Abstract
Brucellosis is a significant infection that causes abortion, decreased milk production, and sterility in livestock, which greatly affects the industry. This study aimed to determine the prevalence of Brucella in buffalo milk samples across various regions of Iran, utilizing serological, molecular, and cultural analyses. A total of 1860 buffalo milk samples were collected from industrial, semi-industrial, and traditional buffalo farms in four major buffalo breeding provinces. The milk ring test agglutination test (MRT) was initially conducted on all milk samples, followed by culture and molecular testing for positive and negative samples in MRT. The study revealed positive results for the presence of Brucella DNA in various provinces of Iran. The MRT had a relatively low sensitivity, with results ranging from 0 to 0.7% in different provinces. However, the AMOS PCR method showed a significantly higher presence of Brucella DNA, ranging from 13 to 46% in these provinces. The highest abundance of Brucella bacterial DNA was found in Ardabil province, while the lowest was in West Azerbaijan province. Brucella abortus was the most commonly detected bacteria, followed by Brucella melitensis. Interestingly, the B. abortus vaccine strain RB51 was detected in 26.3% of positive samples of B. abortus. The culture assay of milk samples further confirmed the presence of B. melitensis biovar 1 in one sample from Khuzestan province. Overall, the study emphasizes that the AMOS PCR method is the most sensitive in detecting Brucella-exposed milk, while the sensitivity of milk sample culture and MRT is relatively lower.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brucellosis is one of the most common zoonotic infections with lifetime sterility and high rates of morbidity in animals and human (Moreno et al. 2023). Brucella abortus is the main cause of brucellosis in buffalo. The primary and most susceptible host of B. abortus is cattle, although it has the potential to infect other animals including buffaloes, sheep, goats, camels, horses, dogs, and wild ruminants. (Gentile 1957; Sousa et al. 2017; Dadar et al. 2019). Moreover, B. melitensis has been identified in the blood of buffaloes in Egypt as well (Hosein et al. 2018). Infected buffaloes can shed the bacteria during the abortion, potentially contaminating the rest of the herd and serving as a source of infection. Additionally, buffaloes are capable of excreting live Brucella spp. bacteria in their milk (Adone et al. 1998; Almashhadany 2019). The primary infection of brucellosis in ruminants is most symptomatic, with a lower incidence of frequent abortions. However, milk, vaginal and uterine discharges, as well as aborted material, typically contain a large quantity of bacteria (Radostits et al. 2007). Brucellosis in buffaloes is characterized by clinical signs such as abortions. These abortions are often accompanied by the presence of white or grayish mucoid discharges from the vagina. Generally, these abortions occur during the last trimester of pregnancy. (Das et al. 1990). Brucellosis in buffaloes can be diagnosed through both indirect and direct methods. The direct approach involves nucleic acid detection using molecular techniques and bacterial culture. Real-time Polymerase Chain Reaction (Real-time PCR) has been established as a reliable method with high levels of specificity and sensitivity for directly identifying Brucella spp. in Iranian buffalo (Dehkordi et al. 2012, 2014). The indirect method involves the use of serological techniques to detect anti-Brucella antibodies in the serum. Serological methods are ideal for this purpose due to their fast results, low cost, and convenience. The World Organisation for Animal Health (WOAH) has recommended screening tests such as the Rose Bengal test (RBT) and the milk ring test (MRT), as well as confirmatory tests like the serum agglutination test (SAT), complement fixation tests (CFT), and 2-Mercaptoethanol (2-ME) for detecting brucellosis in buffaloes, which are the same as those used for detecting it in cattle. However, Iranian studies have also mentioned other serological methods such as RBT, SAT, 2-ME, and i-ELISA for evaluating Brucella antibodies in serum samples from water buffaloes. (Azarkamand et al. 2017; Nowroozi-Asl et al. 2007). The water buffalo (Bubalus bubalis) holds significant economic importance as livestock in various regions of Iran. Based on available information, there are three primary classifications of Iranian buffaloes: the North ecotype, found in Gylan and Mazendaran provinces, the Azary ecotype, found in Ardabil and Eastern/Western Azarbaijan provinces, and the Khuzestan ecotype, found in Khuzestan province.(Dadar et al. 2021). To date, only a limited number of studies conducted in Iran have reported the presence of B. abortus and B. melitensis in the semen, blood, and aborted fetuses of buffaloes through both serological tests and real-time PCR. (Dehkordi et al. 2012, 2014; Nowroozi-Asl et al. 2007; Azarkamand et al. 2017). Additionally, there is limited information available on the prevalence of Brucella infection in the milk of water buffaloes in Iran. Consequently, it is crucial to conduct an epidemiological analysis of brucellosis in buffalo herds to establish comprehensive control and prevention measures. The main objective of this study was to assess the occurrence of Brucella spp. in industrial, semi-industrial, and traditional buffalo farms across various regions of Iran using serological, molecular, and cultural techniques.
Materials and methods
Ethical considerations
Administrative authorizations were obtained from the Iranian Veterinary Organization in Tehran, Iran to conduct this study. The dairy buffalo farmers provided their informed consent for the purpose of this study.
