Abstract
Microorganisms can interfere with meat quality, being a public health problem. The aim of this study was to characterize the antimicrobial susceptibility profile of Staphylococcus spp. isolated from utensils of a bovine slaughterhouse and to evaluate the antibacterial and antibiofilm activity of the essential oil of Rosmarinus officinalis L. (rosemary). Samples of surfaces and utensils used during slaughter in the northwest of the state of Paraná, Brazil were collected. After isolation and differentiation of the isolates by the coagulase test, the antimicrobial susceptibility test, Staphylococcus aureus identification and mecA gene research were performed. The study for biofilm production was carried out by the method of adhesion in borosilicate tube and by adhesion in polystyrene plate. Subsequently, the inhibitory activity of the R. officinalis essential oil and its ability to inhibit biofilm were investigated. Twenty-two of the samples collected were identified as coagulase-negative Staphylococcus and five as coagulase-positive Staphylococcus. There was resistance to all antibiotics tested, with clindamycin (33.33%) and rifampicin (29.6%) showing the highest rate. None of the samples was confirmed as Staphylococcus aureus or for the presence of the mecA resistance gene. The essential oil inhibited the growth of 48% of the isolates at a concentration of 16,000 µg/mL. Of these isolates, 33% were positive for biofilm production and this biofilm was also inhibited by the essential oil. This work revealed that multidrug-resistant Staphylococcus and biofilm producers are present in the slaughter environment and are susceptible to the essential oil of R. officinalis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the year 2021 alone, the world produced about 61.5 million tons of beef. As Brazil is the second largest producer in the world, its production corresponds to 17% of world production, just behind the United States, responsible for 20% of world production (Agrosaber 2021; Brasil 2021; IBGE 2021).
But in addition to producing, it is necessary for this food to be safe, being essential for the health and well-being of consumers. The hygienic-sanitary quality of meat products depends on standards to be followed at all points in the production chain and their origin must be recognized by regulatory agencies, so that their safety is not affected by technologies and inadequate handling (Santos and Gonçalves 2010; Xavier and Joele 2004). The microbiological contamination of meat and its derivatives is a threat to public health, these contaminants are commonly from the skin, feces and intestinal contents, being essential the control of these microorganisms to guarantee the quality of the meat (Hoffmann et al. 2002).
Staphylococcus are a group of bacteria important worldwide because they present several mechanisms of virulence and resistance. According to Peixoto et al. (2015), Staphylococcus spp. are recognized as the most common cause of infections associated with biofilms, which is a structure responsible for survival and often resistance to the action of disinfectant products. These difficult-to-remove cell communities represent a challenge for the food industries, leading to important health problems and economic losses (Xavier et al. 2003; Ouyang et al. 2012).
Cleaning and disinfection are extremely important to control contamination during production, they must be carried out regularly to eliminate most of the contaminating. The use of natural antimicrobial compounds has increased due to changes in consumer attitudes towards the use of synthetic food preservation agents, detergents and sanitizers that may impact the environment (Lebert et al. 2007).
Rosmarinus officinalis popularly known as rosemary is an aromatic and perennial shrub grown throughout Brazil. Porte and Godoy (2001) report that the use of natural substances such as rosemary essential oil has been highlighted in microbial inhibition, having already been used in sanitizing solutions with effectiveness in reducing cells adhered to surfaces. Other works also report the use of R. officinalis oil in the meat industry as promising. (Ntzimani et al. 2010; Kahraman et al. 2015; Badia et al. 2020) however, there are few reports on the application of this essential oil as an inhibitor of antibiotic-resistant bacteria and an inhibitor of biofilm formation associated with the slaughter environment.
Therefore, this work aimed to characterize the sensitivity profile of Staphylococcus spp. isolated from surfaces and slaughtering utensils against conventional antimicrobials, evaluate their potential for biofilm production, and test the antibacterial and antibiofilm efficiency of Rosmarinus officinalis essential oil.
Material and methods
Study location and definition of the number of samples
In total 41 swabs were collected from utensils and surfaces used during the slaughtering process of a small slaughterhouse in a city in the northwest of the state of Paraná. The samples came from knives, sharpening steels, white and red viscera gutters and tables (one swab each) on a day of high slaughter volume in July 2021. During collection, circular movements were performed with the swab to cover the entire utensil, and then it was inserted into 3 mL of Brain Heart Infusion (BHI) medium and transported refrigerated to the laboratory.
Bacterial culture and isolation
On the same day of collection, the tubes containing BHI (Kasvi, Italy) medium were incubated in an oven at 37 °C for 24 h. After this period, the cultures obtained were streaked onto plates containing Mannitol Salt agar (Kasvi, Italy) and incubated at 37 °C for 24 h for the isolation of aerobic bacteria. The predominant colonies on each plate were isolated and subsequently cultured in BHI medium, incubated (37 °C/24 h) and subsequently stored in BHI plus 10% glycerol at -20 °C.
