Abstract
Newcastle disease virus (NDV) belongs to the Avulavirus genus and Paramyxoviridae family virus that causes acute, highly infectious Newcastle disease in poultry. The two proteins of haemagglutinin neuraminidase (HN) and fusion (F) are key virulence factors with an important role in its immunogenicity. Genotype VII NDV is still among the most serious viral hazards to the poultry industry worldwide. In this study, a commercial vector vaccine (HVT-NDV) was evaluated compared to the conventional vaccination strategy against Iranian genotype VII. This experiment showed that the group receiving the conventional vaccination strategy had higher antibodies, fewer clinical signs, and lower viral loads in tracheal swabs and feces. Also, two vaccine groups showed significant difference, which could have resulted from two extra vaccine doses in the conventional group. However, except for antibody levels in commercial chickens in the Iran new-generation vaccine, this difference was minor. Further, both groups showed 100% protection in the challenge study. Despite the phylogenetic gap between the NDV-F gene placed in the vector vaccine and the challenge virus (genotypes I and VII, respectively), the rHVT-NDV vaccine offered strong clinical protection and decreased challenge virus shedding considerably.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Newcastle disease (ND), one of the most destructive and prevalent viral diseases of poultry, is caused by infections with viruses from the avulavirus group and the species Avian Avulavirus1, often recognized as Newcastle disease virus (NDV) and abbreviated as Avian Paramyxovirus 1 (APMV 1) [1]. NDV’s enveloped virion has a 15 kb single-stranded RNA and replicates only in the cytoplasm. The NDV genome contains six genes encoding six proteins: RNA polymerase, which depends on (L) RNA, hemagglutinin-neuraminidase protein (HN), fusion protein (F), phosphoprotein (P), matrix protein (M), and nucleoprotein (N) [2]. The F and HN proteins are surface glycoproteins and protective antigens of NDV; the F protein controls the viral fusion activity while the HN protein is in charge of virus attachment. NDV strains have been categorized into five pathotypes, including viscerotropic velogenic virus, neurotropic velogenic virus, mesogenic virus, lentogenic virus, and asymptomatic virus enteric, based on pathogenicity to decrease virulence. Pathotype may be defined by in vivo pathogenicity assays, such as intracerebral pathogenicity index (ICPI), mean time to death, and intravenous pathogenicity index.
On the other hand, virulence may be evaluated by analyzing the amino acid sequence of the F0 protein cleavage site (positions 112–117) [3]. Genetically, NDVs are divided into class I and class II. The former appear in wild birds, generally have low virulence, and are seldom spotted in poultry species. According to the nucleotide sequence analysis of the F gene in class II viruses, they are grouped into 18 genotypes (I–XVIII), with V, VII, and VIII being the most commonly circulating genotypes worldwide [4]. These have been combined into three subgenotypes of the genotype VII.
The viruses in charge of the fourth NDV panicoid were combined in a single genotype based on nucleotide distance (VII.1.1), joining the former subgenotypes VIIb, VIId, VIIe, VIIj, and VIIl. An exception is the former subgenotype VIIf categorized as a separate subgenotype, i.e., VII.1.2. The groups of viruses involved in the fifth NDV panicoid (VIIh and VIIi) impact the Middle East, Indonesia, Asia, Europe, and Africa [5]. The disease is controlled using appropriate biosecurity measures, notably prophylactic vaccination in flocks, as well as the collection and isolation of affected and susceptible birds. The disease is controlled by vaccination in most countries with a developed poultry industry where ND has become endemic. Currently, most ND vaccination programs use inactivated or attenuated live lentogenic NDV vaccines, with their advantages and disadvantages. Failure to inhibit infection and shedding, the need for compound vaccine administrations to reach satisfactory immunity, interference with maternally produced antibodies (MDA), and the risk of negative reactions (live vaccines) are among the major shortcomings of conventional vaccines [6, 7].
New-generation vaccines, such as vector vaccines based on turkey herpesvirus (HVT) as the basic scaffold for expressing NDV-encoding immunogenic proteins, have been developed to overcome such drawbacks. A commercial vector vaccine termed r HVT-ND, which expresses the F gene, confer clinical protection against virulent Newcastle disease in specific pathogen-free (SPF) chickens, layers, broilers, and turkeys [7]. Vectormune® ND (rHVT-ND) (Ceva Santé Animale) is approved in the EU and the USA; it is currently used in Southeast Asian countries, including Thailand, Malaysia, Indonesia, and South Korea, as well as South American countries, such as Mexico and Brazil. This study aims to investigate the proposed program with the Vectormune® ND, the routine program used in Iran (a combination of killed and live vaccines) to treat ND.