Sampling
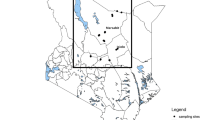
This study analyzed a total of 1860 samples of buffalo milk obtained from industrial, semi-industrial, and traditional buffalo farms in four main buffalo breeding provinces: Khuzestan province (647 samples from 13 targeted districts), West Azerbaijan province (411 samples from 11 targeted districts), East Azerbaijan province (402 samples from 13 targeted districts), and Ardabil province (400 samples from five targeted districts) (Fig. 1). These provinces were chosen because there were no documented cases of buffalo brucellosis in these regions. Additionally, these provinces are significant producers of buffalo milk and have active agricultural sectors. The sampling method employed in this study was systematic random sampling, and the sample collection spanned a year from September 2020 to September 2021. A data collection sheet was utilized to record epidemiological information on buffalo breed, age, and vaccination. All animals involved in this study were female. To obtain milk samples from each buffalo, the teats were first cleaned of manure, soil, or any other environmental debris. The teats were then disinfected by immersing them in a 30-second bath of an effective germicide (70% alcohol), and each teat was thoroughly dried with disposable tissue to ensure no residual disinfectant remained. Approximately 10 ml of milk was collected from all four teats and poured into a sterile falcon tube. The tube was then placed next to an ice bag for transportation and transferred to the Razi Vaccine and Serum Research Institute, specifically to the Brucellosis Department, for further analysis and investigation as part of this study.
Milk ring test
The antigen utilized in this test was prepared by the Razi Vaccine and Serum Research Institute, specifically the Brucellosis Department in Karaj, Iran. The procedure was carried out as previously described (Alton et al. 1975). Briefly, the milk samples and antigens were stored at room temperature (22 °C ± 4 °C). The MRT was performed by mixing 1 ml of well-mixed milk samples with 30µL of the MRT antigen in a test tube. The test was read after incubation for 60 min at 37 °C. Positive and negative controls were included for each test series. Furthermore, the negative and positive predictive values of the MRT were calculated as previously described (Alton et al. 1975).
Culture of milk samples
The milk samples from four provinces, including MRT-positive milk samples (6 samples) as well as 100 MRT-negative samples from each province (400 samples in total) were centrifuged at 2500 g for 15 min. The cream and sediment were then spread separately on a solid selective medium. Each sample was placed on a selective medium containing specific Brucella antibiotics and incubated at 37 °C under CO2 for 14 days. The specific antibiotics used in the culture medium were polymyxin B (2500 international units), bacitracin (12,500 international units), nystatin (50,000 international units), cycloheximide (50 mg), vancomycin (10 mg), and nalidixic acid (2.5 mg). If bacterial growth occurred, classical bacterial typing was performed to identify the genus and species, as described previously(Dadar et al. 2019). The isolated bacteria were typed based on their ability to produce H2S, their requirement for CO2, their reaction to agglutination by acriflavin, their reaction to agglutination by monospecific anti-M serum and anti-A serum, their susceptibility to lysis by specific phages, and their growth in the presence of different concentrations of dyes (specifically, basic fuchsin and thionine).
Molecular typing
Genomic DNA was extracted from all positive milk samples by MRT (6 samples) and 100 negative milk samples by MRT from each province (400 samples) as well as bacterial isolate using the Favorgen Biotech kit (Taiwan), following the manufacturer’s instructions. The DNA concentration was determined using the ND-1000 Nanodrop (Wilmington, DE, USA) at 260/280 nm, and the DNA integrity was assessed by running a 2% agarose gel (Lee et al. 2012). The DNA was then stored at -20 °C for further analysis. For Brucella spp., the AMOS (abortus melitensis ovis suis) polymerase chain reaction was conducted on the extracted DNA under the following PCR thermal conditions: step 1 as initial denaturation (95 °C for 5 min), step 2 as second denaturation (95 °C for 30 s), steps 3 as annealing (55 °C for 60 s), step 4 as extension (72 °C for 3 min), and step 5 as final extension (72 °C for10 min). Steps 2, 3 and 4 were repeated for 35 times (Ewalt and Bricker 2000). The PCR mixture consisted of Taq PCR Master Mix (12.5 µl of Ampliqon, Denmark), a five-primer cocktail (0.2 µM each), ddH2O (7 µl), and template DNA (20 ng). Furthermore, species-level molecular detection of isolated bacteria was performed using Bruce-ladder PCR with the following thermal conditions: step 1 as initial denaturation (95 °C for 5 min), step 2 as second denaturation (95 °C for 30 s), step 3 as annealing (56 °C for 60 s), step 4 as extension (72 °C for 3 min), and step 5 as final extension (72 °C for 10 min). Steps 2, 3, and 4 were repeated 40 times (López-Goñi et al. 2008). The PCR mixture was prepared by combining 12.5 µl of Master Mix Taq PCR (Ampliqon, Denmark), 5 µl of ddH2O, 0.5 µl of each primer from the 16-primer cocktail, and 3 µl of template DNA (10ng). The PCR products were then analyzed by electrophoresis on a 1.5% agarose gel at 90 V for 45 min. Each PCR run included a positive control consisting of B. abortus (544) and B. melitensis (16 M) genomic DNA. The DNA bands were visualized using UV illumination with the Gel Documentation-XR (BioRAD, Hercules, CA).
Differentiation between the B. abortus wild-type and the vaccine strains
Three primers were used to distinguish between wild-type and vaccine strains of B. abortus by targeting the IS711 sequence. By using the primers 5’-TTAAGCGCTGATGCCATTTCCTTCAC-3’, 5’ TTTAGTTTGCCGTAATATAGGTCTAGAACCTGTC-3’, and 5’ GCCAACCAACCCAAATGCTCACAA-3’, it was possible to detect the interruption of the wboA gene by an IS711 element in RB51. This allowed for the identification of the RB51 vaccine strain in comparison to B. abortus (Vemulapalli et al. 1999).
