Abstract
Akabane virus is a teratogenic pathogen transmitted by Culicoides spp. to ruminants. The virus induces anomalies in the central nervous system in the developing fetus, resulting in arthrogryposis-hydranencephaly (A-H) syndrome. During three outbreaks of the disease (2002, 2013, and 2020), 77 calves were born in Varamin, Iran, with A-H syndrome. The presenting neurologic signs were categorized into three main groups, as common, less common, and uncommon signs. The common signs were unawareness of the surroundings, blindness, deep depression, partial failure of suckling, and unintelligent behavior. The less commonly noted signs were hyperexcitability, regurgitation, head pressing, compulsive walking, and kicking, while the uncommon signs comprised protrusion of the tongue, making sounds resembling barking, carnivore-like milk drinking, and deafness. Arthrogryposis, dome-shaped skull, kyphosis, torticollis, lordosis, scoliosis, and spina bifida were the diagnosed skeletal defects. Upon necropsy, hydranencephaly, hydrocephaly, and microencephaly were seen in the calves presenting neurologic signs, while astrocytosis, astrogliosis, focal gliosis, perivascular, perineuronal, and submeningeal edema, perivascular cuffing, non-suppurative meningitis, non-suppurative encephalitis and lymphoplasmacytic infiltration, and perivascular and parenchymal hemorrhage were seen in samples obtained from the brains. RT-PCR detected Akabane virus in the brain tissues of the affected calves. This is the first clinical study of Akabane disease in calves in Iran.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Akabane virus (AKAV) is an arbovirus that belongs to the order Bunyavirales, family Peribunyaviridae, and genus Orthobunyavirus. It has a lipid envelope and three single-stranded negative-sense RNA segments (S, M, and L). The virus was first isolated in Japan in 1959 (Kirkland 2015; Lee et al. 2016; Adams et al. 2017; Yanase et al. 2018; Chen et al. 2021). AKAV is common in many tropical and subtropical areas between 35°N and 35°S (Line 2016) and has been reported in Japan, Eastern and Southeast Asia, the Middle East, Africa, and Australia, and is known as one of the principal etiological agents of congenital abnormalities, abortions and stillbirths in ruminants in these areas (Konno et al. 1982; Uchida et al. 2000; Geoghegan et al. 2014; Lee et al. 2016; Yanase et al. 2017; Chen et al. 2021).
The main vectors of AKAV are mosquitoes, especially Culicoides spp., except for New Zealand and Antarctica (Geoghegan et al. 2014; Kirkland 2015; Yanase et al. 2018). Under favorable environmental conditions, specifically in moist summers or autumns, the outbreak of congenital infections is expected 3–8 months after the virus spreads as a consequence of the abundance of the insect vector and their entry into new areas (Kirkland 2015; Line 2016; Yanase et al. 2017). From 1972 to 1975, Akabane disease was epizootic in Japan and approximately 42,000 calves were affected. The disease mainly flourished from early autumn through the following spring in this area (Konno et al. 1982; Yanase et al. 2017). Recently, the epizootics of Akabane disease have threatened the livestock industry, particularly in Australia and East Asia (Yanase et al. 2018). In endemic areas, there is a high prevalence of antibodies in cattle, buffalo, sheep, goats, camels, and horses (Haligur et al. 2014; Kirkland 2015). In the same vein, in 1969–1970 epizootic Akabane infection has been reported to affect a large number of ruminants i.e. 3,000 dairy calves, 700 lambs, and 600 kids (Ahi et al. 2015). In Chinese Taipei, a high prevalence of Akabane virus infection in pigs has been reported (Kirkland 2015). Akabane virus infection in adult animals develops transient viremia that usually occurs 1 to 6 days after infection. The virus is transmitted through the placenta to the fetus. In this case, fetal infection depends on the age of gestation and the virus strain. Infection in the first three months of gestation in cattle is associated with a low incidence of disease, while infections in the middle to late stages of gestation lead to congenital defects in newborn calves. In cases of highly virulent strains, arthrogryposis-hydranencephaly (A-H) syndrome is seen in up to 80% of newborn calves born to infected cows (Konno and Nakagawa 1982; Uchida et al. 2000; Kirkland 2015; Lee et al. 2016; Yanase et al. 2017). The occurrence of encephalitis in cattle caused by the Iriki strain has been reported in Japan, Taiwan, and Korea (Uchida et al. 2000; Lee 2016).
Congenital abnormality, stillbirth, premature birth, abortion, and reduction of milk production in viremic cows are outcomes of Akabane disease causing an impressive economic loss (Kono et al. 2008; Geoghegan et al. 2014). The aim of this study was to describe Akabane disease in an Iranian dairy farm and illustrate the clinical and pathological features of the disease in the affected calves.
Materials and methods
Study area and animals
During mid-January through the end of April 2002, 2013, and 2020, 77 calves with neurological signs and arthrogryposis (46 calves with neurological signs and 31 calves with skeletal defects and arthrogryposis) from both sexes (40 male and 37 female) were born in a dairy herd in Varamin (a county in the South of Tehran Province, is one of the main dairy areas of Iran).
