Abstract
Hepatitis C virus (HCV) infection is a major global health issue. Although the search for HCV treatments has resulted in great achievements, the current treatment methods have limitations, and new methods and drugs for hepatitis C treatment are still required. The aim of the present study was to investigate the effects of artesunate (ART) on HCV replication and compared these effects with those of ribavirin (RBV) and interferon-2b (IFN). The study was performed in HCV-infection cell models (JFH1-infected Huh7.5.1 and OR6 cell lines). Our results showed that the antimalarial drug ART inhibited HCV replicon replication in a dose- and time-dependent manner at a concentration that had no effect on the proliferation of exponentially growing host cells, and the inhibitory effect on HCV replication was stronger than RBV but weaker than IFN, as determined by qPCR, luciferase assays, and Western blot analysis. Furthermore, the combination of ART and IFN resulted in a greater inhibition of HCV replication. These findings demonstrated that ART had an inhibitive effect on HCV replication and may be a novel supplemental co-therapy with IFN and RBV for HCV and as an alternative strategy to combat resistance mechanisms that have emerged in the presence of DAA agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatitis C virus (HCV) infection is a major global health problem. According to the WHO report, it is estimated more than 185 million HCV-specific antibody-positive people, of whom approximately 130–170 million were believed to be chronically HCV infected in 2005 [1]. HCV is a major cause of chronic liver diseases ranging from liver steatosis, cirrhosis to hepatocellular carcinoma. Currently, no effective vaccine is available for HCV infection. In the early 2000s, the standard of care (SOC) for chronic hepatitis C consisted of a combination of pegylated interferon with ribavirin (RBV) [2]; however, this therapy was not ideal and was associated with severe side effects and a low sustained viral response until direct antiviral agents (DAAs) were appeared and approved by the United States Food and Drug Administration in 2011. The addition of HCV protease inhibitors to the SOC or interferon-free DAAs therapy launched a new therapeutic era for HCV chronic hepatitis and significantly improved the efficacy of treatment [3]. The majority of HCV-infected patients can be cured by these anti-viral treatments. DAAs are not available in many countries and too expensive to afford for many HCV-infected people. It is also reported that pre-existing resistance-associated variants (RAVs) or selected mutations in the targeted proteins affect the DAAs efficiency [4, 5]. Thus, new therapeutic strategies and new drugs with safe, inexpensive, and trouble-free storage are needed to be explored alone or in combination with existing treatments to cure HCV infection.
Artemisinin is a natural product derived from the Chinese medicinal herb Artemisia annua and is a sesquiterpene lactone. Artemisinin derivatives include artesunate (ART), artemether (ARM), and dihydroartemisinin (DHA) [6]. ART is the most promising candidate compound to reduce the worldwide malarial burden. The attractiveness of this compound is its water solubility, safety, tolerability, and low cost [7]. In previous studies, it was revealed that Artemisinin and its derivatives have anti-malarial, anti-bacterial, anti-angiotensin, and anti-cancer properties [8–10]. Artemisinin-mediated oxidative stress has been proposed as a mechanism of action on the basis of anti-malarial activity [11]. Recently, Artemisinin was suggested to have an antiviral effect [12]. Many studies have verified the antiviral effects of Artemisinin and its derivatives in addition to its anti-malarial effects. Artemisinin dimers are potent as non-cytotoxic inhibitors of CMV replication [13, 14]. Artemisinin and ART induced strong inhibition of HBV production at concentrations that did not affect host cell viability. Furthermore, ART had synergic anti-HBV effects with lamivudine [15]. Here, the goal of the present study was to explore in vitro the potential efficacy of ART on HCV as an alternative or complementary therapy.
Materials and methods
Reagents
ART was purchased from Guilin Pharma Corp. (Guangxi, China). IFN was purchased from Yuance Pharmacy (Beijing China). RBV was purchased from Schering-Plough Corp. (Shanghai, China). Dulbecco’s modified minimal essential medium (DMEM), fetal bovine serum (FBS), phosphate-buffered saline (PBS), penicillin–streptomycin and Geneticin (G418) were purchased from Gibco (Grand Island, NY, USA).