Statistical analysis
Data analysis
SPSS software version 22 was used for data analysis. The prevalence of brucellosis was determined by dividing the number of positive samples for each test by the total number of dairy cattle. McNemar analysis was conducted to compare the results of different tests in dairy buffalo using a paired chi-square (x2) analysis. Differential incidences were considered significant if the p-value was less than 0.05. The diagnostic coherence of MRT, culture, and PCR in brucellosis diagnosis was assessed using kappa indexes, which measure overall agreement. Cohen’s Kappa values were interpreted as follows: very good agreement for K = 0.81–1.00, good agreement for K = 0.61–0.80, moderate agreement for K = 0.41–0.60, fair agreement for K = 0.21–0.40, poor agreement for K = 0–0.20, and no agreement for K < 0.(Landis and Koch 1977). Furthermore negative predictive value and positive predictive value of RBPT, SAT and IELISA were also calculated as described previously (Sadhu et al. 2015; Hosein et al. 2017).
Results
Buffalo demographic characteristics
Samples of milk were collected from a total of 1860 buffaloes. Out of these, 647 were from Khuzestan province, accounting for 34.8% of the total. The West Azerbaijan province contributed 411 samples, representing 22.1%, while the East Azerbaijan province contributed 402 samples (21.6%). 400 samples were collected from Ardabil province, making up 21.5% of the total. The most common breed observed was the Azary ecotype (found in Western/Eastern Azarbaijan and Ardabil provinces), accounting for 65.2%. The Khuzestan ecotype was the second most predominant with a representation of 34.8%.
Prevalence of buffalo brucellosis using MRT
The overall incidence of brucellosis using MRT was 0.3% (6 out of 1860). Out of the 1213 buffaloes from the Azary ecotype in Western Azarbaijan, Eastern Azarbaijan, and Ardabil provinces, only one (0.08%) had anti-Brucella antibodies in the milk in the traditional buffalo farms. Out of the 647 buffaloes from the Khuzestan ecotype, 5 (0.7%) also had anti-Brucella antibodies in milk. However, the difference in prevalence between the two ecotypes was statistically significant (P < 0.05). The prevalence of anti-Brucella antibodies at different collection sites was not statistically significant (P > 0.05) in the Western Azarbaijan, Eastern Azarbaijan, and Ardabil provinces. However, Khuzestan province showed a significantly higher prevalence of anti-Brucella antibodies in buffalo milk (P > 0.05).
Prevalence of buffalo brucellosis using PCR
The AMOS PCR targeting IS711 was detected in 30.5% (124 out of 406) of samples. These samples included 123 from traditional buffalo farms, 1 from an industrial farm, and 1 from a semi-industrial farm. The highest infection rates were observed in Ardabil province (46.6%), followed by Khuzestan province (41.9%), Eastern Azarbaijan (20.8%), and Western Azarbaijan (13%). When analyzing the Brucella species, it was discovered that B. abortus DNA was present in 99 samples (24.4%). Co-infections with both B. abortus and B. melitensis were identified in 3.7% of samples, while B. melitensis DNA alone was found in 2.4% of samples (Fig. 2). Additional primers were utilized to discriminate between the wild-type B. abortus and the vaccine strains of RB51 used in buffalo farms. In this reaction, the positive control amplified a 400 bp fragment from field B. abortus and 900 and 1,300 bp fragments from the RB51 DNA segment corresponding to the vaccine strain. The RB51 vaccine was detected in 26.3% (26 out of 99) of milk samples. Of these, 24 samples were from Khuzestan province (92.3%), 1 sample was from Eastern Azarbaijan (3.85%), and 1 sample was from Western Azarbaijan (3.85%). Of the 26 samples, 15 samples only showed the presence of RB51 vaccine DNA, while nine samples showed the presence of both RB51 vaccine DNA and B. abortus DNA simultaneously (Fig. 3).
1% agarose gel was stained with a safe stain to visualize Brucella spp. specific PCR products in milk samples collected from buffaloes. The gel image shows lanes 1–16 representing the buffalo milk samples. The 1 Kb DNA ladder marker (Fermentas, USA) was loaded in the M lane. Lanes 1, 2, 3, 4, 12, 13, 14, and 16 displayed a band of 400 bp, indicating the presence of B.abortus. Lanes 5, 6, 7, 8, 9, 10, 11, and 15 exhibited bands at 400 bp, 900 bp, and 1300 bp, were indicating the presence of both RB51 and B.abortus. The RB51 vaccine and B.ab were used as reference controls for vaccine and B.abortus strain 544 respectively, while C- was used as the negative control. The positive control of B. abortus (B.ab) amplified a 400 bp fragment and the RB51 DNA segment corresponding to the vaccine strain amplified 900 and 1,300 bp fragments from vaccine strain
Prevalence of buffalo brucellosis using milk culture
Overall, the incidence of brucellosis based on milk culture was 0.2% (1/406). Out of 406 buffaloes from the Azary ecotype in Western Azarbaijan, Eastern Azarbaijan, Ardabil, and Khuzestan provinces, only one milk sample from a traditional buffalo farm in Khuzestan province tested positive in the milk culture (Fig. 4). The bacteria isolated from this sample showed agglutination with antiserum M but not with acriflavin and antiserum (A) After 48 h of culture, the colony size of the isolated bacteria was one to two mm. The colonies were translucent and shiny, with a faint blue halo under light. Gram staining revealed red coccobacilli. The isolated bacteria were able to grow in the presence of thionin and fuchsia dyes. Additionally, no production of H2S was observed when the isolated bacteria were exposed to lead acetate paper. The bacteria isolated from buffalo milk samples also showed resistance to phage Tb (RTD dilution and RTD×104 dilution), indicating non-lysis. Based on these characteristics, the isolated Brucella spp. were identified as (B) melitensis biovar 1.