Material and methods
Experimental design
Commercial Ross 308 chickens were grouped into four, with 20 birds in each. During all experiments, each group was kept in separate negative pressure isolators, and offered feed and water ad libitum. As presented in Table 1, the groups were vaccinated and challenged.
Challenge virus
In 2012, ND virus challenge was isolated from a vaccinated broiler farm in Iran and categorized as virulent NDV genotype VII (vNDV VII) (based on the F cleavage site sequence analysis and an intracerebral pathogenicity index of 1.8) and registered in GenBank with NO. JX131360. Through the eye drops/intranasal (ED) route, the animals were challenged with 0.2 mL (105 50% embryonic lethal dose) of NDV strain SG10 [8].
ELISA
Before the challenge, sera were collected from the animals to define the ELISA titers of NDV vaccination utilizing ID Screen® Newcastle Disease Indirect (IDVet, France). After challenge, each group was tested with its relevant kit (ID Vet Screen for VTM-ND kit for the vector group and ID Screen® Newcastle Disease Indirect Conventional for the conventional Newcastle group) according the manufacturer’s guideline.
Mortality rate and clinical signs
Mortality rate and clinical signs (principally respiratory symptoms, e.g., conjunctivitis, sneezing, swollen head, rales, and nasal discharge; enteric symptoms, e.g., greenish diarrhea; nervous symptoms, e.g., torticollis and recumbency) (No clinical signs: 0/mild: 1/moderate: 2/severe: 3) were observed daily for 14 days post-challenge (dpc). Further, the dead animals were examined for post-mortem (PM) lesions, including congested pectoral muscles, proventricular hemorrhage, congestion and exudate in the tracheal mucosa, intestinal ulceration, and ileocecal hemorrhage.
RNA extraction, cDNA synthesis, and quantitative real-time PCR
Tracheal and cloacal swabs were collected for virus isolation 5 days after the challenge. Viral RNA was isolated from the tissues utilizing the Cinna PureRNA extraction kit (Sinaclone, Iran), according to the manufacturer’s instructions. The RevertAid cDNA first-strand synthesis kit (Thermo Scientific, Canada) was utilized to synthesize cDNA. This study used real-time PCR to detect IBV based on 5′-UTR. Real-time PCR amplification was carried out utilizing the amplification kit (Bioneer, South Korea) with forward primer(5′-CGCTGTTGCAACCCCAAG-3′) and reverse primer (5′-CCGCAAGATCCAAGGGTCT-3′), as well as a double-labeled Taqman probe (FAM-AAGCGTTTCTGTCTCCTTCCTCCA-BHQ1) [9]. PCR cycle parameters include 95 °C for 2 min, 40 cycles for 15 s at 95 °C, and 1 min at 60 °C. The reaction was carried out in a QIAGEN Rotor-Gene Q (Corbett Rotor-Gene 6000, USA).
Statistical analysis
Data were collected for body weight loss, ELISA assay, and rRT-PCR. They were expressed as means ± standard deviation (SD). Means were analyzed by one-way ANOVA and Kruskal–Wallis test utilizing GraphPad Prism version 9 (GraphPad Software, USA). Differences were statistically significant at P: 0.05.
Results
NDV antibody detection
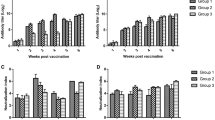
In order to assess the vaccination program’s impact on the immune system response, antibody titers to NDV were detected by the ELISA method. As expected, no antibodies were detected in the non-vaccinated and negative control groups (Fig. 1). According to the results, there were significant differences (P < 0.05) between Vector (GMT: 3031 ± 1772) and conventional (GMT: 5026 ± 2336) (Fig. 1).
ELISA titer. Newcastle Disease titer of the commercial broiler chickens, non-vaccinated (Negative + Challenged), vaccinated with Conventional (Live and Inactivated ND Vaccine) and Vector-based (Vectormmune ND and Live vaccine) and challenged with GVII NDV isolate, at the day of 42 Days, before Challenge
Survival rate
No deaths were observed in both the vector and conventional groups, indicating that the protection was 100%. In contrast, in the control-non-vaccinated-challenged group, an 80% death rate was observed (Fig. 2A).