Identification of isolates
Each isolate was submitted to the analysis of macroscopic characteristics and Gram stain to visualize the morphology. Subsequently, catalase and coagulase tests were performed to differentiate between coagulase-positive Staphylococcus and coagulase-negative Staphylococcus (Quinn et al. 1994).
Antimicrobial susceptibility test
Antimicrobial susceptibility tests were performed using the agar disk diffusion methodology according to the criteria established by the Brazilian Antimicrobial Sensitivity Testing Committee (BRCAST 2020). Single colonies were seeded on BHI medium for overnight growth. On the day of the experiments, the bacterial inoculum was standardized according to the 0.5 McFarland scale and the bacterial suspension was seeded on plates containing Mueller Hinton agar (Kasvi, Italy) with the aid of a swab. The discs impregnated with antimicrobials chosen based on the “Categorization of antibiotics for prudent and responsible use in animals” of the European Medicines Agency (EMA 2019), being them; amikacin (30 µg), amoxicillin (10 µg), amoxicillin + clavulanic acid (20/10 µg), cefoxitin (30 µg), ceftiofur (30 µg), clindamycin (2 µg), chloramphenicol (30 µg), erythromycin (15 µg), meropenem (10 µg), norfloxacin (10 µg), oxacillin (1 µg), rifampicin (5 µg) were placed and the plates incubated (37 °C/18–24 h). Inhibition halos were measured (mm) and the results obtained were recorded.
Identification of Staphylococcus aureus
DNA was extracted using the PureLink® Genomic DNA Kit (Invitrogen, USA) following the manufacturer's recommendations. Polymerase chain reaction (PCR) was performed for coagulase positive Staphylococcus samples to verify which of these isolates were Staphylococcus aureus. The reactions were performed according to the parameters of Martineau et al. (1998) using the primer Sa442-1 (5'-AAT CTT TGT CGG TAC ACGATA TTC TTC ACG-3' and the primer Sa442-2 (5'-CGT AAT GAGATT TCA GTA GAT AAT ACA ACA-3'). The amplification of the products was visualized by electrophoresis on a 2% agarose gel stained with GelRed 10,000 x (Biotium, USA) using a 100 bp molecular marker and the products visualized as a single band of 241 bp.
Detection of the mecA gene
PCR reactions were performed on samples of Staphylococcus spp. resistant to oxacillin and cefoxitin by the antibiogram test and the DNA was also obtained using the commercial kit. The primers mecA1 (5'-AAA ATC GAT GGT AAA GGT TGG-3') and mecA2 (5'-AGT TCT GCA GTA CCG GAT TTG-3') were used – employing the parameters described by Murakami et al. (1991); and amplification of the 533 bp product was visualized on 2% agarose gel stained with gel red.
Study of biofilm production
Biofilm production research by the borosilicate tube adhesion method
The investigation of biofilm production was carried out using the method of Christensen et al. (1982) with modifications. The isolated staphylococcal colonies were inoculated into tubes containing 2.0 mL of Trypticase Soy broth (TSB) (Kasvi, Italy) and incubated at 37 °C for 48 h, without shaking. Subsequently, the contents were discarded and 1.0 mL aliquots of 0.4% aqueous crystal violet solution were added to each tube. After gentle agitation, to ensure the staining of the material adhered to the inner surface of the tubes, the dye was discarded. The positive result was indicated by the presence of a layer of colored material, adhered to the inner wall of the tubes. The presence of a colored ring only on the liquid–air contact surface was not considered a positive result.
Research on the production of biofilm by the adhesion method on a polystyrene plate
The biofilm production research method on plaques proposed by Christensen et al. (1985) was used with some modifications. This method has spectrophotometric bases, based on the reading of the optical density (OD) of the adherent material produced by the bacterium. Cultures in TSB were used, incubated for 24 h and later diluted 1:1 with TSB, prepared with 2% glucose. Flat-bottomed 96-well plates were used. The wells were filled in quadruplicate with 200μL of the diluted culture, using a multichannel pipette. In all tests, one sample was used as a positive control, one as a negative control and one with sterile TSB. The plates were incubated for 24 h at 37 °C and then the contents of each well were carefully aspirated using a multichannel pipette, and then washed four times with 200μL of phosphate buffer saline (PBS), pH 7.4. The plate was dried at room temperature for one hour. Then, the wells were stained with 2% crystal violet for one minute, and then the volume was aspirated and the excess dye was removed by washing the plates three times with distilled water using a multichannel pipette. Then, the plates were dried at room temperature for 60 min, and the optical density was read in an Elisa reader, Molecular Devices model Spectra mass 384 plus in a 540 nm filter.