Comparison of culture, MRT and PCR results
The overall prevalence of brucellosis, as determined by MRT, culture, and PCR, was 0.32%, 0.25%, and 30.54% respectively (see Table 1). Out of the six samples that tested positive using MRT, all six (100%) were also confirmed positive by PCR, while none (0%) were PCR negative (see Table 1). Out of the 400 samples that tested negative using MRT, 118 (4.7%) were PCR positive and 282 (95.3%) were PCR negative. The difference in results between PCR and MRT assays was statistically significant (p < 0.05) (Table 2).
The assays were validated by amplifying the positive control samples of B. abortus and B. melitensis, resulting in the production of bands of 498 and 731 bp, respectively. In all provinces, the prevalence of B. abortus was found to be higher than that of B. melitensis. There was no significant difference observed between the provinces in terms of the prevalence of B. abortus and B. melitensis. However, it was noted that the simultaneous prevalence of B. abortus and B. melitensis was higher in Ardabil province compared to the other provinces. The isolated bacterium was confirmed to be wild-type B. melitensis based on the findings from the AMOS-PCR test, where a PCR product of 731 bp was obtained. Additionally, the Bruce-ladder test also identified the isolated bacterium as wild-type B. melitensis, as evidenced by the presence of PCR products with sizes of 1682, 794, 587, 450, 152, and 1,071 bp.
Kappa index and comparison of agreement between Culture, MRT and PCR methods with McNemar’s test
The Kappa statistical analysis indicated that there was poor agreement between culture and PCR (k = 0.0112) and between MRT and PCR (k = 0.0259). However, a fair agreement was observed between culture and MRT tests (k = 0.222). McNemar analysis showed a significant difference in the positive and negative results obtained using different methods. Furthermore, our findings suggest that PCR occasionally yields false positives as a result of detecting the RB51 vaccine, despite generally demonstrating higher sensitivity than culture and MRT (Table 3).
Discussion
Water buffaloes that originally come from India and China have successfully spread across the globe and have become a significant and valued source of food. Through experimental research, it has been discovered that female water buffaloes can contract brucellosis by consuming B. abortus (Xavier et al. 2009). Keeping various livestock in close proximity can also promote bacterial transmission. Moreover, since water buffaloes are accustomed to walking in muddy areas, they may play a significant role in the spread of brucellosis (Catozzi 2020). Moreover, the prevalence of disease in buffalo populations is significantly influenced by the level of natural genetic resistance. Notably, the presence of the natural macrophage resistance-associated protein 1 gene (Nramp1) is closely associated with brucellosis resistance in cattle (Adams and Templeton 1998). The Nramp1BB genotype has been shown to offer protective effects in buffaloes against brucellosis. Therefore, it is anticipated that the prevalence of this genotype will influence the incidence of brucellosis in these animals (Borriello et al. 2006). However, it has been revealed that the genotype of Nramp1AA confers susceptibility to B. abortus (Capparelli et al. 2007). Furthermore, significant alteration of lymphocytes has been reported in buffalo with brucellosis (Grandoni et al. 2023). Despite the role of buffalo milk as a source of infection in humans, few studies have been investigated on the presence of Brucella spp. in the milk of buffaloes in Iran. The aim of this study was to investigate the presence of Brucella spp. in milk samples of buffaloes in Iran. In southern Iran, buffalo breeding and milk production are important sources of income (Dadar et al. 2021). This study revealed the overall individual seroprevalence of dairy buffalo brucellosis in Iran at low levels of 0.3% through MRT. The overall occurrence is lower than that in the previous studies from Iraq (Abbas and Aldeewan 2009; Almashhadany 2019), where the prevalence of Brucella antibodies in milk ranged from 7.5 to 24.2% using MRT assay in buffaloes. A study conducted in the Kurdistan region of Iraq involved the random collection of 80 buffalo milk samples from lactating females. The results indicated a 7.5% overall prevalence of Brucella antibodies. The study recommended utilizing MRT as a standard screening tool for brucellosis in dairy factories, farms, and milk collection centers (Almashhadany 2019). In another study conducted in Basra province, Iraq, researchers collected a total of four hundred and twenty samples of raw buffalo milk from various sites. These samples were then assessed using the MRT method to detect the presence of Brucella antibodies. The findings revealed that approximately 24.2% of the samples tested positive for these antibodies (Abbas and Aldeewan 2009). In Pakistan, a comprehensive study was conducted to assess the presence of B. melitensis and B. abortus in both buffalo and bulk tank samples. The evaluation involved analyzing a total of 300 samples, utilizing MRT and ELISA commercial kits (I-ELISA). Among the milk samples collected from buffaloes, the prevalence of the pathogens was found to be 15%. To ascertain the accuracy of MRT, Milk I-ELISA was used as the standard golden test. The results showed that MRT exhibited a sensitivity of 78.9% and a specificity of 100% for buffalo samples. Overall, this research sheds light on the prevalence and diagnostic performance of B. melitensis and B. abortus in Pakistan’s buffalo population, offering valuable insights for potential control and prevention measures (Khan et al. 2018). Although, Chand and his colleagues reported that MRT has less sensitivity in diagnosing B. melitensis (Chand et al. 2005), it is very useful in cows. However, the reliability of the experiment decreased when dealing with large herds, specifically those with more than 100 lactating cows. Additionally, it is essential to consider that MRT (Milk Ring Test) can produce false-positive results due to various factors. These factors include mastitis, colostrum presence, milk samples collected towards the end of lactation, as well as hormonal disorders. (Mahajan et al. 2011). The presence of Brucella spp. contamination in Tabriz City was investigated by analyzing 40 buffalo milk samples using the i-ElISA and MRT assays. The study revealed that 12 samples (14.11%) were contaminated with Brucella spp. These findings suggest that there is a significant level of Brucella spp. contamination in the milk distributed throughout the city. Therefore, it is crucial to take proactive measures and implement careful and proper planning to prevent the distribution of contaminated milk (Azarkamand et al. 