All affected calves were carefully examined and vital parameters were recorded. Accordingly, they were divided into 2 groups; those suffering from neurological signs and those born with arthrogryposis. In 52 cases the birth weight was recorded and the daily weight gain was recorded in 24 affected calves (in 53 cases, the measurement was unsuccessful due to the difficulty of nursing and early culling).
40 calves with neurological signs were humanely euthanized (for welfare reasons and difficulty in nursing) and their skulls were examined for gross and microscopic lesions. In 27 cases, blood samples were collected from the jugular vein in EDTA tubes for hematological analysis.
Sampling, viral RNA preparation, and RT-PCR
23 samples of the developed brain tissue were collected (approximately 10 g) for the detection of Akabane virus RNA. RNA was extracted using the MBST kit (Molecular Biological System Transfer, Iran) from 0.05 g of brain tissue according to the manufacturer’s instructions. Akabane virus RNA was identified using Reverse Transcription-Polymerase Chain Reaction (Qiagen, Hilden, Germany) with forward and reverse primers for the Akabane genome (Table 1). Moreover, these specimens were examined, as described above, to detect the Schmallenberg virus. Cerebrospinal fluid (CSF) was collected into a sterile container, and tested in aerobic and anaerobic cultures.
Pathological examination
40 calves with neurological signs were systematically necropsied and their central nervous system was precisely examined. In all cases, the developed brain tissue and the surrounding structures were evaluated for gross pathology (other heads were not examined because of the owner’s dissent). During necropsy, 71 specimens of different regions of the remaining brain tissues (frontal, parietal, and occipital cortices, periventricular zone, corpus striatum, thalamus, hippocampus, rostral colliculi, caudal colliculi, pons, cerebellum, deep nuclei of the cerebellum, medulla oblongata, cervical spinal cord) were obtained and fixed in 10% neutral buffered formalin, processed routinely for histology, embedded in paraffin, sectioned at 5 μm thickness, and stained with hematoxylin and eosin (H&E staining). Histologic slides were prepared and reviewed using a light microscope. In 6 hydranencephaly cases, where fluid-filled sacs had replaced the poorly developed brain tissue, the volume of the fluid (CSF) was measured by a syringe, and the remaining brain tissue was weighed and 5 ml of the accumulated fluid was submitted for cytological and bacteriological analysis. It is worthwhile to mention that due to the owner’s refusal, none of the calves with arthrogryposis were necropsied.
Statistical analysis
Statistical analysis was performed by independent samples t-test method (SPSS-26 software, IBM Co., Armonk, NY, USA) for analyzing the differences between birth weight, daily weight gain, and weight at the slaughter of calves with hydranencephaly and normal calves. The differences were considered statistically significant with p < 0.05.
Results
Clinical features
In all calves, vital parameters such as body temperature (ranging from 38.5 to 39.2 ˚C), heart rate (mean: 120.6/min), and respiratory rate (mean: 35.7/min) were within normal limits. Two distinct signs were observed; i.e., neurological signs and skeletal defects and arthrogryposis.
Mean birth weight and mean daily weight gain were recorded in 52 and 24 affected calves, respectively. Mean weight at slaughter in 14 affected calves (12 three-month-old and 2 six-month-old) was recorded (Table 2). In other patients, records of birth weight, daily weight gain, and weight at slaughter were missed due to early culling. While the mean birth weight difference 52 affected and normal calves was not significant (p = 0.32), and the mean daily weight gain between 24 affected calves was significantly lower than normal calves (p = 0.00) at the same time and farm. There were statistically significant differences between weight at slaughter in 3 and 6 months old affected calves with normal ones (p = 0.001).
In addition, in 27 calves the pale mucous membranes revealed anemia (mean of hematocrit and erythrocytes: 15.9% and 3.55 × 106/µl, respectively). In addition to anemia, leukocytosis and neutrophilia were remarkable hematological findings in these calves (Table 3). Emaciation and poor body condition were seen in 29 cases.
Neurological signs
In the present study, the frequency of neurological signs in calves with hydranencephaly was classified into three groups: common, less common and uncommon signs.
Commons signs: unawareness of the surroundings and blindness were observed in all calves (46 cases) with neurological signs. In these animals, eye-preservation reflexes (menace reflex, corneal reflex and palpebral reflex) were absent, but the pupillary light reflex was present, indicating cortical blindness. Deep depression was seen in 43 cases. Patients were unable to respond to environmental stimuli. Partial failure of suckling was seen in 30 cases and milk drinking was difficult for all affected calves. Unintelligent behaviors, such as drinking milk from the bottom of the bucket, were observed in 10 affected calves. In these calves, asphyxia and aspiration pneumonia followed each milk-drinking session. Also, shedding of hairs on lower and upper jaws and alopecia were observed in these animals.