To determine the cut-off point, the same procedure described above was used with a whole plate containing sterile TSB. After reading, the mean (m) and standard deviation of the plate (SD) were determined. To calculate the cut-off point, the standard deviation was multiplied by three and the mean value of the optical density (OD) of the sterile TSB sample was added at the same wavelength according to the formula described below:
The samples were classified into: non-adherent, OD equal to or less than the cut-off point OR adherent OD greater than the cut-off point.
Activity of Rosmarinus officinalis essential oil in inhibiting bacterial growth and biofilm production
Essential oil
The essential oil of R. officinalis popularly known as rosemary was acquired by Laszlo Aromaterapia LTDA. The chemical analysis provided by the company indicated camphor, α-pinene and 1.8 cineole as major components with 25.4%, 21.7% and 20.0% of the essential oil, respectively.
Antibacterial activity of essential oil in vitro
The minimum inhibitory concentration (MIC) of R. officinalis essential oil was determined by the broth microdilution test (CLSI 2018). Therefore, all Staphylococcus spp. isolates were tested. and S. aureus strains ATCC 29213 and ATCC 25213. Polystyrene plates with 96-well bottom "U" were used and each well received known amounts of essential oil in Mueller Hinton Broth (MHB) (Kasvi, Italy) plus Tween 80 (0.02 g/mL) in order to obtain concentrations of 32,000 µg/mL, 16,000 µg/ml, 8,000 µg/ml, 4,000 µg/ml, 2,000 µg/ml, 1,000 µg/ml, 500 µg/ml and 250 µg/ml. Then, standardized inoculum was added to obtain 105 colony forming units per mL and incubated at 37 °C/24 h. Control assays were also performed to verify the sterility of MHB, essential oil and the viability of bacteria. After incubation, 10 µL of 10% triphenyltetrazolium chloride developer was added to each well and the plates were incubated at 37 °C for 30 min. The MIC was defined as the lowest concentration of essential oil to inhibit bacterial growth.
Evaluation of the inhibition of biofilm production
The effect of R. officinalis essential oil (EO) on biofilm formation was evaluated in polystyrene microplates using the broth microdilution method followed by quantification of biofilm formation at 540 nm (Christensen et al. 1985; Xu et al. 2011).
For this purpose, cultures of isolates classified as adherent (item Study of biofilm production) were used in TSB medium (incubated for 24 h) diluted 1:1 with TSB + 2% glucose + Tween 80 (0.02 g/mL) together with the EO at concentrations of 32,000 µg/mL, 16,000 µg/mL, 8,000 µg/mL, 4,000 µg/mL, 2,000 µg/mL, 1,000 µg/mL, 500 µg/mL and 250 µg/mL. In a comparative way, a commercial detergent used in the sanitization routine of the respective slaughterhouse was also tested (chlorinated alkaline detergent; active sodium hydroxide 4.5%) in concentrations from 6 to 1.5% as indicated by the manufacturer.
The wells were filled in quadruplicate with 200μL of the diluted culture and the systems were incubated statically at 37 °C for 18–24 h. After the incubation period, the growth medium was removed and the wells were washed with PBS to remove planktonic cells. The biofilm was stained and the excess dye was washed with distilled water, then the absorbance was recorded in a microplate reader.
In all tests, one sample was used as a positive control, one as a negative control and one with sterile TSB. The minimum biofilm inhibitory concentration (MBIC) was the lowest concentration that inhibited biofilm formation.
Results
Twenty-seven Staphylococcus spp. were isolated from four tables, seven sharpeners, 16 knives, being 22/41 coagulase negative Staphylococcus (81.5%) and 5/41 coagulase positive Staphylococcus (18.5%).
Resistance to all tested antibiotics was observed. One isolate was resistant to 08/12 antibiotics and five isolates were resistant to 09/12 antibiotics tested (Table 1).
A 22.2% resistance to the antibiotics: amoxicillin, amoxicillin + clavulanic acid, cefoxitin, oxacillin, amikacin and meropenem was observed. For ceftiofur, erythromycin, norfloxacin and chloramphenicol the percentages of resistance were 14.8%, 11.1%, 7.4% and 3.7%, respectively. Antimicrobials to which Staphylococcus spp. showed the highest percentage of resistance were clindamycin (33.33%) and rifampicin (29.63%) (Table 1).
Of the five coagulase positive Staphylococcus isolated, none were confirmed as Staphylococcus aureus in the polymerase chain reaction (PCR). Among the Staphylococcus spp. resistant to oxacillin and cefoxitin, none were positive for the presence of the mecA resistance gene.