2017). Furthermore, regulation of One Health approaches through strict control of animal, human, and environment health proposed to tackle brucellosis and control the reemerged brucellosis in buffaloes (Mazzeo et al. 2023). The findings of our study revealed a notably lower prevalence of Brucella in milk samples compared to previous studies conducted in Iran and neighboring countries. Interestingly, the study identified the highest incidence of positive MRT results in buffalo milk samples from Khuzestan province, closely followed by East Azerbaijan. However, both Ardebil and West Azerbaijan demonstrated zero positive MRT results. It’s worth noting that Brucella spp., being slow-growing bacteria, were the isolates obtained and identified in this study, specifically as B. melitensis biovar 1. These results align with previous findings, as B. melitensis biovar 1 has been previously reported in milk samples from a buffalo herd in Sicily(Adone et al. 1998). Brucella species and biotypes may vary from one area to another in buffaloes. In India (Das et al. 1990) and Italy (Di Giannatale et al. 2008), B. abortus biovar 1 was detected in buffaloes. In Turkey, B. abortus biovar 3 was isolated from buffaloes (Özen et al. 2021). In Argentina, B. abortus biovar 5 has also been reported in an aborted buffalo fetus(Martínez et al. 2014). In another study, B. abortus biovar 6 was identified in Italian buffaloes (Adone et al. 1998). However, the isolation of Brucella is difficult, time-consuming, and potentially dangerous for laboratory workers. Therefore, most recent studies have used culture-independent diagnostic methods, such as ELISA and PCR to detect infection (Amoroso et al. 2011; Fusco et al. 2009; Baltazar-Pérez et al. 2022). In this study, we evaluated the feasibility of PCR as a diagnostic tool to detect Brucella species in buffalo milk with positive and negative milk ring tests. The results showed that all the negative samples in the MRT were also negative in microbial culture, while out of 400 negative milk samples in the MRT, 118 samples (29.5%) were positive in the PCR test. All positive samples in the MRT showed positive results in the PCR assay. The presence of Brucella DNA in buffalo milk alone does not indicate contamination and can be caused by exposure of buffaloes to infected animals or the excretion of the vaccine in buffalo milk. The results of the present study also showed agreement with those of Dehkordi et al., which showed that the highest prevalence of Brucella spp. DNA belonged to B. abortus through the TaqMan real time PCR (Dehkordi et al. 2012). He showed the sensitivity and specificity of the real-time PCR method was 100%. Moreover, in agreement to our results, statistical analysis of Dehkordi et al., showed a significant difference between B. abortus and B. melitensis. The results showed that PCR is significantly faster than the current standard methods for the isolation of Brucella species (Dehkordi et al. 2014). Molecular investigations of Brucella spp. were performed on Iranian buffalo sperm samples by PCR and real-time PCR. DNA samples were extracted and primers were designed using the IS711 target for PCR. In total, 14.28% of sperm samples were positive in Brucela species. A total of 14 (15.3%) and 1 (1.1%) of the sperm samples were positive for B. abortus and B. melitensis, respectively (Dehkordi et al. 2014). In another study, the milk of seropositive and seronegative buffaloes was tested using PCR and Real-time PCR methods, and the molecular findings were compared with the results of bacteriological and serological tests. They showed that although culture had higher sensitivity than PCR tests, some buffaloes were positive by serological tests and negative by PCR testing and milk culture. Therefore, the mutual use of molecular and bacteriological methods has increased the detection of positive samples (Marianelli et al. 2008). However, detection limits for Brucella in the milk of cow is variable in different literature from 2.8 × 104 cfu/mL (Rijpens et al. 1996) to 2 × 103 cfu/mL (Sreevatsan et al. 2000), and between 5 and 50 cfu/mL(Romero and Lopez-Goñi 1999). Furthermore, different limits have been observed for various Brucella species. A detection limits of 1000 cfu/ml for B. melitensis and 100 cfu/ml for B. abortus were reported in a study on milk samples that artificially infected with B. melitensis or B. abortus (Hamdy and Amin 2002; Romero and Lopez-Goñi 1999). Therefore, various factors such as the Brucella species tested, the DNA extraction protocol, the amount of sample processed, and the molecular assay used may affect PCR sensitivity (Marianelli et al. 2008).
B. abortus vaccine strain RB51 in the milk of water buffaloes was also detected in 26.3% of the positive samples of B. abortus in Khuzestan province (92.3%), Eastern Azarbaijan (3.85%), and Western Azarbaijan (3.85%). Similarly, the RB51 strain isolated from buffalo milk farms in southern Italy posed the risk of this vaccine to milk consumers following illegal vaccination or public health implications for farm workers (Averaimo et al. 2022). B. abortus RB51 is naturally resistant to rifampin and can be caused serious infection in humans. Under experimental conditions, the shedding of RB51 in milk has been reported in water buffalo only after a triple dose injection of vaccine during the first week (Longo et al. 2009). Our results showed the first identification of the RB51 vaccine in the milk of water buffalo under field conditions in Iran. These results demonstrate that illegal administration of the B. abortus RB51 vaccine in buffaloes might cause public health concerns. In our study, we evaluated the combined use of serological, molecular, and cultural tests for diagnosing brucellosis in a buffalo population across different regions of Iran. By comparing serological and bacteriological tests, we found that only one out of 1860 buffaloes tested positive for infection (positive serology and positive culture). Additionally, five animals showed positive serum results but negative culture, suggesting potential exposure to infected animals without actual infection. This highlights the importance of using multiple diagnostic methods since relying on a single test could lead to overestimating the true prevalence of Brucella. Serological tests, despite being the main diagnostic method for brucellosis screening in the field, have limitations. They cannot distinguish between animals that are truly infected and those that have been vaccinated, which is crucial for an accurate diagnosis. To address this issue, we propose the simultaneous use of two direct detection methods (culture and PCR) along with an indirect detection method (MRT). This combined approach proves to be more effective in detecting Brucella in buffalo milk compared to relying on a single test alone.