Cells and expression plasmids
Huh7.5.1 cells and JFH1 are the gifts from Dr. Chung (Harvard University). OR6 is a gift from Dr. Zhao (Chinese Academy of Sciences). Huh7.5.1 is a human hepatoma cell line, and JFH1 harbors the full-length HCV genome (genotype 2a) [16, 17]. The process of establishing the JFH1-infected Huh7.5.1 cell line has been described previously [18], briefly, Huh7.5.1 cells were cultured in DMEM supplemented with 10 % FBS, 100 U/ml penicillin, 100 μg/ml streptomycin at 37 °C in a humidified 5 % CO2. JHF1 (MOI = 0.4) were added to Huh7.5.1 cells. After 2 weeks post JHF1 infection, JHF1 replicons stably in Huh7.5.1 cells and HCV RNA is detected in the supernatant. The OR6 cells contain the full-length genotype of 1b HCV RNA coupled with Renilla Luciferase gene, with HCV replicates in OR6 cells Renilla Luciferase also expressed [19], and were cultured in DMEM supplemented with 10 % FBS and 400 µg/ml of G418, 100 IU/ml penicillin, 100 μg/ml streptomycin at 37 °C in a humidified 5 % CO2 condition.
Cell proliferation assay
Cell proliferation was analyzed using the MTT assay. The JFH1-infected Huh7.5.1 cells were seeded at a density of 5 × 104 cells per well, and OR6 cells were seeded at a density of 1 × 104 cells per well into 96-well plates containing DMEM and were left to adhere overnight. OR6 cells were supplemented with the appropriate concentrations of G418. Serial dilutions of the test drugs in complete DMEM without G418 were added 24 h after seeding. Cells were allowed to proliferate for 48 h at 37 °C and 5 % CO2. A total of 10 ml of 5 mg/ml MTT was added, and the cells were incubated in the dark at 37 °C for 2 h. The absorbance was then determined at a wavelength of 492 nm (Bio-Rad Laboratories, Inc, Hercules, CA, USA). The 50 % cytotoxic concentration (CC50) was defined as the concentration that induced a 50 % reduction in host cell viability.
Anti-HCV assay
For anti-HCV experiment in OR6 cells, OR6 cells were seeded at a density of 2 × 105 cells per well in 6-well cell culture plates in DMEM supplemented with 400 μg/ml G418. Following incubation for 24 h at 37 °C and 5 % CO2, the cell culture medium was removed, and serial dilutions of the test drugs in complete DMEM without G418 were added to a total volume of 2 ml. The cell culture medium was changed daily. After 3 days of incubation at 37 °C and 5 % CO2, cell culture fluid was removed, and monolayers were washed once with phosphate buffered saline. For anti-HCV experiment in Huh7.5.1 cells, the established JFH1-infected Huh7.5.1 cells were seeded at a density of 2 × 105 per well in 6-well cell culture plates in DMEM. Following incubation for 24 h at 37 °C and 5 % CO2, the culture media were removed, and serial dilutions of the test drugs in DMEM were added in a total volume of 2 ml without G418. The cell culture fluid was changed daily.
Western blotting
For the Western blot analysis of total cell lysates, cells were harvested and washed with ice-cold PBS. The protein concentration in the lysates was measured using a BCA protein assay kit (Thermo Fisher Scientific, Rockford, IL, USA) in accordance with the manufacturer’s instructions. Cell lysate samples were separated using 12 % SDS-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). The membrane was reacted with 1:1000 incubated primary antibody overnight followed with horseradish peroxidase-conjugated secondary antibody. The primary antibodies used were mouse anti-NS5A (Virogen, Watertown, MA, USA) and mouse anti-β-actin (Sigma Life Science and Biochemicals, St. Louis MO, USA). The secondary antibodies used were HPR-conjugated ECL sheep anti-mouse IgG purchased from Cell Signaling (Danvers, MA, USA). The protein bands were visualized using an enhanced chemiluminescence kit (PerkinElmer, Boston, MA, USA), and quantified using VisionWorks LS software (Upland, CA, USA), β-actin acting as the internal control.