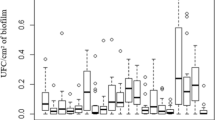
The essential oil of R. officinalis was able to inhibit Staphylococcus spp. isolated from slaughter tools and surfaces at concentrations ranging from 250 µg/mL or less to 32,000 µg/mL. Most of the isolates (48.15%) had their growth inhibited at a concentration of 16,000 µg/mL while the MIC90% was 15,570 µg/mL (Table 2).
Nine Staphylococcus spp. (33.3%) being a table isolate, four from sharpening steels and four from knives were positive for the production of biofilm both by the method of adherence to the borosilicate tube and by the test of adherence to the polystyrene plate. There was no association between the resistance profile of the isolates and the biofilm production capacity (data not shown).
When exposed to R. officinalis essential oil, biofilm production was inhibited in all isolates, and only one isolate from a knife (F3) was able to continue producing biofilm at the lowest concentration tested, that is, the MBIC for this sample was 500 µg/mL and for all others it was < 250 µg/mL (Table 3).
On the other hand, when biofilm-producing bacteria were treated with the detergent, five isolates stopped producing biofilm at the highest concentration tested (6%). For the other isolates, the detergent was not able to contain biofilm production at any of the concentrations evaluated. The positive control biofilm was inhibited by all concentrations of the two treatments (Table 3).
Discussion
In this study, Staphylococcus spp. were present in about 65.85% of the utensils used in slaughter, with a predominance of coagulase-negative Staphylococcus. Some of these bacteria proved to be resistant to several antibiotics and were also able to produce biofilm, showing the potential danger of these contaminants.
Stocco et al. (2017) collected swabs at 25 points of the production process of a slaughterhouse, being isolated Escherichia coli, Salmonella spp. and Staphylococcus aureus. These contaminations evidenced failures in the hygiene of equipment and utensils, and Staphylococcus, associated with cross-contamination from handlers.
Unfortunately, inadequate hygienic-sanitary practices during meat handling are common in slaughterhouses (Germano 2003). Such practices facilitate the transmission of microorganisms to the meat, compromising the final quality of the product and generating a risk to consumer health (Moura et al. 2015). Beef has been highlighted as a source of foodborne diseases and pathogens can be detected at various points in the supply chain. Determining the source of these pathogens and how they behave in meat production and processing are important parts of approaches to ensuring food safety (Fegan and Jenson 2018).
In this study, we did not confirm the presence of S. aureus in samples from the bovine slaughterhouse using the PCR technique. Although S. aureus is considered the most virulent of staphylococci, there are at least seven other species of coagulase-positive staphylococci (S. aureus, S. delphini, S. intermedius, S. pseudintermedius, S. lutrae, S. schleiferi ssp. coagulans, S. hyicus) of importance in human and animal diseases. Unfortunately, laboratory methods based on phenotypic testing do not differentiate coagulase-positive staphylococci due to the significant similarity of phenotypic characteristics in certain representatives of this group (Balbutskaya et al. 2017).
Our results revealed that 51.85% Staphylococcus spp. isolates from the bovine slaughter environment showed some degree of resistance to the tested antibiotics. Coagulase-negative staphylococci are a threat to food safety because they can harbor various enterotoxins and resistance genes. It has already been demonstrated that equipment, hands and nasal cavities of employees of cattle farms and slaughterhouses are critical points for the isolation of coagulase-negative staphylococci with a predominance (78.6%) of multidrug resistance (Gizaw et al. 2020). Although the rates are not alarming, among the isolates of this work, 22.2% can be considered multi-resistant or even extensively resistant according to the classification proposed by Magiorakos et al. (2012).
Among the factors that can be identified as responsible for the multi-resistance of bacteria, we can mention the therapeutic and non-therapeutic use of antibiotics in production animals, use in sub-doses with the aim of prophylaxis for production animals and application as growth promoters, among other factors (Quadros 2018). Antimicrobial resistance in animal production has been reported over the years (Aarestrup et al. 1998; Roth et al. 2019) and is a reason for alerting the productive sector (Ibarra et al. 2018; Callaway et al. 2021). In Brazil, the National Plan for the Control of Residues and Contaminants (PNCRC) is responsible for promoting the chemical safety of foods of animal origin, through tests that include a wide range of authorized and prohibited veterinary drugs, in order to raise awareness among producers of antimicrobial use and the risk of resistance. However, the real scenario of this problem is not known since there are few studies addressing antimicrobial resistance in isolates from the bovine slaughter environment (Souza et al. 2013).
Our research also did not detect the mecA gene in isolates resistant to oxacillin and cefoxitin. This result can be explained by the presence of methicillin resistance determined by another gene, mecC. This variant has already been described in S. aureus and other staphylococcal species and is encoded by a distinct SCCmec chromosomal mobile element (Ito et al. 2012).