A Survival Rate. The percent of survival rate of the commercial broiler chickens, non-vaccinated (Negative + Challenged), vaccinated with Conventional (Live and Inactivated ND Vaccine) and Vector-based (Vectormmune ND and Live vaccine) and challenged with GVII NDV isolate, after 14 days post-challenge. B Clinical Score. Average of the clinical score of the commercial broiler chickens, non-vaccinated (Negative + Challenged), vaccinated with Conventional (Live and Inactivated ND Vaccine) and Vector-based (Vectormmune ND and Live vaccine) and challenged with GVII NDV isolate, after 14 days post-challenge (No clinical sign: 0/Mild:1/Moderate:2/Sever:3)
Clinical evaluation
Clinical observations were recorded and scored 5 days after the challenge; the clinical scores results are shown in Fig. 2B. Significant differences (P < 0.05) were observed between the non-vaccinated-challenged group (score 2.6) compared to the conventional (score 0.3) and the vector (score 0. 5) groups. No significant difference was observed between the conventional (score 0.3) and vector (score 0. 5) groups (Fig. 2B).
Real-time PCR of tracheal swabs
The CT value for the conventional group (34.14 ± 12.25) was higher than that of the Vector group (27.5 ± 6.71), indicating a lower viral load in this group [not significant (P > 0.05)]. Meanwhile, this value in the non-vaccinated-challenged and negative control groups was (15.36 ± 3.12) and (48.33 ± 1.98), respectively. There was a significant difference between CT of the non-vaccinated-challenged and vaccinated groups (P > 0.05) (Fig. 3A).
A Real-time PCR of the tracheal swab. Viral load detection of Newcastle Disease(ND) virus in the tracheal swab of the commercial broiler chickens, non-vaccinated (Negative + Challenged), vaccinated with Conventional (Live and Inactivated ND Vaccine) and Vector-based (Vectormmune ND and Live vaccine) and challenged with GVII NDV isolate, after 5 days post-challenge. B Real-time PCR of the fecal swab. Viral load detection of Newcastle Disease(ND) virus in the fecal swab of the commercial broiler chickens, non-vaccinated (Negative + Challenged), vaccinated with Conventional (Live and Inactivated ND Vaccine) and Vector-based (Vectormmune ND and Live vaccine) and challenged with GVII NDV isolate, after 5 days post-challenge
Real-time PCR of fecal swabs
The CT value for the conventional group (43.21 ± 11.15) was higher than that of the vector group (35.93 ± 11.06), indicating a lower viral load in this group [not significant (P > 0.05)]. Meanwhile, the CT values in the non-vaccinated-challenged and negative control groups were (21.00 ± 5.12) and (49.21 ± 1.31), respectively. There was a significant difference between CT of the non-vaccinated-challenged and vaccinated groups (P > 0.05) (Fig. 3B).
Discussion
The poultry industry is changing rapidly, thus increasing the challenges significantly over the years. For producers, efficiency has become more of a survival strategy than a differentiator. In today’s difficult environments, it is critical to produce more with fewer resources. In addition, there are high disease pressures, high-stocking densities on farms in very densely populated areas, poorly skilled labor, pressure to reduce antibiotic use, and other challenges in the industry. Recently, Iranian chicken farmers have failed to control virulent ND outbreaks (with different genotypes VII d, VIIi, VII L, VII g, VIIj) despite good biosecurity and vaccination practices [3, 8, 10,11,12].
High mortality rates have been reported in broiler chickens or breeders and layers in the country. ND outbreaks in vaccinated Iranian chicken flocks call into question the efficiency of these vaccines [3, 8].
Vectormune® ND is a recombinant HVT vector vaccine using turkey herpesvirus (HVT) as the vector and cloning the fusion gene (F) of NDV. The “F” protein (for “fusion”) is the epitope on the NDV surface, allowing it to attach to and invade target cells. Simultaneously, it is a major element in the virus virulence and a vital protective antigen. It is much more difficult for NDV to infect cells and cause damage when immunity to the “F” protein is established. This explains the high efficacy of Vectormune® ND. Vectormune® ND is the best-in-class solution to overcome the problems, including interference with maternally derived antibodies (MDA), vaccine delivery challenges, side effects, and viral circulation (CEVA Cante Animal), for which other ND vaccines fall short.
The HVT has several advantages over other viral vectors for transmitting foreign antigens to the chicken. First, it is routinely applied as a vaccine for 1-day-old chickens, unlike the fowlpox virus. Vaccine production, storage, and administration are well-established procedures in the poultry industry. Second, the lack of HVT pathogenicity in chickens is well-documented; therefore, it is considered an extremely safe virus to be used as a vector. Third, for several weeks after vaccination, HVT forms persistent viremia in chickens; thus, foreign antigens can be expected to move in the immune system of vaccinated chickens over a prolonged period. Fourth, HVT does not spread horizontally, even in chicken populations with high-stocking densities [13, 14].