Luciferase assay
The OR6 replication cell line represents a successful model of HCV infection and harbors a full-length genotype 1b HCV RNA with the Renilla Luciferase as a reporter. The Renilla Luciferase activity reflected the amount of HCV RNA synthesized. Cells were lysed in Renilla Luciferase Lysis Buffer (Promega, Madison, WI, USA). HCV replication in OR6 cells was determined by monitoring Renilla Luciferase activity and analyzed using a Luciferase Reporter assay system (Promega, Madison, WI, USA); the specific steps performed were according to the instruction. The 50 % effective concentration (EC50) was defined as the concentration of compound that reduced the Luciferase signal by 50 %.
Quantitative PCR (qPCR)
The supernatant was collected on the 7th and 14th day. RNA was purified from 200 μl of supernatant, and HCV RNA was detected by using Hepatitis C Viral RNA Quantitative Fluorescence Diagnostic kit (Sansure Biotech, Changsha, China), with a detection limit ≥25 HCV RNA IU/ml. The viral titer of the supernatant was expressed as international units (IU/ml); the specific steps performed were according to the instruction.
Statistical analysis
All results are expressed as the mean ± standard deviation. Statistical comparison is made using Student’s t test after analysis of variance. Differences are considered statistically significant at P < 0.05.
Results
JFH1-infected Huh7.5.1 cells expressed HCV virus stably
At 48-h post-infection HCV RNA replication was analyzed by quantitative PCR. The results showed a significant increased in HCV RNA levels in supernatants during the first 5 days of post-infection and at 7th day reached the peak (7.58 × 107 IU/ml), at the 12th day decreased to 3.62 × 107 IU/ml, then maintained at this level (about 7logs). At the same time, we observed the cells shape, at the 6th day some of them were died and then we changed medium to continue to culture the cells and the cells recovered to normal (data was not showed). The JFH1-infected Huh7.5.1 cell line was in a stable state, and the expression of the virus was in a steady level. We used the stable cell lines for the followed experiment.
Drug’s effect on the cells
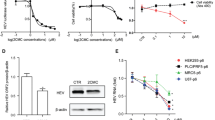
Treatment-induced cytotoxicity was determined by an MTT assay. The data showed that a low dose of ART (<25 μmol/l) had no effect on inhibiting the proliferation of growing JFH1-infected Huh 7.5.1 cells and OR6 cells according to the results obtained. In Huh7.5.1 cells, the CC50 ≈ 264.40 ± 12.46 μmol, in OR6 cells, the CC50 ≈ 190.66 ± 11.62 μmol (Table 1). As shown in Fig. 1a, b, the low dose of ART (<25 μmol/l) inhibited the proliferation of growing cells to the same extent as 10 μg/ml RBV and 500 U/ml IFN in the JFH1-infected Huh7.5.1 cells and OR6 cells (Fig. 1c, d).
Effect of cell viability on host cells induced by drugs. The MTT assay was used to measure the cytotoxicity of different concentrations of ART, IFN, RBV in JFH1-infected Huh 7.5.1 cells (a, c) and OR6 cells (b, d). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared with the negative control group. Data are expressed as mean values ± SD. Each experiment was repeated at least three times
ART inhibited HCV replication in OR6 cells
We next determined the extent of the anti-HCV effect of ART in OR6 cells. ART inhibited HCV replication in a concentration-dependent manner in OR6 cells, as monitored by measuring the Luciferase activity. The EC50 of ART was calculated to be 10.81 ± 0.63 μmol (Table 1). Then, we compared the anti-HCV effect of ART with that of RBV and IFN and explored whether ART can potentiate the anti-HCV activity of IFN. We treated OR6 cells with RBV (10 μg/ml), IFN (500 U/ml), and ART (12.5 μmol/l) combined with IFN (500 U/ml) for 3 days, as shown in Fig. 2a and Table 2. These results indicated that the inhibitive effect of ART on HCV replication was stronger than RBV but weaker than IFN. ART had a synergistic inhibitive effect on HCV with IFN.
ART inhibited HCV replication in OR6 cells. a Luciferase assay was used to measure the Renilla Luciferase activity in OR6 cells. b Western blot analysis was used to determine the expression of NS5A protein. β-actin was used as a protein loading control. ****P < 0.0001 compared with the negative control group. Data are expressed as mean values ± SD. Each experiment was repeated at least three times
To further confirm the results, the expression levels of HCV NS5A protein in OR6 cells that were treated with ART, RBV, or IFN were measured by Western blot. β-actin acted as the internal control, as shown in Fig. 2b. The expression of NS5A was also downregulated. ART reduced NS5A protein expression in a concentration-dependent manner in OR6 cells, confirming that ART exerted an antiviral action against HCV. The extent of the reduction in the expression of NS5A treated with RBV, IFN, and ART combined with IFN was consistent with the results of the Luciferase assay.