Less common signs: hyperexcitability was seen in 3 cases, making it difficult to touch and examine. Regurgitation and head pressing were also seen in 3 cases. Compulsive walking was seen in 3 cases and these calves continued to move without aimlessly. Kicking was found in 3 cases; the calves were kicking around unexpectedly.
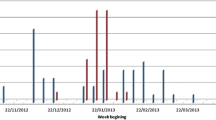
Uncommon signs: protrusion of the tongue was present in 2 cases. Making sounds resembling barking and carnivore-like milk drinking were seen in 1 case. Also, deafness was found in 1 case; this calf did not respond to any vocal stimuli. However, calves with depression also had difficulty responding to sound stimuli. These calves were normally delivered (Fig. 1; Table 4).
Skeletal defects and arthrogryposis
Of these, 28 calves exhibited rigid extension or contraction of one or more limbs (degree of contraction varied between joints of limbs) and a decrease in the size of muscle (arthrogryposis). These animals were unable to stand.
Despite dystocia in some cases, fetotomy was not necessary. The most remarkable gross malformations in the vertebral column were scoliosis in the cervical area (1 case), lordosis in the thoracic area (1 case), kyphosis in the thoracic area (3 cases), torticollis (2 cases), and spina bifida (1 case).
Dome-shaped skulls were observed in 4 cases (Fig. 1; Table 4). These calves were unable to stand and died on the first day after birth.
Abortion was recorded in 32 dams and the fetuses and placenta were discarded and not studied.
Reverse transcription-polymerase chain reaction (RT-PCR)
Out of the 23 samples of the remaining brain tissue submitted for RT-PCR, 13 cases (56.52%) were positive for the Akabane virus and 10 cases (43.48%) were negative (Table 1). Also, Schmallenberg virus was not detected in any specimen. Aerobic and anaerobic bacterial cultures of CSF were negative in all samples.
Post-mortem examination
Three types of central nervous system disorders (hydranencephaly, microencephaly, and hydrocephalus) were seen in post-mortem examination.
In all 40 skulls, the calvaria and dura mater were removed. In 30 cases (75%), cerebral hemispheres were absent and replaced by huge fluid-filled sacs, indicating hydranencephaly; 4 cases (10%) showed hydrocephalus. Microencephaly was detected in 6 cases (15%). The fluid was watery and clear and there was no accumulation of cerebrospinal fluid in any part of the subarachnoid space. In the CSF analysis, specific gravity was within normal limits and cell count was 5–18 / µl.
In calves with hydranencephaly, dorsal and lateral surfaces of the fluid-filled sacs were surrounded by a thin membrane with blood vessels running through the fluid content. In the depth of these sacs and proximity to the ventricles, the structure of the formed nervous tissue (diencephalon) was seen (Fig. 2). There were various types of hydranencephaly in both cerebral hemispheres. In 6 cases, the major changes were observed in the telencephalon. The lesions extended from the frontal lobe to the occipital lobe. In 2 cases, the frontal and occipital lobes were formed incompletely, while these structures were absent in other cases. The parietal lobe was partially hypoplastic in one case and the temporal lobe was not formed in any cases. In some areas, including the frontal, parietal, and occipital cortices, the gyri were still present but were smaller and wider than normal in appearance which depicted attenuated gyri. In all cases, cerebral peduncles were completely formed. In 3 cases pyriform lobes were completely formed while in 3 calves incomplete tissue formation was seen. Thalamus, rostral and caudal colliculi, cerebellum, pons, medulla oblongata, and falx cerebri were completely formed and no apparent changes were observed. Also, the corpus callosum and fornix were present in all cases. The weight of the remaining brain tissue of affected calves was lower than the normal value, with the heaviest brain tissue seen in calf NO. 5 (158 g) and the lightest noted in calf NO. 1 (69 g). The maximum volume of fluid was measured in calf NO. 4 (230 ml) and the lowest volume of fluid was observed in calf NO. 3 (110 ml) (Table 5). The macroscopic findings of the brain were sufficient to identify hydranencephaly.
In 4 cases with dome-shaped skulls, bilateral ventriculomegaly (hydrocephalus) and thinning of cranial bones were seen (Fig. 3).
In calves with microencephaly, the diameter of the cranial bones was increased. The size of the cranial cavity was considerably reduced and cerebral hemispheres were smaller than normal (Fig. 4).
Histopathological examination
Histological examination of residual portions of the brain in calves with hydranencephaly and hydrocephalus revealed no remarkable lesions. Histopathological examination of the membranous covering of the hypoplastic hemisphere of the telencephalon showed vascular structures, arachnoid and pia matter. The superficial tissue of the cortex was flat and microgyric. In the gray matter, the number of neurons was reduced. Moreover, astrocytosis, astrogliosis, and focal gliosis were seen. Cerebral edema was diagnosed as perivascular, perineuronal, and submeningeal edema. There was lymphocytic perivascular cuffing in both gray and white matter, indicative of viral inflammation. In one case, non-suppurative meningitis was diagnosed. Non-suppurative encephalitis (lymphoplasmacytic infiltration) was noted in the frontal lobe of another case. In most sections, perivascular and parenchymal hemorrhage was seen. The cerebellum, pons, and medulla oblongata were normal in all cases (Fig. 5).