Conclusion
In conclusion, our results indicate that employing a combination of diagnostic techniques yields better outcomes for identifying Brucella infections in buffalo populations. By utilizing both direct and indirect methods, we can improve the accuracy of diagnosis and subsequently enhance the management and control of brucellosis in these animals. In order to reduce the risk of brucellosis in Iranian buffalo, it is important to implement effective control measures such as vaccination and improved husbandry practices. Additionally, it is important to create awareness among farmers about the risk of brucellosis and the need for regular testing of their animals. The results of this study indicate that Brucella spp. is present in buffalo milk in Iran, and there is a need for further studies to investigate the prevalence of the disease in buffaloes in the country. In addition, appropriate control measures should be taken to reduce the risk of transmission of Brucella spp. from buffalo milk to humans.
Data Availability
Data and materials are available upon request.
Data Availability
The data supporting the results reported in this study are available upon request from the corresponding author.
References
Abbas B, Aldeewan A (2009) Occurrence and epidemiology of Brucella spp. in raw milk samples at Basrah province, Iraq. Bulg J Vet Med 12:136–142
Adams L, Templeton J (1998) Genetic resistance to bacterial diseases of animals. Rev sci tech 17:200–219. https://doi.org/10.20506/rst.17.1.1085
Adone R, Ciuchini F, Cerri D, Andreani E, Lillini E (1998) Isolation of Brucella melitensis biovar 1 from milk samples of seropositive buffaloes [Sicily]. Selezione Veterinaria (Italy)
Almashhadany D (2019) The significance of milk ring test for identifying Brucella antibodies in cows and buffaloes’ raw milk at Erbil governorate, Kurdistan region, Iraq. Iraqi J Vet Sci 33:395–400. https://doi.org/10.33899/ijvs.2019.163085
Alton G, Maw J, Rogerson B, McPherson G (1975) The serological diagnosis of bovine brucellosis: an evaluation of the complement fixation, serum agglutination and rose bengal tests. Aust Vet J 51(2):57–63. https://doi.org/10.1111/j.1751-0813.1975.tb09404.x
Amoroso MG, Salzano C, Cioffi B, Napoletano M, Garofalo F, Guarino A, Fusco G (2011) Validation of a real-time PCR assay for fast and sensitive quantification of Brucella spp. in water buffalo milk. Food Control 22:1466–1470. https://doi.org/10.1016/j.foodcont.2011.03.003
Averaimo D, De Massis F, Savini G, Garofolo G, Sacchini F, Abass A, Tittarelli M, Migliorati G, Petrini A (2022) Detection of Brucella abortus Vaccine strain RB51 in Water Buffalo (Bubalus bubalis) milk. Pathogens 11:748. https://doi.org/10.3390/pathogens11070748
Azarkamand B, Pourmahdi Borujeni M, Gharibi D, Ghorbanpour M (2017) Comparison of serological methods for the diagnosis of brucellosis in water buffalo. Iran J Vet Res 13:5–12. https://doi.org/10.22055/IVJ.2017.36718.1612
Baltazar-Pérez J, Utrera-Quintana F, Camacho-Ronquillo J, González-Garduño R, Jiménez-Cortez H, Villa-Mancera A (2022) Prevalence of Neospora caninum, Toxoplasma gondii and Brucella abortus in water buffalo (Bubalus bubalis): climatic and environmental risk factors in eastern and southeast Mexico. Microb Pathog 173:105871. https://doi.org/10.1016/j.micpath.2022.105871
Borriello G, Capparelli R, Bianco M, Fenizia D, Alfano F, Capuano F, Ercolini D, Parisi A, Roperto S, Iannelli D (2006) Genetic resistance to Brucella abortus in the water buffalo (Bubalus bubalis). Infect Immun 74:2115–2120. https://doi.org/10.1128/IAI.74.4.2115-2120.2006
Capparelli R, Borriello G, Marabelli R, Roperto S, Roperto F, Iannelli D (2007) The Nramp1 AA genotype confers susceptibility to Brucella abortus in water buffalo. Mamm Genome 18:137–143. https://doi.org/10.1007/s00335-006-0103-x
Catozzi C (2020) Water buffalo microbiota and immunity during infectious diseases
Chand P, Rajpurohit B, Malhotra A, Poonia J (2005) Comparison of milk-ELISA and serum-ELISA for the diagnosis of Brucella melitensis infection in sheep. Vet Microbiol 108(3–4):305–311. https://doi.org/10.1016/j.vetmic.2005.04.006
Dadar M, Alamian S, Behrozikhah AM, Yazdani F, Kalantari A, Etemadi A, Whatmore AM (2019) Molecular identification of Brucella species and biovars associated with animal and human infection in Iran. Vet Res Forum 10:315–321. https://doi.org/10.30466/vrf.2018.89680.2171
Dadar M, Wareth G, Neubauer H (2021) Brucellosis in iranian buffalo. Ger J Vet Res 1:13–16. https://doi.org/10.51585/gjvr.2021.2.0009
Das V, Paranjape V, Corbel M (1990) Investigation of brucellosis-associated abortion in dairy buffaloes and cows in Bombay. Indian J Anim Sci 60:1193–1194