Another point to consider for meat safety is the ability to form biofilms that play an important role in staphylococci virulence. However, studies reporting the biofilm formation of coagulase-negative staphylococci isolated from animals are still very scarce (Silva et al. 2022).
In this research, 33.3% of Staphylococcus spp. isolates from the bovine slaughter environment were positive for the production of biofilm, isolated these compounds mainly by coagulase negative staphylococci. In meat processing environments, the presence of biofilms has already been detected on food-contact surfaces and also on non-food-contact surfaces such as drains and water hoses, including biofilms formed by several species (Wagner et al. 2020).
Antimicrobials used as growth promoters in animal production have the onus of selecting resistant strains and the use of sublethal doses to induce staphylococci to produce biofilm. Once formed, the biofilm can spread antimicrobial-resistant strains and their resistance genes through contaminated animal foods. Therefore, the potential for persistent biofilm contamination in the meat production chain is a problem to be faced (Silva-de-Jesus et al. 2022).
In the search for new agents to promote food safety, we evaluated the antimicrobial effect of rosemary essential oil (R. officinalis) on Staphylococcus spp. from slaughtering utensils and surfaces, and all isolates, including those resistant to antibiotics, were inhibited (MIC90% 15,570 µg/mL). In addition, R. officinalis essential oil was also able to inhibit the biofilm production of the isolates (MBIC 250 µg/mL), with the exception of an isolate from a knife whose MBIC was 500 µg/mL.
In a comparative way, a commercial sanitizer used in the routine of the slaughterhouse under study was tested. Positively, when biofilm-producing bacteria were treated with this product, four isolates stopped producing biofilm, but only at the highest concentration tested, revealing an inefficiency of the agent for cleaning the site.
Sanitizers are chemical products that aim to eliminate microorganisms, altering the integrity of the bacterial cell wall or achieving metabolic reactions within the microorganism's cells. Thus, as with antibiotics, continuous or uninterrupted exposure to sanitizers at sublethal doses can lead to the development of bacterial resistance. Thus, it is necessary to rotate active ingredients, as well as periodic control of their effectiveness (Colla et al. 2014).
No relationship was shown between antimicrobial resistance and biofilm formation in this research. However, Silva et al. (2022), when evaluating samples of coagulase-negative staphylococci from different animal species, observed that multidrug-resistant (MDR) strains produced more biofilms than non-MDR strains and that cefoxitin-resistant isolates produced significantly more biofilm than susceptible isolates (SILVA et al. 2022) showing the urgency of new strategies to control the spread of these pathogens.
Progressively, consumers have been changing attitudes and perspectives regarding the use of synthetic agents, be they food preservatives, detergents or sanitizers, which reflects in the growth of interest and searches for natural antimicrobial compounds, making this research area essential to guarantee safety food (Lebert et al. 2007; Rocha et al. 2014).
Studies of the antibacterial and antibiofilm activity of R. officinalis have shown promise. Manilal et al. (2021) observed that the ethanolic extract of the leaves of R. officinalis successfully prevented the growth of both clinical isolates and strains of pathogens derived from meat. In addition, Rocha et al. (2014) tested the essential oil of R. officinalis and observed that the growth of S. aureus was inhibited by the lowest concentration tested (7.5 µL/mL) with a bactericidal effect. There are also reports of the action of R. officinalis essential oil against the biofilm produced by Escherichia coli (Lagha et al. 2019), Salmonella Enteritidis (Lira et al. 2020) and methicillin-resistant Staphylococcus aureus (Abdallah et al. 2020). In this work, the antibiofilm activity of the essential oil was better than the inhibitory activity, showing that the mechanisms of these actions are different.
It is suggested that the addition of essential oils before biofilm formation eliminates planktonic cells and may reduce the adhesion of the polystyrene surface, which becomes less susceptible to cell adhesion. Contact with the oil also modifies bacterial surface proteins by inhibiting the initial fixation phase. Essential oils diffuse through the polysaccharide matrix of the pre-formed biofilm, destabilizing it (Nostro et al. 2007).
The chemical composition of the oil can also make it more effective against pre-formed or mature biofilms (Abdallah et al. 2020). In a study where α-pinene was also one of the major components of the essential oil evaluated, the antibacterial, antibiofilm and quorum sensing modulating activity was found (Chatterjee and Vittal 2021) corroborating our results that showed the potential of natural agents as adjuvants for safety when handling food.
The results found evidence the presence of staphylococci, predominantly the coagulase negative ones, in utensils and surfaces used during bovine slaughter. These bacteria can be resistant to some antibiotics used in both humans and animals and even to antibiotics for veterinary use only. Even the use of antibiotics is not so frequent in bovine production, it is a point of attention that multidrug-resistant bacteria are already in circulation in these productive environments. On the other hand, the essential oil of R. officinalis showed an inhibitory capacity against Staphylococcus spp. and may also prevent the formation of biofilm in vitro. Our findings show that essential for R. officinalis, as it acts differently from synthetic antimicrobials, it may be promising in agents used in cleaning and, thus, allow a lower resistance to the products already available. This natural product can be an important tool in the control of meat-borne pathogens.