Histopathological lesions of the remaining brain tissue from calves infected with Akabane virus (H&E). A Perivascular cuffing (PVC) in the cerebral cortex. B Multifocal parenchymal hemorrhage (arrows) in the gray matter. C Astrocytosis and astrogliosis in the cerebral cortex. Astrocytes (arrowheads) have replaced necrotic neurons in the gray matter. Neurons (arrows) are sparsely present between these cells. D Perivascular (arrow) and perineuronal edema (arrowheads)
The proximal part of the cervical spinal cord was also histopathologically examined, which only showed focal gliosis (Table 6).
Discussion
There are two endemic zones for AKAV; (1) from East Asia to Southeast Asia and Australia, and (2) from the Middle East to South Africa (Tzeng et al. 2022). Reports on Akabane disease in the Middle East are scarce. However, seropositive cases were reported in Turkey, Syria and Jordan in 1979 and 1980 (Taylor and Mellor 1994) and in Saudi Arabia in 1998 (Abu Elzein et al. 1998). The disease was first reported as a causative agent of A-H syndrome in 1992 in Iran (Ahourai et al. 1992). Additionally, Alsaad et al. (2017) reported this syndrome in Iraq. The spread of the virus and the occurrence of the disease are seasonal (Lee et al. 2002) and the presence of the virus is related to the insect vector’s population and the regional temperature (Kirkland 2015). Infection occurs in the summer and early autumn when vectors become active, which is depending on the location of countries (from April through November in New South Wales in Australia and from September through March in Japan) (Konno et al. 1982), but in Iran the disease occurs from mid-January through the end of April. Three different species of Culicoides have been identified in Turkey (C. schultzei, C. longipennis, and C. circumscriptus) which are considered AKAV vectors (Dağalp et al. 2021; Tzeng et al. 2022). Also, C. oxystoma and C. brevitarsis have been identified as vectors in Japan and Australia, respectively (Uchida et al. 2000; Kirkland 2015); there is no information about the vectors in Iran.
AKAV can cross the placenta in ruminants and infect fetuses (Hartley et al. 1977; Haligur et al. 2014). Fetal immunity will develop in the second half of pregnancy and results in minimal lesions (Konno et al. 1982); otherwise, results in fetal infection are congenital malformations, especially in the central nervous system of the neonate (Hartley et al. 1977; Haligur et al. 2014).
Congenital A-H syndrome is recognizable based on clinical, gross, and histopathological findings. Also, the causative agent can be confirmed by Immunohistochemistry (Kono et al. 2008; Haligur et al. 2014; Lee et al. 2016) and RT-PCR (Nietfeld 2012; Haligur et al. 2014; Kirkland 2015; Dağalp et al. 2021), as used in the present study. It has been revealed that viruses circulating in Asia have a common gene pool that is distinguishable from existing strains in Africa and Oceania (Kono et al. 2008). Recently serologic investigation showed antibodies to AKAV in ruminants in different zones of Iran (Ahi et al. 2015; Kojouri et al. 2015). Golchin et al. (2023) have detected AKAV using RT-PCR in Iran, similar to the present study.
Abortion, stillbirth, premature birth, and neonatal deaths are the first presentation of the disease in the herd. Central nervous system disorders will be expected at the end of the outbreak (Konno et al. 1982; Kirkland 2015). The most affected calves have been reported to deliver alive (Konno et al. 1982). In the present study, H-A syndrome, stillbirth and abortion were recorded, but aborted fetuses were eliminated by the owner before necropsy.
Calves with severe arthrogryposis may have reduced birth weight due to muscular atrophy, but in the present study, birth weight was normal in successfully delivered calves. In contrast to our findings, Kurogi et al. (1977) stated that affected calves with AKAV had lower birth weights. Also, Blood (1956) stated weight loss and reduction in body size in 5 calves with hydranencephaly. Abnormalities presented in these calves, including blindness, depression, abnormal drinking, and suckling; may explain reduced daily weight gain and reduced slaughter weight in calves with hydranencephaly.
Impaired formation of various parts of the brain was responsible for the neurological signs, leading to disorders in the innervation of organs, while peripheral nerve structures were normal. It has been proven that cortical blindness happens due to occipital lobe damage and in the absence of gross or microscopic ocular lesions (Whittem 1957). Previous studies have sporadically reported these signs in newborns affected by hydranencephaly (Blood 1956; Whittem 1957; Kurogi et al. 1977; Konno et al. 1982; Kirkland 2015; Alsaad et al. 2017). All studied patients were blind and unaware of their surroundings. Additionally, some of the affected calves suffered a partial failure of suckling, with ensuing difficulty in feed uptake, cachexia, and death. As stated in previous studies, surviving animals are euthanized (Kirkland 2015) because of difficult nursery or will pass away at 6 months of age due to blindness and other neurologic disorders (Alsaad et al. 2017). Similarly, the present study showed that under proper nursing care, calves suffering from hydranencephaly can be viable, however difficult nursing led to the slaughter of presented calves in 6 months of age.