Dehkordi FS, Saberian S, Momtaz H (2012) Detection and segregation of Brucella abortus and Brucella melitensis in aborted bovine, ovine, Caprine, Buffaloes and Camelid fetuses by application of conventional and real-time polymerase chain reaction. Thai J Vet Med 42:13
Dehkordi FS, Khamesipour F, Momeni M (2014) Brucella abortus and Brucella melitensis in iranian bovine and buffalo semen samples: the first clinical trial on seasonal, senile and geographical distribution using culture, conventional and real-time polymerase chain reaction assays. Kafkas Univ Vet Fak Dergisi 20:821–828
Di Giannatale E, De Massis F, Ancora M, Zilli K, Alessiani A (2008) Typing of Brucella field strains isolated from livestock populations in Italy between 2001 and 2006. Vet Ital 44:383–388
Ewalt DR, Bricker BJ (2000) Validation of the abbreviated BrucellaAMOS PCR as a Rapid Screening Method for differentiation of Brucella abortus Field strain isolates and the vaccine strains, 19 and RB51. J Clin Microbiol 38:3085–3086. https://doi.org/10.1128/JCM.38.8.3085-3086.2000
Fusco G, Amoroso M, Tavano R, Corrado F, Conte F, Iovane G, Capuano F (2009) Identification of Brucella sp. by real time PCR in buffalo milk: preliminary data. In: XI Congresso Nazionale SI Di. LV, Parma, Italia, 30 settembre-2 ottobre 2009, 2009. Società Italiana di Diagnostica di Laboratorio Veterinaria (SIDiLV), pp 146–147
Gentile A (1957) Brucellosis in buffaloes. Vet Ital 8:591–596
Grandoni F, Signorelli F, Martucciello A, Napolitano F, De Donato I, Donniacuo A, Di Vuolo G, De Matteis G, Del Zotto G, Davis WC, De Carlo E (2023) In-depth immunophenotyping reveals significant alteration of lymphocytes in buffalo with brucellosis. Cytometry Part A 103:528–536. https://doi.org/10.1002/cyto.a.24710
Hamdy ME, Amin A (2002) Detection of Brucella species in the milk of infected cattle, sheep, goats and camels by PCR. VET J 163:299–305. https://doi.org/10.1053/tvjl.2001.0681
Hosein H, Rouby S, Menshawy A, AbdAl-Ghany A (2017) Sensitivity and specificity of the commonly used diagnostic procedures of bovine brucellosis. Vet Sci 3:45–52. https://doi.org/10.17582/journal.vsrr/2017.3.3.45.52
Hosein H, Zaki HM, Safwat NM, Menshawy AM, Rouby S, Mahrous A, Madkour BE-d (2018) Evaluation of the General Organization of Veterinary Services control program of animal brucellosis in Egypt: an outbreak investigation of brucellosis in buffalo. Veterinary World 11:748. https://doi.org/10.14202/vetworld.2018.748-757
Khan TI, Ehtisham-ul-Haque S, Waheed U, Khan I, Younus M, Ali S (2018) Milk Indirect-ELISA and milk Ring Test for Screening of Brucellosis in Buffaloes, Goats and Bulk Tank milk samples collected from two districts of Punjab, Pakistan. Pak Vet J 38:105–108. https://doi.org/10.29261/pakvetj/2018.021
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174. https://doi.org/10.2307/2529310
Lee PY, Costumbrado J, Hsu CY, Kim YH (2012) Agarose gel electrophoresis for the separation of DNA fragments. JoVE (Journal of Visualized Experiments) 20:e3923. https://doi.org/10.3791/3923-v
Longo M, Mallardo K, Montagnaro S, De Martino L, Gallo S, Fusco G, Galiero G, Guarino A, Pagnini U, Iovane G (2009) Shedding of Brucella abortus rough mutant strain RB51 in milk of water buffalo (Bubalus bubalis). Prev Vet Med 90:113–118. https://doi.org/10.1016/j.prevetmed.2009.03.007
López-Goñi I, García-Yoldi D, Marin C, De Miguel M, Munoz P, Blasco J, Jacques I, Grayon M, Cloeckaert A, Ferreira A (2008) Evaluation of a multiplex PCR assay (Bruce-ladder) for molecular typing of all Brucella species, including the vaccine strains. J Clin Microbiol 46:3484–3487. https://doi.org/10.1128/JCM.00837-08
Mahajan S, Agrawal R, Pande N (2011) A comparative evaluation of different diagnostic tests for brucellosis in buffaloes. Buffalo Bull 30:75–78
Marianelli C, Martucciello A, Tarantino M, Vecchio R, Iovane G, Galiero G (2008) Evaluation of molecular methods for the detection of Brucella species in water buffalo milk. J Dairy Sci 91:3779–3786. https://doi.org/10.3168/jds.2008-1233
Martínez D, Thompson C, Draghi G, Canavesio V, Jacobo R, Zimmer P, Elena S, Nicola AM, de Echaide ST (2014) Pheno-and genotyping of Brucella abortus biovar 5 isolated from a water buffalo (Bubalus bubalis) fetus: first case reported in the Americas. Vet Microbiol 173:172–176. https://doi.org/10.1016/j.vetmic.2014.07.011
Mazzeo A, Tremonte P, Rossi N, Ferrara C, Mascolo C, Lombardi SJ, Sorrentino E (2023) Modulation of the One Health Approach to Tackle Brucellosis in Buffaloes and cattle in two Italian Territories with different characteristics. J Buffalo Sci 12:55–69. https://doi.org/10.6000/1927-520X.2023.12.07