This is the first study on commercial chickens with new-generation vaccines in Iran. This study compared the vaccine Vectormune® ND with the program commonly used in Iranian broiler flocks, a combination of live lentogenic and inactivated vaccines. Its results showed that the group receiving the traditional vaccination had higher antibodies, fewer clinical signs, and lower viral loads in tracheal swabs and feces. However, except for the antibody level in the new-generation vaccine in Iran on commercial chickens, this difference was insignificant. Moreover, both groups showed 100% protection in the challenge trial. Also, there was a significant difference between two vaccine groups, with the conventional having higher titers, which can result from receiving two extra doses of ND (day 17 via spray and day 25 via drinking water) in conventional group.
Several independent and commercial studies of new-generation vaccines have been conducted worldwide. The study by Morgans et al. was one of the first in this field, in which chickens receiving a single intra-abdominal inoculation at 1 day of age with recombinant HVT expressing the NDV fusion protein showed an immunological response, while at 28 days of age with the neurotropic velogenic NDV strain Texas GB, they were protected against lethal intramuscular challenge (> 90%). Against the same challenge, recombinant HVT expressing NDV hemagglutinin-neuraminidase showed limited protection (47%). When challenged ocular, chickens vaccinated with recombinant HVT vaccines provided less protection against NDV replication in the trachea [14]. In another study, the research team provided the details for adding the foreign gene to the appropriate site of the HVT genome.
A general recombination vector for foreign gene integration in HVT was created based on an insertion site mapped in one of the open reading frames of the unique short region. A robust promoter component derived from the lung-terminal repeat sequence of Rous sarcoma virus-induced recombinant virus-driven expression of individual Newcastle disease virus antigen [15]. It should be noted that competition between different vaccine companies in the field of vaccines and vectors is based on technical and clonal strategies and the appropriate site for introducing foreign genes into the vector to enhance vaccine efficacy. Morgan et al. compared the vector and normal vaccines in SPF and commercial chickens. The HVT/F and NDV strain Hitchner B1 vaccines showed, respectively, 73% and 80% protection against NDV in broilers, while both vaccines showed 100% protection in SPF layers [16].
One of the advantages of the vector vaccine is its use during the embryonic period. According to Reddy et al., rHVT is safe for both ED18 and post-hatch vaccination (ND and MD); this vaccine may induce longer-lasting immunity compared to conventional live NDV vaccines since it is persistent [17]. The start of protective immunity in chicks differs after vaccination with a recombinant herpesvirus. When the vaccinated birds are challenged at 4, 7, 10, and 14 DPV, the recombinant vaccine, respectively, offers 0%, 35–75%, 85%, and 94–100% protection [18]. Palya et al. showed that the rHVT-NDV vaccine had good clinical protection and considerably decreased challenge virus shedding despite the phylogenetic distance between the NDV-F gene inserted into the vector vaccine and the challenge virus (genotype I or V) [19]. In commercial laying hens, the emergence and persistence of immunity by a recombinant ND vaccine employing HVT viruses as a vector (rHVT-ND) have been studied up to 72 weeks after a single administration or as part of two distinct vaccination regimens, including conventional killed ND and live vaccines.
A single vaccination with the rHVT-ND vaccine at 1 day of age offered complete or nearly complete (95–100%) clinical protection against NDV challenges from 4 weeks of age until the last challenge was administered at 72 weeks of age [20]. Ferreira et al. indicated that the combination of recombinant rHVT-ND-IBD with live vaccine at 1 day of age was better because there were no clinical signs, antibody levels were higher before and after provocation, and viral shedding was reduced at any time after provocation at 3 or 4 weeks of age with California virus 2018 [21]. This vaccine also has a good protective response in turkeys. ELISA detected the formation of a humoral immune response to vaccination as early as 4 weeks of age. The challenge strain was a novel NDV genotype IV from Morocco.
Unvaccinated turkeys developed ND-specific symptoms and stunting with no subsequent mortality, whereas vaccinated ones showed protection as early as 3 weeks of age, as demonstrated by the absence of clinical symptoms, improved weight gain, and decreased virus shedding [22].