Data availability
All data generated or analyzed during this study is included in this published article (and its accompanying information files).
References
Aarestrup FM, Bager F, Jensen NE, Madsen M, Meyling A, Wegner HC (1998) Surveillance of antimicrobial resistance in bacteria isolated from food animals to antimicrobial growth promoters and related therapeutic agents in Denmark. APMIS 106:606–622. https://doi.org/10.1111/j.1699-0463.1998.tb01391
Abdallah FB, Lagha R, Gaber A (2020) Biofilm Inhibition and Eradication Properties of Medicinal Plant Essential Oils against Methicillin-Resistant Staphylococcus aureus Clinical Isolates. Pharmaceuticals (Basel) 13:1–15. https://doi.org/10.3390/ph13110369
Agrosaber (2021) Brasil responderá por 17% da produção mundial de carne bovina em 2021. https://agrosaber.com.br/brasil-respondera-por-17-da-producao-mundial-de-carne-bovina-em-2021/. Accessed 17 Dec 2021
Badia V, Oliveira MSR, Polmann G, Milkievicz T, Galvão AC, da Silva RW (2020) Effect of the addition of antimicrobial oregano (Origanum vulgare) and rosemary (Rosmarinus officinalis) essential oils on lactic acid bacteria growth in refrigerated vacuum-packed Tuscan sausage. Braz J Microbiol 51:289–301. https://doi.org/10.1007/s42770-019-00146-7
Balbutskaya AA, Dmitrenko OA, Skvortsov VN (2017) The modern characteristics of species identification of coagulase-positive bacteria of Staphylococcus genus. Klin Lab Diagn 62(8):497–502. https://doi.org/10.18821/0869-2084-2017-62-8-497-502
Brasil (2021) Ministério da Agricultura e Pecuaria. Abate de bovinos e suínos cresceu no segundo trimestre do ano. https://www.gov.br/pt-br/noticias/agricultura-e-pecuaria/2021/08/abate-de-bovinos-e-suinos-cresceu-no-segundo-trimestre-do-ano. Accessed 18 Nov 2021
BrCAST (2020) Brazilian Committe on Antimicrobial Susceptibility Testing - BrCAST. Tabela de pontos de corte clínicos. BrCAST 2020–01–05–2020. Versão 10, 2020
Callaway TR, Lillehoj H, Chuanchuen R, Gay CG (2021) Alternatives to antibiotics: A symposium on the challenges and solutions for animal health and production. Antibiotics (Basel) 10:471. https://doi.org/10.3390/antibiotics10050471
Chatterjee B, Vittal RR (2021) Quorum sensing modulatory and biofilm inhibitory activity of Plectranthus barbatus essential oil: a novel intervention strategy. Arch Microbiol 203:1767–1778. https://doi.org/10.1007/s00203-020-02171-9
Christensen GD, Simpson WA, Bisno AL, Beachey EH (1982) Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun 37:318–326. https://doi.org/10.1128/iai.37.1.318-326.1982
Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, Beachey EH (1985) Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol 22:996–1006. https://doi.org/10.1128/jcm.22.6.996-1006.1985
CLSI. Clinical and Laboratory Standards Institute (2018) Performance Standards for Antimicrobial Susceptibility Testing: 28th Informational Supplement. Clinical and Laboratory Standards Institute. PA. M100–S12
Colla FL, Mion L, Parizotto L, Santos LA, Pilotto F, Rodrigues LB, Nascimento VP, Santos LR (2014) Perfil de sensibilidade aos antimicrobianos e eficácia de sanitizantes frente aos isolados de Salmonella spp. oriundos de carcaças suínas no Rio Grande do Sul. Pesq Vet Bras 34:320–324. https://doi.org/10.1590/S0100-736X2014000400003
EMA. European Medicines Agency (2019) Categorisation of Antibiotics for Use in Animals for Prudent and Responsible Use https://www.ema.europa.eu/en/documents/report/categorisation-antibiotics-use-animalsprudent-responsible-use_en.pdf. Accessed 22 Mar 2022
Fegan N, Jenson I (2018) The role of meat in foodborne disease: Is there a coming revolution in risk assessment and management? Meat Sci 144:22–29. https://doi.org/10.1016/j.meatsci.2018.04.018
Germano MIS (2003) Treinamento de manipuladores de alimentos: fator de segurança alimentar e promoção de saúde. Ed. Varela, São Paulo, p 2003
