.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11262_2024_2095_MOESM1_ESM.tiff
Supplementary file1 (TIFF 2377 KB) Fig. 1 Expression of co-stimulatory molecules in cells from animals immunized with GST protein.
11262_2024_2095_MOESM2_ESM.tiff
Supplementary file2 (TIFF 5172 KB) Fig 2. Expression of co-stimulatory molecules. A) Splenocytes from normal animals, B) Splenocytes from GST-NS3pro1-immunised animals, C) Splenocytes from GST-NS3pro2-immunised animals, D) Splenocytes from GST-NS3pro3-immunised animals, and E) B) Splenocytes from GST-NS3pro4-immunised animals. Splenocytes were stimulated with Con A, concanavalin A; BSA, bovine serine albumin; H-Ag, homologous antigen; GST-NS3pro1-4 (protein mixture) and non-stimulated (NS).
11262_2024_2095_MOESM3_ESM.tiff
Supplementary file3 (TIFF 2721 KB) Fig 3. CD25 expression in non-immunised animals and animals immunised with the recombinant proteins
11262_2024_2095_MOESM4_ESM.tiff
Supplementary file4 (TIFF 4254 KB) Fig 4. IL-2 expression in non-immunised animals and animals immunised with the recombinant proteins
11262_2024_2095_MOESM5_ESM.tiff
Supplementary file5 (TIFF 5924 KB) Fig 5. Determination of apoptosis by Annexin V binding. Splenocytes from non-immunized, pool-immunized and immunized mice with each of the GST-NS3pro1,2,3 and 4 proteins were stimulated under the following conditions: NS (black color graph), dexamethasone 20 μg/mL (red color graph), with the protein pool 10 μg/mL (blue color graph) and with homologous antigens as appropriate 10 μg/mL (purple color graph), PS translocation was determined by flow cytometry by Annexin V binding.
11262_2024_2095_MOESM6_ESM.tiff
Supplementary file6 (TIFF 5924 KB) Fig 6. Staining for caspases 3 and 7. Splenocytes from unimmunized, pool-immunized and Immunostained mice immunized with each of the GST-NS3pro1, 2,3 and 4 proteins were stimulated under the following conditions: S/E (black line), dexamethasone 20 μg/mL (red line), with the protein pool 10 μg/mL (blue line) and with homologous antigens as appropriate 10 μg/mL (purple line), caspases 3 and 7 were determined by flow cytometry staining.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Romero-Cruz, V.A., Ramos-Ligonio, A., García-Alejandro, K. et al. Immunization of recombinant NS3 protein (protease region) of dengue virus induces high levels of CTLA-4 and apoptosis in splenocytes of BALB/c mice. Virus Genes 60, 475–487 (2024). https://doi.org/10.1007/s11262-024-02095-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-024-02095-2