Even in countries or regions with very low ND field pressure, the spread of mild live vaccines ND within or between herds can be detrimental to producer profitability. With this in mind, the use of Vectormune® ND is also greatly beneficial to producers in low-pressure areas. By replacing the live attenuated vaccines with this vector vaccine, the spread of lentogenic NDV strains among chickens is reduced, resulting in improved performance. In areas of high infection pressure, where ND outbreaks result in unacceptable losses, Vectormune® ND is superior to conventional vaccines since it evades MDA NDV, is used in hatcheries (i.e., in a well-controlled environment with well-trained personnel), induces long-lasting immunity, and significantly reduces shedding [23].
This vector vaccine has all these benefits with no post-vaccination reactions. However, even with a safe and effective vaccine such as Vectormune® ND, it is more necessary than ever to look at the situation holistically at ND and not just focus on the microorganism and/or the efficacy of the vaccine. It is also necessary to consider biosecurity limitations, the urgent need for farm workers’ training, legislation, and, if possible, disease eradication. Without such a comprehensive approach, ND will continue to cause enormous losses to producers for a long time [23,24,25,26]. In its current form, the HVT/F vaccine could be useful in controlling velogenic NDV, where systemic immunity is critical but difficult to achieve with conventional vaccines. Moreover, a recombinant HVT/F vaccine could be beneficial in countries where live NDV vaccination is not recommended, but ND may not be completely eradicated.
Data availability
Not applicable.
References
Absalón A, Cortés-Espinosa DV, Lucio E, Miller P, Afonso C (2019) Epidemiology, control, and prevention of Newcastle disease in endemic regions: Latin America. Trop Anim Health Prod 51:1033–1048
Suarez DL, Miller PJ, Koch G, Mundt E, Rautenschlein S (2020) Newcastle disease, other avian paramyxoviruses, and avian metapneumovirus infections. Dis Poult 109–166
Ghalyanchilangeroudi A, Hosseini H, Jabbarifakhr M, Fallah Mehrabadi MH, Najafi H et al (2018) Emergence of a virulent genotype VIIi of Newcastle disease virus in Iran. Avian Pathol 47:509–519
Sultan HA, Elfeil WK, Nour AA, Tantawy L, Kamel EG et al (2021) Efficacy of the Newcastle disease virus genotype VII. 1.1-matched vaccines in commercial broilers. Vaccines 10:29
Dimitrov KM, Abolnik C, Afonso CL, Albina E, Bahl J et al (2019) Updated unified phylogenetic classification system and revised nomenclature for Newcastle disease virus. Infect Genet Evol 74:103917
Mayers J, Mansfield KL, Brown IH (2017) The role of vaccination in risk mitigation and control of Newcastle disease in poultry. Vaccine 35:5974–5980
Śmietanka K, Tyborowska J, Olszewska-Tomczyk M, Domańska-Blicharz K, Minta Z et al (2019) A recombinant Turkey herpesvirus expressing F and HN genes of Avian Avulavirus-1 (AAvV-1) genotype VI confers cross-protection against challenge with virulent AAvV-1 genotypes IV and VII in chickens. Viruses 11:784
Hosseini H, Langeroudi AG, Torabi R (2014) Molecular characterization and phylogenetic study of Newcastle disease viruses isolated in Iran, 2010–2012. Avian Dis 58:373–376
Rasoli M, Yeap SK, Tan SW, Moeini H, Ideris A et al (2014) Alteration in lymphocyte responses, cytokine and chemokine profiles in chickens infected with genotype VII and VIII velogenic Newcastle disease virus. Comp Immunol Microbiol Infect Dis 37:11–21
Sabouri F, Vasfi Marandi M, Bashashati M (2018) Characterization of a novel VIIl sub-genotype of Newcastle disease virus circulating in Iran. Avian Pathol 47:90–99
Jabbarifakhar M, Mousavi M, Ziafati Z, Rezaee H, Hosseini H et al (2018) (2018) Emergence of genotype VIIg of velogenic Newcastle disease virus in Iran. First Rep 12:40–46
Esmaelizad M, Mayahi V, Pashaei M, Goudarzi H (2017) Identification of novel Newcastle disease virus sub-genotype VII-(j) based on the fusion protein. Adv Virol 162:971–978
Maas H, Van Vloten J, Vreede-Groenewegen A, Orthel F (1978) Absence of Marek’s disease virus antigen in the feather follicle epithelium of vaccinated chicks demonstrated by immunofluorescence. Avian Pathol 7:79–86