Only in two calves kept up to 6 months of age, hypersensitivity was seen which exaggeratedly reacted during the clinical examination. Previous studies have noted depression in patients, but there are no reports regarding hypersensitivity in Akabane patients. Although learning disability was seen in all affected calves, in the 2 six-month-old calves, there was a slight learning ability with increasing age. Among the observed clinical signs, deafness (Alsaad et al. 2017) and protrusion of the tongue (Blood 1956) have been described in calves with Akabane disease, but bark-like sounds and kicking were not reported in any papers. However, tremor and kicking may be explained by demyelination of the motor nerves of the spinal cord (Constable et al. 2017).
Arthrogryposis is the most common skeletal defect in calves with Akabane disease, which is commonly seen in the middle stage of epizootics and may be seen in fetuses infected between 105 and 150 days of gestation (Alsaad et al. 2017). Calves that have been infected earlier in gestation show more severe arthrogryposis in four limbs, in contrast to the ones affected in later stages, which may have mild arthrogryposis in one limb (Alsaad et al. 2017). This condition is caused by degeneration and loss of motor nerves in the ventral horn of the spinal cord (Whittem 1957; Moriguchi et al. 1976; Kirkland 2015), as a consequence of polymyositis, myodegeneration, and neurogenic muscle atrophy which is revealed by reduced muscular tonicity of the limbs and restricted movement. Neuronal damage may be caused by the direct cytopathic effects of AKAV or tissue response to the virus. This is accompanied by partial fixation and contraction of the joints; hence this condition is referred to as “curly calf” syndrome (Haligur et al. 2014; Kirkland 2015; Alsaad et al. 2017). In AKAV-infected calves lacking arthrogryposis, the spinal cord remains intact (Moriguchi et al. 1976). In the present study, no lesions were seen in the histopathological examination of the cervical spinal cord. Similar to previous studies (Moriguchi et al. 1976; Haligur et al. 2014; Alsaad et al. 2017), joint deformity was not seen at the articular surfaces of affected limbs. The spinal cord was not sampled in calves with arthrogryposis, due to the owner’s refusal.
Torticollis, scoliosis, and brachygnathism in AKAV-infected calves are less common compared to small ruminants (Kirkland 2015), however, were diagnosed in the present study. Scoliosis, lordosis, and spina bifida were less common skeletal defects in calves. Previous studies have reported these defects in calves with AKAV (Haligur et al. 2014; Kirkland 2015; Alsaad et al. 2017). Concurrent skeletal defects such as lordosis and torticollis were seen in calves with hydranencephaly, similar to a study conducted by Haligur et al. (2014) in an aborted AKAV-infected fetus. Skeletal defects in calves may cause dystocia, which in severe cases requires fetotomy (Kirkland 2015; Alsaad et al. 2017). Although in this study some calves with arthrogryposis were delivered with dystocia, fetotomy was not necessary.
Calves with dome-shaped skulls look normal at first glance, but they are mostly blind and unable to suck and respond to stimuli (Alsaad et al. 2017). In the present study, stance inability was seen in the calves and they died much earlier than other affected calves. Hence, recorded clinical signs are minimal. Whittem (1975) stated that calves with hydranencephaly have no abnormalities in their skull bones, with no disorders in CSF circulation and intracranial pressure. However, domed skulls may be found in patients with AKAV with a slight increase in skull size (Blood 1956; Alsaad et al. 2017). In the present study, the 4 dome-shaped skulls were diagnosed as hydrocephalus upon necropsy.
The acute phase response in calves with A-H syndrome leads to the disruption of physiologic and biochemical processes (Alsaad et al. 2017). Leukocytosis as neutrophilia in the leukogram may be explained by this condition or stress in our patients. On the other hand, inflammatory cytokines have been shown to suppress the bone marrow, inducing anemia in severe inflammation (Morceau et al. 2009). We believe that difficulty in forage intake and probably the above mentioned factors have led to anemia and pale mucous membranes.
Macroscopic observation of the brain is sufficient for the diagnosis of hydranencephaly (Konno et al. 1982; Maxie 2016). Primary inflammatory foci and circulation of cerebrospinal fluid cause continual erosion and are involved in the development of hydranencephaly (Whittem 1957; Konno et al. 1982). Cerebral defects can vary from small cystic lesions to the complete absence of hemispheres (Kirkland 2015). Upon necropsy, cerebral hemispheres were markedly lost and replaced by CSF-filled sacs. However, in some cases, lobes were incompletely formed (with minimal gyri and sulci). These findings were similar to previous results, but the stages of lobar development were variable (Konno et al. 1982; Haligur et al. 2014). Moreover, Inoue et al. (2024) reported similar necropsy findings in porcine fetuses infected with AKAV.