ART inhibited HCV replication in Huh7.5.1 cells
To determine the abundance of HCV RNA in the media of treated cells, fluorescence quantitative PCR was used. The supernatant of JFH1-infected Huh7.5.1 cells was collected from the culture, and the RNA was extracted and detected using the Hepatitis C Viral RNA Quantitative Fluorescence Diagnostic kit. When JFH1-infected Huh7.5.1 cells were exposed to ART, and an untreated group was used as a negative control for anti-HCV activity, the abundance of HCV RNA released to the culture medium was significantly decreased in a concentration-dependent and time-dependent manner (Fig. 3a). The EC50 of ART for the inhibition of HCV replication was 125.82 ± 16.25 μmol (Table 1). We further compared the anti-HCV effect of ART with RBV and IFN. We next treated JFH1-infected Huh7.5.1 cells with ART (12.5 μmol/l), RBV (10 μg/ml), and IFN (500 U/ml) and used an untreated group as a negative control for anti-HCV activity. ART decreased HCV RNA 1–2logs in the first week and 2–3logs in the second week. RBV only decreased HCV RNA 1log until the second week. IFN decreased HCV RNA 4logs in the first week and was undetectable in the second week. Compared to a negative control group, the dataset showed significant differences (P < 0.05) (Fig. 3b). These results indicated that the inhibitive effect of ART on HCV replication was stronger than RBV but weaker than IFN. This result was consistent with the inhibition of HCV replication in OR6 cells.
ART inhibited HCV replication in Huh7.5.1 cells. a The effect on HCV replicon replication in Huh7.5.1 cells induced by different concentrations of ART. b Compared the effect on HCV replicon replication in Huh7.5.1 cells induced by ART, IFN, RBV. Fluorescence quantitative PCR was used to detected the HCV RNA level of the supernatant. In the first week (open bars), all the experimental groups compared to the negative control group # P < 0.05; in the second week (filled bars), all the experimental groups compared to the negative control group × P < 0.05; in the same concentration group, compared the HCV RNA level of the first week to the second week, *P < 0.05, ****P < 0.0001, negative control group ▽ P > 0.05. Data are expressed as mean values ± SD. Each experiment was repeated at least three times
Next we explored whether ART potentiates the anti-HCV activity of IFN. We conducted the experiment by applying the combination of different doses of ART and IFN to the JFH1-infected Huh7.5.1 cells to test the synergistic effect on HCV replication. Fluorescence quantitative PCR was used to detect the HCV RNA level of the supernatant. Our results showed that low dose of IFN combined with low dose of ART obtained the same anti-HCV activity as high dose of IFN alone (Fig. 4a, b). Then we measured the expression levels of HCV NS5A protein in JFH1-infected Huh 7.5.l cells by Western blot. The extent of the reduction in the expression of NS5A was consistent with the results of the Fluorescence quantitative PCR assay (Fig. 4c). It demonstrated that ART has a synergistic inhibitive effect on HCV with IFN.
ART potentiated the anti-HCV activity of IFN. a, b Fluorescence quantitative PCR was used to detect the HCV RNA level of the supernatant. Effect of IFN (open bars) or 12.5 μmol/l ART combined with IFN (filled bars) on HCV replicon replication in Huh 7.5.1 cells. **P < 0.01, ***P < 0.001, ****P < 0.0001 (HCV RNA level obtained after treatment with IFN versus HCV RNA level obtained after treatment with 12.5 μmol/l ART + IFN). c Western blot analysis was used to determine the expression of NS5A protein. β-actin was used as a protein loading control. Data are expressed as mean values ± SD. Each experiment was repeated at least three times
Discussion
Artemisinin is a natural product derived from the Chinese herb, Artemisia annua. The World Health Organization officially recommends artemisinin and its derivatives for the treatment of malaria, particularly as a part of combination therapies with other antimalarial drugs. Previous studies have reported the inhibitory activity of artemisinin on subgenomic HCV replicon replication without adverse effects on host cells [20]. This result was similar to those seen in our study that a low dose of ART (<25 μmol/l) had no effect on inhibiting the proliferation of JFH1-infected Huh7.5.1 cells and OR6 cells. ART is a semisynthetic derivative of artemisinin that has improved water solubility. ART was well tolerated by patients. The reported data have shown that ART at a high dose of 8 mg/kg (≈20 μmol/kg) or 600–1200 mg (≈1562–3125 μmol) per day was safe in clinic [21, 22]. The concentration at which ART was active against HCV (12.5 μmol/l) in our present experiment was similar to that which was previously reported for human cytomegalovirus [23], and the inhibitory concentrations were close to the drug concentrations reached in the plasma of patients when this drug was used in antimalaria treatments (≈7 μmol/l) [9].