Morgan RW, Gelb Jr J, Schreurs CS, Lütticken D, Rosenberger JK et al (1992) Protection of chickens from Newcastle and Marek’s diseases with a recombinant herpesvirus of turkeys vaccine expressing the Newcastle disease virus fusion protein. Avian diseases 858–870
Sondermeijer PJ, Claessens JA, Jenniskens PE, Mockett AA, Thijssen RA et al (1993) Avian herpesvirus as a live viral vector for the expression of heterologous antigens. Vaccine 11:349–358
Morgan RW, Gelb Jr J, Pope CR, Sondermeijer PJ (1993) Efficacy in chickens of a herpesvirus of turkeys recombinant vaccine containing the fusion gene of Newcastle disease virus: onset of protection and effect of maternal antibodies. Avian Dis 1032–1040
Reddy S, Sharma J, Ahmad J, Reddy D, McMillen J et al (1996) Protective efficacy of a recombinant herpesvirus of turkeys as an in ovo vaccine against Newcastle and Marek’s diseases in specific-pathogen-free chickens. Vaccine 14:469–477
Heckert RA, Riva J, Cook S, McMillen J, Schwartz RD (1996) Onset of protective immunity in chicks after vaccination with a recombinant herpesvirus of turkeys vaccine expressing Newcastle disease virus fusion and hemagglutinin-neuraminidase antigens. Avian Dis 770–777
Palya V, Kiss I, Tatar-Kis T, Mato T, Felföldi B et al (2012) Advancement in vaccination against Newcastle disease: recombinant HVT NDV provides high clinical protection and reduces challenge virus shedding with the absence of vaccine reactions. Avian Dis 56:282–287
Palya V, Tatár-Kis T, Mató T, Felföldi B, Kovács E et al (2014) Onset and long-term duration of immunity provided by a single vaccination with a turkey herpesvirus vector ND vaccine in commercial layers. Vet Immunol Immunopathol 158:105–115
Ferreira HL, Reilley AM, Goldenberg D, Ortiz IR, Gallardo RA et al (2020) Protection conferred by commercial NDV live attenuated and double recombinant HVT vaccines against virulent California 2018 Newcastle disease virus (NDV) in chickens. Vaccine 38:5507–5515
El Khantour A, Darkaoui S, Tatár-Kis T, Mató T, Essalah-Bennani A et al (2017) Immunity elicited by a turkey herpesvirus-vectored Newcastle disease vaccine in turkey against challenge with a recent genotype IV Newcastle disease virus field strain. Avian Dis 61:378–386
Sandikli M-S (2021) Why vector (rhvt-nd) vaccine for newcastle disease?
Kgotlele T, Modise B, Nyange JF, Thanda C, Cattoli G, Dundon WG (2020) First molecular characterization of avian paramyxovirus-1 (Newcastle disease virus) in Botswana. Virus Genes 56(5):646–650. https://doi.org/10.1007/s11262-020-01770-4
Ferreira HL, Taylor TL, Absalon AE, Dimitrov KM, Cortés-Espinosa DV, Butt SL, Marín-Cruz JL, Goraichuk IV, Volkening JD, Suarez DL, Afonso CL (2019) Presence of Newcastle disease viruses of sub-genotypes Vc and VIn in backyard chickens and in apparently healthy wild birds from Mexico in 2017. Virus Genes 55(4):479–489. https://doi.org/10.1007/s11262-019-01663-1
Chellappa MM, Dey S, Gaikwad S, Pathak DC, Vakharia VN (2017) Rescue of a recombinant Newcastle disease virus strain R2B expressing green fluorescent protein. Virus Genes 53(3):410–417. https://doi.org/10.1007/s11262-017-1433-3
Acknowledgements
The authors gratefully acknowledge Dr. Habibi and Dr. Hamed Abdolahi (Iranian veterinary organization) for their extensive technical support. The main grant source for doing the project was supplied by Ceva Santé Animale, Iran, Savapars (exclusive distributor in Iran).
Funding
The main Grant source for the project was supplied by Ceva Santé Animale, Iran, Savapars (exclusive distributor in Iran).
Author information
Authors and Affiliations
Contributions
MKJ: wrote the main manuscript text; MHBF: analyzed and investigated; AH: analyzed and investigated; HH: edited; SC: edited.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Edited by Takeshi Noda.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rajab, M.K., Fard, M.H.B., Ghalyanchilangeroudi, A. et al. Comparison of HVT-ND recombinant and convection-based Newcastle disease vaccination programs in the protection against the genotype VII NDV challenges: an experimental study. Virus Genes 60, 126–133 (2024). https://doi.org/10.1007/s11262-023-02038-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-023-02038-3