Although Golchin et al. (2023) have diagnosed cerebellar hypoplasia upon gross and microscopic examination in AKAV-positive calves, in the present study, there was no cerebellar defect, and lesions were limited to the cerebral hemispheres and spinal cord (focal gliosis).
Various factors such as the causative agent, pathogenicity, and fetal age (immune system development) appear to play a role in the severity of lesions in patients with hydranencephaly (Hartley et al. 1977; Uchida et al. 2000; Haligur et al. 2014). Infection in the second half of gestation is expected to cause limited lesions (Konno et al. 1982), where some structures of the brain will remain intact. Due to the destruction of the cerebral hemispheres in hydranencephaly, the brain’s weight is decreased. The maximum and minimum recorded brain weights in calves with hydranencephaly were 158 g and 69 g, respectively. On the other hand, CSF volume measured 110–230 ml. Although, the volume of CSF in the cranial cavity of calves with hydranencephaly was reported 50 to 75 ml (Kurogi et al. 1977; Haligur et al. 2014). The incidence of microencephaly, occurring at the latest stages of the outbreak, is low in AKAV infection (Constable et al. 2017). In the present study, this defect was only seen in 6 calves.
Histopathological findings of hydranencephaly in Akabane cases do not have much diagnostic value as many parts of the brain are destroyed (Kirkland 2015), but they can help understand the process of disease and tissue changes (Maxie 2016). The thickness of the cerebral cortex and the neuronal population was reduced in partially developed lobes where they were replaced by astrocytosis, astrogliosis, and focal gliosis. In addition, each patient showed either non-suppurative encephalitis or meningitis. The microscopic findings in our study were similar to that obtained from previous studies in affected calves and porcine fetuses (Hartley et al. 1977; Konno et al. 1982; Haligur et al. 2014; Kirkland 2015; Lee et al. 2016; Golchin et al. 2023; Inoue et al. 2024). Gliosis and perivascular cuffing in neural tissues were found only in viral hydranencephaly (Schild et al. 2011), and were present in our study. The presence of microgliosis and astrocytosis in the perivascular area indicates an inflammatory process in the brain (Lee and Zhang 2016), however, the presence of inflammatory cells is not common in viral hydranencephaly, because most of the inflammatory reactions occur at the early stages of infection and are eliminated before birth (Kirkland and George 2018). Some sections revealed multifocal hemorrhages that were similar to the results of Moriguchi et al. (1976). The authors believe that the hemorrhages may have been caused by repeated injury to the brain tissue following the frequent circulation of CSF and exaggerated movements of the patient that cause harsh fluid displacement within the sacs. Similar to previous studies, all lesions were limited to cerebral hemispheres, while cerebellum, pons, and medulla oblongata were intact in all cases (Kirkland 2015; Alsaad et al. 2017). However, Haligur et al. (2014) stated the main lesions in the medulla oblongata, pons, and midbrain of aborted fetuses due to AKAV. Interestingly, cerebellar hypoplasia has been reported in the affected calves by Golchin et al. (2023).
It is worthwhile to mention that throughout the disease episodes, no cases were observed in the small ruminants, camels, or equine populations in the area. Since there is no commercial vaccine available, prevention of the disease is challenging (Alsaad et al. 2017). Eventually, it must be mentioned that the disease is likely to stay latent for 5 to 10 years and outbreaks can be expected when a lot of naive pregnant cattle come to contact with a large population of vectors (Blood 1956; Constable et al. 2017). Thus, an outbreak of this disease is expected within 10 years in Iran.
Conclusion
AKAV infection has distinct neurological and skeletal manifestations. In areas possessing Culicoides species, AKAV infection must be included in the differential diagnoses along with other teratogenic viral infections.
Data availability
No datasets were generated or analysed during the current study.