It was revealed that Artemisinin and its derivatives have anti-malarial, anti-bacterial, anti-angiotensin, and anti-cancer properties [8–10], and many studies have verified that they possessed anti-viral effect [15]. Our study indicated that ART possessed anti-HCV activity in a concentration- and time-dependent manner. The anti-HCV activity of ART was weaker than that of IFN but stronger than that of RBV. In the synergistic experiment, low dose of IFN combined with low dose of ART obtained the same anti-HCV effect as high dose of IFN alone. These data indicated that the ART can potentiate the anti-HCV activity of IFN. ART harbors an endoperoxide bridge whose cleavage results in the generation of reactive oxygen species (ROS) and/or carbon-centered free radicals [24, 25] may be one of the mechanisms by which ART inhibits HCV replication. Previous study reported that ROS disrupted active HCV replication complexes and reduced the amount of NS3 and NS5A in the subcellular fraction where active HCV RNA replication complexes existed [26]. ROS are also known to affect Keap1/Nrf2/ARE and NF-κB/Sp1 signaling pathways [27]. The Keap1/Nrf2/ARE is the center of antioxidant; HCV replication was accompanied by activation of Nrf2/ARE pathway in response to oxidative stress [28]. Therefore, we speculated that ART may possess the ability to anti-HCV by interfering with Keap1/Nrf2/ARE, but it still need to be elucidated. In addition, HCV nonstructural protein 5A (NS5A) could stimulate NF-κB and Sp1 pathway in response to oxidative stress [29]. It is also reported that a reduction in HCMV-induced protein synthesis and a reduction in the DNA binding activity of NF-κB and Sp1 were observed following ART treatment, the efficiency of HCMV replication was closely connected with NF-κB and Sp1 activation pathways [23], whether ART inhibited NF-KB pathway to HCV replication is waiting to be proved.
In summary, our present data evidenced that ART is a efficient inhibitor of HCV replication and has a synergistic effect when used with IFN in the HCV cell models. The mechanism of ART on HCV replication is not clear and need further exploring. Our results also suggested ART may be a supplemental therapy with IFN for HCV.
References
D.L. Thomas, Nat. Med. 19, 850–858 (2013)
A. Craxi, A. Licata, Semin. Liver Dis. 23(Suppl 1), 35–46 (2003)
M.G. Ghany, D.R. Nelson, D.B. Strader, D.L. Thomas, L.B. Seeff, Hepatology 54, 1433–1444 (2011)
H.W. Reesink, S. Zeuzem, C.J. Weegink, N. Forestier, A. van Vliet, D.R.J. van de Wetering, L. McNair, S. Purdy, R. Kauffman, J. Alam, P.L. Jansen, Gastroenterology 131, 997–1002 (2006)
J.M. Pawlotsky, Hepatology 53, 1742–1751 (2011)
N.J. White, Science 320, 330–334 (2008)
M. Adjuik, A. Babiker, P. Garner, P. Olliaro, W. Taylor, N. White, Lancet 363, 9–17 (2004)
X. Zhou, W.J. Sun, W.M. Wang, K. Chen, J.H. Zheng, M.D. Lu, P.H. Li, Z.Q. Zheng, Anticancer Drugs 24, 920–927 (2013)
K.T. Batty, T.M. Davis, L.T. Thu, T.Q. Binh, T.K. Anh, K.F. Ilett, J. Chromatogr. B Biomed. Appl. 677, 345–350 (1996)