Moreno E, Middlebrook EA, Altamirano-Silva P, Al Dahouk S, Araj GF, Arce-Gorvel V, Arenas-Gamboa Á, Ariza J, Barquero-Calvo E, Battelli G, Bertu WJ (2023) If you’re not confused, you’re not paying attention: Ochrobactrum is not Brucella. J Clin Microbiol 61:e00438–e00423. https://doi.org/10.1128/jcm.00438-23
Nowroozi-Asl A, Oliaei A, Poormahmood-Shalgahian M (2007) A serological survey of brucella spp. in water buffalo in Khoozestan province, Iran. Ital J Anim Sci 6:825–827. https://doi.org/10.4081/ijas.2007.s2.825
Özen H, İlhan Z, Usta M, Karaman M, İlhan F (2021) Brucellosis in a water buffalo (Bubalus bubalis) herd in Balikesir province of Turkey: a bacteriological and pathological investigation. Thai J Vet Med 51:133–140. https://he01.tci-thaijo.org/index.php/tjvm/article/view/247441
Radostits O, Gay C, Hinchcliff K, Constable P (2007) Diseases associated with bacteria. Veterinary Medicine: a textbook of the diseases of cattle, horses, sheep, pigs, and goats. Can Vet J 10:1007–1060
Rijpens NP, Jannes G, Van Asbroeck M, Rossau R, Herman L (1996) Direct detection of Brucella spp. in raw milk by PCR and reverse hybridization with 16S-23S rRNA spacer probes. Appl Environ Microbiol 62:1683–1688. https://doi.org/10.1128/aem.62.5.1683-1688.1996
Romero C, Lopez-Goñi I (1999) Improved method for purification of bacterial DNA from bovine milk for detection of Brucella spp. by PCR. Appl Environ Microbiol 65:3735–3737. https://doi.org/10.1128/AEM.65.8.3735-3737.1999
Sadhu DB, Panchasara H, Chauhan H, Sutariya D, Parmar V, Prajapati H (2015) Seroprevalence and comparison of different serological tests for brucellosis detection in small ruminants. Vet World 8:561. https://doi.org/10.14202/vetworld.2015.561-566
Sousa MG, Salvarani FM, Bomjardim HA, Brito MF, Barbosa JD (2017) Brucellosis in water buffaloes. Pesqui Vet Bras 37:234–240. https://doi.org/10.1590/s0100-736x2017000300006
Sreevatsan S, Bookout JB, Ringpis F, Perumaalla VS, Ficht TA, Adams LG, Hagius SD, Elzer PH, Bricker BJ, Kumar GK (2000) A multiplex approach to molecular detection of Brucella abortus and/or Mycobacterium bovis infection in cattle. J Clin Microbiol 38:2602–2610. https://doi.org/10.1128/JCM.38.7.2602-2610.2000
Vemulapalli R, McQuiston JR, Schurig GG, Sriranganathan N, Halling SM, Boyle SM (1999) Identification of an IS711 element interrupting the wboA gene of Brucella abortus vaccine strain RB51 and a PCR assay to distinguish strain RB51 from other Brucella species and strains. Clin Diagn lab Immunol 6:760–764. https://doi.org/10.1128/CDLI.6.5.760-764.1999
Xavier M, Paixão T, Poester F, Lage A, Santos R (2009) Pathological, immunohistochemical and bacteriological study of tissues and milk of cows and fetuses experimentally infected with Brucella abortus. J Comp Pathol 140:149–157. https://doi.org/10.1016/j.jcpa.2008.10.004
Acknowledgements
The authors would like thanks Amin Nemati Azar, Jafar Shirazi, Mehran Pedernejad, Mohammad Antik Chi for kindly providing the milk samples from differnt parts of Iran.
Funding
This work was supported by a grant from the Razi vaccine and serum research institute (Grant # 3-18-1857-005-000134).
Author information
Authors and Affiliations
Contributions
Maryam Dadar and Akram Bahrainipour designed and funded the study; Maryam Dadar, Akram Bahrainipour, Karim Amiri, Faranak Abnaroodheleh and Saeed Alamian performed the experiments; Maryam Dadar and Ali Reza Yousefi analyzed and interpreted the results; The first draft and final draft of the manuscript was written by Maryam Dadar and all authors commented and contributed to the preparation of the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All milk samples were obtained following the procedures on Institutional Animal Care and Use Committee from Iranian veterinary organization by Ethics Committee (Approval number, 99030922; Approval date, 22Feb2020). All methods were performed in accordance with the relevant guidelines and regulations. Before beginning work on the present study, we contacted the buffalo owners and written informed consent was obtained from the owners for the participation of their animals in this study. During collecting milk specimens, these animals were not disturbed.
Consent to participate
Written informed consent was obtained from the buffalo owners.
Consent to publish
Buffalo-owners signed informed consent regarding publishing their animals’ data.
Conflict of Interest
The authors declare no conflict of interest.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dadar, M., Bahreinipour, A., Alamian, S. et al. Serological, cultural, and molecular analysis of Brucella from Buffalo milk in various regions of Iran. Vet Res Commun 48, 427–436 (2024). https://doi.org/10.1007/s11259-023-10228-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-023-10228-5