References
Abu Elzein EME, A1-Afaleq AI, Mellor PS, E1-Bashir AM, Hassanein MM (1998) Study of Akabane infection in Saudi Arabia by the use of sentinel ruminants. J Comp Path 119:473–478
Adams MJ, Lefkowitz EJ, King AMQ, Harrach B, Harrison RL, Knowles NJ, Kropinski AM, Krupovic M, Kuhn JH, Mushegian AR, Max Nibert M, Sabanadzovic S, Sanfacon H, Siddell SG, Simmonds P, Varsani A, Zerbini FM, Gorbalenya AE, Davison AJ (2017) Changes to taxonomy and the International Code of Virus classification and nomenclature ratified by the International Committee on Taxonomy of Viruses. Arch Virol 162:2505–2538. https://doi.org/10.1007/s00705-017-3358-5
Ahi MR, Pourmahdi-Borujeni M, Haji-Hajikolaei MR, Seifi-Abad-Shapouri MR (2015) A serological survey on antibodies against Akabane virus in sheep in southwest of Iran. Iran J Virol 9:22–27. https://doi.org/10.21859/isv.9.2.20
Ahourai P, Gholami MR, Ezzi A, Kargar R, Khedmati K, Aslani A, Rahmani F, Zarrin-Naal E (1992) Bovine congenital arthrogryposis & hydranencephaly outbreaks attributed to Akabane virus infection in Iran. Arch Razi Inst 42:51–56. https://doi.org/10.22092/ari.1992.109107
Akashi H, Onuma S, Nagano H, Ohta M, Fukutomi T (1999) Detection and differentiation of Aino and Akabane Simbu serogroup bunyaviruses by nested polymerase chain reaction. Arch Virol 144:2101–2109. https://doi.org/10.1007/s007050050625
Alsaad KM, Alautaish HHN, Alamery MAY (2017) Congenital arthrogryposis-hydranencephaly syndrome caused by Akabane virus in newborn calves of Basrah Governorate, Iraq. Vet World 10:1143–1148. https://doi.org/10.14202/vetworld.2017.1143-1148
Blood DC (1956) Arthrogryposis and hydranencephaly in newborn calves. Aust Vet J 32(6):125–131
Chen D, Wang D, Wei1 F, Kong Y, Deng J, Lin X, Shaoqiang WS (2021) Characterization and reverse genetic establishment of cattle derived Akabane virus in China. BMC Vet Res 17:349–357. https://doi.org/10.21203/rs.3.rs-547132/v1
Constable PD, Hinchcliff KW, Done SH, Grunberg W (2017) Veterinary medicine. Elsevier, Missouri
Dağalp SB, Dik B, Doğan F, Farzani TA, Ataseven VS, Acar G, Şahinkesen I, Özkul A (2021) Akabane virus infection in Eastern Mediterranean Region in Turkey: Culicoides (Diptera: Ceratopogonidae) as a possible vector. Trop Anim Health Prod 53:231–240. https://doi.org/10.1007/s11250-021-02661-y
Geoghegan JL, Walker PJ, Duchemin JB, Jeanne I, Holmes EC (2014) Seasonal drivers of the epidemiology of arthropod-borne viruses in Australia. Negl Trop Dis 8:3325. https://doi.org/10.1371/journal.pntd.0003325
Golchin D, Sasani F, Moosakhani F, Badiei A, Zafari M, Partovi Nasr M (2023) Congenital cerebral and cerebellar anomalies in relation to bovine viral diarrhoea virus and Akabane virus in newborn calves. Acta Vet Hung 71(1):34–40. https://doi.org/10.1556/004.2023.00764
Haligur M, Hasircioglu S, Ozmen O, Kale M, Aydogan A (2014) Immunohistochemical evaluation of akabane virus infection in aborted and new-born calves. Vet Med 59(5):230–238. https://doi.org/10.17221/7516-VETMED
Hartley WJ, De Saram WG, Della-Porta AJ, Snowdon WA, Shepherd NC (1977) Pathology of congenital bovine epizootic arthrogryposis and hydranencephaly and its relationship to akabane virus. Aust Vet J 53(7):319–325. https://doi.org/10.1111/j.1751-0813.1977.tb00240.x
Inoue D, Hayashima A, Suzuta F, Motomura Y, Kawamoto Y, Yoshino F, Morita K, Hirai Y, Iwamatsu S, Nakazato S, Kimura K, Yanase T (2024) Congenital malformations caused by Akabane virus in porcine fetuses in southern Japan. Vet Res Commun 48:449–457. https://doi.org/10.1007/s11259-023-10230-x
Kirkland PH (2015) Akabane virus infection. Rev Sci Tech 34:403–410. https://doi.org/10.20506/rst.34.2.2366
Kirkland PD, George TD (2018) Diseases caused by Akabane and related Simbu-group viruses in: infectious diseases of livestock. https://anipedia.org/resources/diseases-caused-by-akabane-and-relatedsimbu-group-viruses/1171
Kojouri GA, Davoodi Z, Momtaz H (2015) Serological and molecular detection of Akabane virus in Iran. Iran. J Appl Anim Sci 5:737–740
Konno S, Nakagawa M (1982) Akabane disease in cattle: congenital abnormalities caused by viral infection. Experimental disease. Vet Pathol 19:267–279. https://doi.org/10.1177/030098588201900305
Konno S, Moriwaki M, Nakagawa M (1982) Akabane disease in cattle: congenital abnormalities caused by viral infection. Spontaneous disease. Vet Pathol 19:246–266. https://doi.org/10.1177/030098588201900304