W. Jiang, B. Li, X. Zheng, X. Liu, X. Pan, R. Qing, Y. Cen, J. Zheng, H. Zhou, J. Antibiot. 66, 339–345 (2013)
P.M. O’Neill, G.H. Posner, J. Med. Chem. 47, 2945–2964 (2004)
T. Efferth, M.R. Romero, D.G. Wolf, T. Stamminger, J.J. Marin, M. Marschall, Clin. Infect. Dis. 47, 804–811 (2008)
R. Arav-Boger, R. He, C.J. Chiou, J. Liu, L. Woodard, A. Rosenthal, L. Jones-Brando, M. Forman, G. Posner, PLoS One 5, e10370 (2010)
R. He, B.T. Mott, A.S. Rosenthal, D.T. Genna, G.H. Posner, R. Arav-Boger, PLoS One 6, e24334 (2011)
M.R. Romero, T. Efferth, M.A. Serrano, B. Castano, R.I. Macias, O. Briz, J.J. Marin, Antiviral Res. 68, 75–83 (2005)
K.J. Blight, J.A. McKeating, C.M. Rice, J. Virol. 76, 13001–13014 (2002)
V. Lohmann, F. Korner, J. Koch, U. Herian, L. Theilmann, R. Bartenschlager, Science 285, 110–113 (1999)
L.D. Presser, A. Haskett, G. Waris, Virology 412, 284–296 (2011)
M. Ikeda, K. Abe, H. Dansako, T. Nakamura, K. Naka, N. Kato, Biochem. Biophys. Res. Commun. 329, 1350–1359 (2005)
J. Paeshuyse, L. Coelmont, I. Vliegen, J. Van Hemel, J. Vandenkerckhove, E. Peys, B. Sas, E. De Clercq, J. Neyts, Biochem. Biophys. Res. Commun. 348, 139–144 (2006)
R.S. Miller, Q. Li, L.R. Cantilena, K.J. Leary, G.A. Saviolakis, V. Melendez, B. Smith, P.J. Weina, Malar J. 11, 255 (2012)
R. Price, M. van Vugt, L. Phaipun, C. Luxemburger, J. Simpson, R. McGready, F. ter Kuile, A. Kham, T. Chongsuphajaisiddhi, N.J. White, F. Nosten, Am. J. Trop. Med. Hyg. 60, 547–555 (1999)
T. Efferth, M. Marschall, X. Wang, S.M. Huong, I. Hauber, A. Olbrich, M. Kronschnabl, T. Stamminger, E.S. Huang, J. Mol. Med. 80, 233–242 (2002)
S.R. Meshnick, Y.Z. Yang, V. Lima, F. Kuypers, S. Kamchonwongpaisan, Y. Yuthavong, Antimicrob. Agents Chemother. 37, 1108–1114 (1993)
G.H. Posner, C.H. Oh, D. Wang, L. Gerena, W.K. Milhous, S.R. Meshnick, W. Asawamahasadka, J. Med. Chem. 37, 1256–1258 (1994)
J. Choi, K.J. Lee, Y. Zheng, A.K. Yamaga, M.M. Lai, J.H. Ou, Hepatology 39, 81–89 (2004)
T. Zima, M. Kalousova, Alcohol. Clin. Exp. Res. 29, 110S–115S (2005)
D. Burdette, M. Olivarez, G. Waris, J. Gen. Virol. 91, 681–690 (2010)
G. Waris, A. Livolsi, V. Imbert, J.F. Peyron, A. Siddiqui, J. Biol. Chem. 278, 40778–40787 (2003)
Acknowledgments
We thank Dr. Chung (Harvard University) for providing Huh7.5.1 cells and JFH1 and Dr. Zhao (Chinese Academy of Sciences) for providing OR6 cells. This work was supported by Grants from the National Natural Science Fund of China (81370542), Chinese National Key Clinical Specialty Project (No. 2013), and Chinese National Science and Technology Major Project (No. 2008ZX10202, http://www.nmp.gov.cn/) to GZ Gong.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Paul Schnitzler.
Rights and permissions
About this article
Cite this article
Dai, R., Xiao, X., Peng, F. et al. Artesunate, an anti-malarial drug, has a potential to inhibit HCV replication. Virus Genes 52, 22–28 (2016). https://doi.org/10.1007/s11262-015-1285-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-015-1285-7