Gizaw F, Kekeba T, Teshome F, Kebede M, Abreham T, Hayishe H, Waktole H, Tufa TB, Edao BM, Ayana D, Abunna F, Beyi AF, Abdi RD (2020) Distribution and antimicrobial resistance profile of coagulase-negative staphylococci from cattle, equipment, and personnel on dairy farm and abattoir settings. Heliyon 6(3):e03606. https://doi.org/10.1016/j.heliyon.2020.e03606
Hoffmann FL, Mansor AP, Coelho AR, Vinturim TM (2002) Microbiologia de carcaças e carnes mecanicamente separadas (CMS), obtidas em abatedouro de aves da região de São José do Rio Preto, SP. Hig Aliment 1:45–50
Ibarra R, Rich KM, Adasme M, Kamp A, Singer RS, Atlagich M, Estrada C, Jacob R, Zimin-Veselkoff N, Escobar-Dodero J, Mardones FO (2018) Animal production, animal health and food safety: Gaps and challenges in the chilean industry. Food Microbiol 75:114–118. https://doi.org/10.1016/j.fm.2017.10.004
IBGE. Instituto Brasileiro de Geografia e Estatística. Estatística da Produção Pecuária (2021). https://ftp.ibge.gov.br/Producao_Pecuaria/Fasciculo_Indicadores_IBGE/2021/abate-leite-couro-ovos_202103caderno.pdf. Accessed 22 Mar 2022
Ito T et al (2012) Guidelines for reporting novel mecA gene homologues. Antimicrob Agents Chemother 56:4997–4999. https://doi.org/10.1128/AAC.01199-12
Kahraman T, Issa G, Bingol EB, Kahraman BB, Dumen E (2015) Effect of rosemary essential oil and modified-atmosphere packaging (MAP) on meat quality and survival of pathogens in poultry fillets. Braz J Microbiol 46:591–599. https://doi.org/10.1590/S1517-838246220131201
Lagha R, Ben Abdallah F, Al-Sarhan BO, Al-Sodany Y (2019) Antibacterial and biofilm inhibitory activity of medicinal plant essential oils against Escherichia coli isolated from UTI patients. Molecules 24:1161. https://doi.org/10.3390/molecules24061161
Lebert I, Leroy S, Talon R (2007) Effect of industrial and natural biocides on spoilage, pathogenic and technological strains grown in biofilm. Food Microbiol 24:281–287. https://doi.org/10.1016/j.fm.2006.04.011
Lira MC, Rodrigues JB, Almeida ETC, Ritter AC, Tondo E, Torres SM, Schaffner D, de Souza EL, Magnani M (2020) Efficacy of oregano and rosemary essential oils to affect morphology and membrane functions of noncultivable sessile cells of Salmonella Enteritidis 86 in biofilms formed on stainless steel. J Appl Microbiol 128:376–386. https://doi.org/10.1111/jam.14423
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. https://doi.org/10.1111/j.1469-0691.2011.03570.x
Manilal A, Sabu KR, Woldemariam M, Aklilu A, Biresaw G, Yohanes T, Seid M, Merdekios B (2021) Antibacterial activity of Rosmarinus officinalis against multidrug-resistant clinical isolates and meat-borne pathogens. Evid Based Complement Altern Med 2021:1–10. https://doi.org/10.1155/2021/6677420
Martineau F, Picard FJ, Roy PH, Ouellette M, Bergeron MG (1998) Species-specific and ubiquitous-DNA-based assays for rapid identification of Staphylococcus aureus. J Clin Microbiol 36:618–623. https://doi.org/10.1128/JCM.36.3.618-623.1998
Moura ESR, Abrantes MR, Mendes CG, Oliveira ARM, Sousa ES, Silva JBA (2015) Perfil higiênico-sanitário e perigos microbiológicos em abatedouros públicos. Braz J Vet Med 37:203–208
Murakami K, Minamide W, Wada K, Nakamura E, Teraoka H, Watanabe S (1991) Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J Clin Microbiol 29:2240–2244. https://doi.org/10.1128/jcm.29.10.2240-2244.1991
Nostro A, Roccaro AS, Bisignano G, Marino A, Cannatelli MA, Pizzimenti FC, Cioni PL, Procopio F, Blanco AR (2007) Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Med Microbiol 56:519–523. https://doi.org/10.1099/jmm.0.46804-0
Ntzimani AG, Giatrakou VI, Savvaidis IN (2010) Combined natural antimicrobial treatments (EDTA, lysozyme, rosemary and oregano oil) on semi cooked coated chicken meat stored in vacuum packages at 4 °C: microbiological and sensory evaluation. Innov Food Sci Emerg Technol 11:187–196. https://doi.org/10.1016/j.ifset.2009.09.004
Ouyang Y, Li J, Dong Y, Blakely LV, Cao M (2012) Genome-wide screening of genes required for Listeria monocytogenes biofilm formation. J Biotech Res 1:13–25
Peixoto MMR, Gressler LT, Sutili FJ, Costa MM, Vargas AC (2015) Ação dos desinfetantes sobre a adesão e biofilme consolidado de Staphylococcus spp. Pesq Vet Bras 35:105–109. https://doi.org/10.1590/S0100-736X2015000200001