Kono R, Hirata M, Kaji M, Gato Y, Ikeda S, Yanasa T, Kato T, Tanaka S, Tsutsui T, Imada T, Yamakawa M (2008) Bovine epizootic encephalomyelitis caused by Akabane virus in southern Japan. BMC Vet Res 4:20. https://doi.org/10.1186/1746-6148-4-20
Kurogi H, Inaba Y, Takahashi E, Sato K, Satoda K, Goto Y, Omori T, Matumoto M (1977) Congenital abnormalities in newborn calves after inoculation of pregnant cows with Akabane virus. Am Soc Mic 17(2):338–343. https://doi.org/10.1128/iai.17.2.338-343.1977
Lee T, Zhang S (2016) Microgliosis in the injured brain: infiltrating cells and reactive microglia both play a role. Neurosci 1–6. https://doi.org/10.1177/1073858415572079
Lee JK, Park JS, Choi JH, Park BK, Lee BC, Hwang WS, Kim JH, Jean YH, Haritani M, Yoo HS, Kim DY (2002) Encephalomyelitis associated with Akabane virus infection in adult cows. Vet Pathol 39:269–273. https://doi.org/10.1354/vp.39-2-269
Lee H, Jeong H, Park S, Yang MS, Kim J, Bae J, Kwon Y, Kim MS, Oem JK, Lee MH, Lim CW, Kim B (2016) Experimental infection of cows with newly isolated Akabane virus strain (AKAV–7) causing encephalomyelitis. Vet Res 47:62. https://doi.org/10.1186/s13567-016-0349-6
Line S (2016) The Merck veterinary manual, 11th edition, Merck & Co. Inc., Kenilworth, USA
Maxie MG (2016) Nervous system. Jubb, Kennedy and Palmar’s pathology of domestic animals. Elsevier, Missouri, pp 272–274
Morceau F, Dicato M, Diederich M (2009) Proinflammatory cytokine-mediated anemia: regarding molecular mechanisms of erythropoiesis. Mediators Inflamm 2009(1–11). https://doi.org/10.1155/2009/405016
Moriguchi R, Izawa H, Soekawa M (1976) A pathological study on calves with arthrogryposis and hydranencephaly. Zentralbl Veterinarmed 23:190–199. https://doi.org/10.1111/j.1439-0450.1976.tb00675.x
Nietfeld JC (2012) Neuropathology and diagnostics in food animals. Vet Clin North Am Food Anim Pract 28:515–534. https://doi.org/10.1016/j.cvfa.2012.07.008
Schild AL, Fiss L, Damé MC, Uzal FA, Soares MP, Schuch LF, Flores EF, Riet-Correa F (2011) Congenital hydranencephaly and cerebellar hypoplasia in water buffalo in southern Brazil. J Vet Diagn Invest 23(3):603–609. https://doi.org/10.1177/1040638711403426
Taylor WP, Mellor PS (1994) The distribution of Akabane virus in the Middle East. Epidemiol Infect 113:175–185
Tzeng H, Tsai C, Ting L, Liao K, Tu W (2022) Molecular epidemiology of Akabane virus in Taiwan. Vet med sci 1–8. https://doi.org/10.1002/vms3.887
Uchida K, Murakami T, Sueyoshi M, Tsuda T, Inai K, Acorda JA, Yamaguchi R, Tateyama S (2000) Detection of akabane viral antigens in spontaneous lymphohistiocytic encephalomyelitis in cattle. J Vet Diagn Invest 12:518–524. https://doi.org/10.1177/104063870001200605
Whittem JH (1957) Congenital abnormalities in calves: arthrogryposis and hydranencephaly. J Path Bact 23:375–387. https://doi.org/10.1002/path.1700730208
Yanase T, Kato T, Hayama Y, Akiyama M, Itoh N, Horiuchi S, Hirashima Y, Shirafuji H, Yamakawa M, Tanaka S, Tsutsui T (2017) Transition of Akabane virus genogroups and its association with changes in the nature of disease in Japan. Transbound Emerg Dis 1–10. https://doi.org/10.1111/tbed.12778
Yanase T, Kato T, Hayama Y, Shirafuji H, Yamakawa M, Tanaka S (2018) Oral susceptibility of Japanese Culicoides (Diptera: Ceratopogonidae) species to Akabane Virus. J Med Entomol 20(10):1–7. https://doi.org/10.1093/jme/tjy201
Acknowledgements
The authors would like to thank Mr. Mahdi Nowrouzi for his assistance with sample collection.
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
Conceptualization: M.G and P.D.R. Methodology: M.G, P.D.R., M.M. and S.H.M. Formal analysis and investigation: M.G., A.R., P.D.R., M.M. and S.H.M. Writing - original draft preparation: M.G and P.D.R. Writing - review and editing: M.G., A.R., P.D.R. and S.H.M. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All experimental protocols were approved by the ethics committee of the University of Tehran (Faculty of Veterinary Medicine, No. 854 T 1398). Animals handling and procedures were performed following the guiding principles for biomedical research involving animals. Written informed consent was provided by the owner of the dairy farm for diagnostic work-up and participation in this research.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gorjidooz, M., Raoofi, A., Rahimabadi, P.D. et al. Study of Akabane disease in an Iranian dairy herd: a re-emerging disease. Vet Res Commun (2024). https://doi.org/10.1007/s11259-024-10487-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11259-024-10487-w