Porte A, Godoy RLO (2001) Alecrim (Rosmarinus officinalis L.): Propriedades antimicrobiana e química do óleo essencial. CEPPA Bull 19:193–210
Quadros CL (2018) Salmonella spp. isoladas em abatedouro frigorífico de suínos: resistência a sanitizantes e antimicrobianos. Dissertação (Mestrado em Agronomia) - Universidade de Passo Fundo, Passo Fundo
Quinn PJ et al (1994) Clinical veterinary microbiology. Wolfe, London
Rocha CR, Careli RT, Silva RP, Almeida AC, Martins ER, Oliveira EMB, Duarte ER (2014) Óleo essencial de Rosmarinus officinalis L. como sanitizante natural para controle de bactérias sésseis em superfície utilizada para corte de alimentos. Rev Inst Adolfo Lutz 73:338–44. https://doi.org/10.18241/0073-98552014731624
Roth N, Käsbohrer A, Mayrhofer S, Zitz U, Hofacre C, Domig KJ (2019) The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview. Poult Sci 98:1791–1804. https://doi.org/10.3382/ps/pey539
Santos IC, Gonçalves ECBA (2010) Qualidade de carnes in natura na recepção de uma rede de supermercados e de implantação de ações educativas para os manipuladores dos produtos. Hig Aliment 24:38–44
Silva V, Correia E, Pereira JE, González-Machado C, Capita R, Alonso-Calleja C, Igrejas G, Poeta P (2022) Exploring the biofilm formation capacity in S. pseudintermedius and Coagulase-negative Staphylococci Species. Pathogens 11:689. https://doi.org/10.3390/pathogens11060689
Silva-de-Jesus AC, Ferrari RG, Panzenhagen P, Conte-Junior CA (2022) Staphylococcus aureus biofilm: the role in disseminating antimicrobial resistance over the meat chain. Microbiology (Reading) 168:10. https://doi.org/10.1099/mic.0.001245
Souza MV, Lage ME, Prado C (2013) Resíduos de antibióticos em carne bovina. Enciclopédia Biosfera 9:1917–1938
Stocco CW, Almeida L, Barreto EH, Bittencourt JVM (2017) Controle de qualidade microbiológico no processamento de frigorífico bovino. Rev Espacios 38:1–14
Wagner EM, Pracser N, Thalguter S, Fischel K, Rammer N, Pospíšilová L, Alispahic M, Wagner M, Rychli K (2020) Identification of biofilm hotspots in a meat processing environment: Detection of spoilage bacteria in multi-species biofilms. Int J Food Microbiol 328:108668. https://doi.org/10.1016/j.ijfoodmicro.2020.108668
Xavier VG, Joele MRSP (2004) Avaliação das condições higiênico sanitárias da carne bovina in natura comercializada na cidade de Belém, PA. Hig Aliment 18:64–73
Xavier JB, Picioreanu C, Almeida JS, Van Loosdrecht MCM (2003) Monitorização e modelação da estrutura de biofilmes. Bol Biotecnol 76:1–13
Xu X, Zhou XD, Wu CD (2011) The tea catechin epigallocatechin gallate suppresses cariogenic virulence factors of Streptococcus mutans. Antimicrob Agents Chemother 55:1229–1236. https://doi.org/10.1128/AAC.01016-10
Acknowledgements
We would like to thank the Coordination for the Improvement of Higher Education Personnel (Coordination for the Improvement of Higher Education Personnel—CAPES) and the Graduate Support Program for Institutions of Private Education (PROSUP) for the granting of a school fee.
Funding
This work was supported by Universidade Paranaense (Universidade Paranaense—UNIPAR) and Fundação Araucária.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Karolaine Bezerra, Lídia Kazue Iukava, Jacqueline Midori Ono, Sandra Geane Pereira de Souza, Isabela Carvalho dos Santos, Lidiane Nunes Barbosa. The first draft of the manuscript was written by Karolaine Bezerra, Isabela Carvalho do Santos, Lidiane Nunes Barbosa, Jacqueline Midori Ono and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that there were no conflicts of interest for this article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bezerra, K., Iukava, L.K., Ono, J.M. et al. Resistance profile and biofilm production capacity of Staphylococcus spp. beef slaughterhouse isolates and their sensitivity to Rosmarinus officinalis essential oil. Vet Res Commun 47, 911–919 (2023). https://doi.org/10.1007/s11259-022-10057-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-022-10057-y