Abstract
There are many reports on the deleterious effects of herbicides on aquatic organisms which lead to tremendous biological, environmental and economical damage. In this regard, in the present study, the protective effect of summer savory (Satureja hortensis) essential oil (SEO) against pretilachlor, one of the most used herbicides was investigated in common carp (Cyprinus carpio). The fish assigned to six treatment groups (T1: control treatment; T2: 25% LC50 pretilachlor herbicide; T3: 50% LC50 pretilachlor herbicide; T4: 1% SEO; T5: 25% LC50 pretilachlor herbicide + 1% SEO; and T6: 25% LC50 pretilachlor herbicide + 1% SEO) for 21 days. The results showed that the SEO-containing treatments significantly increased the survival rate (SR) (P < 0.05). The highest final weight (FW), specific growth rate (SGR), and feed conversion ratio (FCR) were observed in the T4 treatment (P < 0.05). There was a significant increase in glucose (GLU) level in pretilachlor treatments and a significant decrease in SEO-containing treatments compared to the control (P < 0.05). The significantly highest total protein (TP) content was observed in T4 treatment containing SEO. Cholesterol (CHOL) and triglyceride (TRIG) levels decreased in SEO-containing treatments with the lowest level in T4 treatment (P < 0.05). Alternative complement pathway activity (ACH50), activity levels of superoxide dismutase (SOD), and glutathione peroxidase (GPX) showed an increasing trend in SEO-containing treatments with the highest level in T4 treatment (P < 0.05). The activity of liver enzymes showed a significantly lowest level in T4 treatment. To conclude, our findings revealed that the use of SEO in fish exposed to pretilachlor herbicide could improve growth, strengthen the immune system and exert a protective effect on common carp.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Following the increasing use of herbicides in modern agriculture, a large proportion of these herbicides are accumulated in surface waters through surface runoff, leaching, and drift leading to environmental hazards for aquatic organisms and human health (Jiang et al. 2016; Gharaei et al. 2020; Saha et al. 2021; Stara et al. 2021; Suchiang 2021; Vali et al. 2022). Exposure of the fishes to pesticides results in behavioral, physical, morphological, physiological disorders, and suppression of the immune system, which leads to unfavorable consequences on fish growth and reproduction (Srivastava et al. 2016; Soni and Verma 2018; Kumari 2020; Suchiang 2021; Dar et al. 2022; Merola et al. 2022).
Pretilachlor (2-chloro-2’,6’ -diethyl-N- (2 propoxyethyl) acetanilide) is one of the most widely used chloroacetamide herbicides for pre-emergence control of undesirable weeds in corn, cotton, soybeans and many other crops (Jiang et al. 2016; Soni and Verma 2018). There are many studies on evaluation of the negative impact of pretilachlor on physiology after acute or chronic exposure in different aquatic species showing that male and female sex hormones decrease during exposure and there is increase in cortisol, and liver enzymes and free radicals related to reactive oxygen species (ROS) (Soni and Verma 2018, 2020).
Common carp (Cyprinus carpio) is an economically important species in the world, accounting for 71.9% of freshwater production, and its production has increased from 2.9 million tons in 2008 to 4.1 million tons in 2017, with an increase of almost 30% (Mohammadi et al. 2020).
Despite the significant progress, common carp culture has been always encountered challenges such as changes in water quality, pollution by pesticides, and nutritional problems. Numerous studies have shown that the use of herbal additives can be significantly beneficial in overcoming these problems (Galina et al. 2009; Pandey et al. 2012; Reverter et al. 2014; Myszka et al. 2019; Alagawany et al. 2021; Lumsangkul et al. 2022; Rashidian et al. 2022a, b).
Plant essential oils, with their abundant antioxidant and antimicrobial properties, can exert positive effects on growth performance, resistance to environmental stress, infectious diseases, stimulation of nonspecific immune system, and some blood parameters in livestock, poultry, and cultural aquatics (Dügenci et al. 2003; Kapoorchali et al. 2009; Awad and Awaad 2017; Abdel-Latif et al 2020;a,b Mohammadi et al. 2020; Abdel-Tawwab and El-Araby 2021; Ghafarifarsani et al. 2021 a; Yousefi et al. 2021a; Yousefi et al. 2021b; Abdel-Latif et al. 2022; Kumar et al. 2022; Raissy et al. 2022; Rashidian et al. 2022a,b). Numerous studies have shown that various plant extract additives could increase immunity, including increased serum complement levels, plasma protein content, serum globulin, and lysozyme (Greathead 2003; Wu et al. 2007; Windisch et al. 2008; Alishahi et al. 2011; Harikrishnan et al. 2011; Abdel-Tawwab and El-Araby 2021; Ghafarifarsani et al. 2021 a,b; Alagawany et al. 2021; Ghafarifarsani et al. 2022; Raissy et al. 2022). It has been also demonstrated that improving the flavor of the diet by plant compounds stimulated pancreatic enzymes and growth, caused weight gain, and helped digest and absorb important nutrients (Frankic et al. 2009; Abdel-Tawwab et al. 2010).
There have been some reports that summer savory (Satureja hortensis) has many beneficial properties including stimulating growth and appetite and boosting the immune system (Akbarzadeh 2003). The genus Satureja belongs to the family Lamiaceae (Hernández-Contreras and Hernández 2020), which is widely used in food preparation and has a special role in the pharmaceutical industry and traditional medicine (Taherian et al. 2019) and is rich in thymol and carvacrol (Hernández-Contreras and Hernández 2020).
Recent studies have shown that the toxicity of pesticides in fish may be associated with increased ROS production, which causes oxidative damage to biological systems (Yonar and Sakin 2011). Oxidative stress refers to an imbalance between the production and neutralization of ROS by antioxidant mechanisms within an organism (Puangkaew et al. 2005; Valavanidis et al. 2006); specification of antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT) and glutathione S-transferase (GST) can be used to identify and highlight this stress (Puangkaew et al. 2005; Blahová et al. 2013; Hamed et al. 2021).
On the other hand, fish epidermal mucus contains a variety of biologically active agents such as lysosomes, flavoenzymes, immunoglobulins, and antimicrobial peptides ((Van Doan et al. 2019; Rashidian et al. 2021). Mucous secretions by trapping high concentrations of toxins prevent their introduction into the fish body (Magnadottir 2006; Subramanian et al. 2007). Therefore, studying the parameters of the mucosal immune system will help to understand the biological conditions of fish and reduce the immune function of fish due to stress caused by pollutants (Magnadottir 2006).
Accordingly, the present study aimed to investigate the protective effect of summer savory (Satureja hortensis) essential oil (SEO) on growth and survival parameters, liver enzymes, immune serum, mucosal immune system, and biochemical profiles of common carp (Cyprinus carpio) exposed to the pretilachlor herbicide.
Materials and methods
Preparation of herbal extract
The SEO was purchased ready-made from Tabib Daru Company (Kashan-Iran). The chemical composition of SEO was Carvacrol (29.6%), gamma-Terpinene (26.3%), Para-Cymene (14.1%), alpha-Terpinene (9.7%), Myrcene (2.5%), alpha-Pinene (2.1%) and alpha-thujene (1.9%) determined by Gas chromatography-Mass Spectrometry (GC-MS, model- Shimadzu-9 A).
The basic diet components (Faradaneh Company, Iran) including fish meal (10%), soybean meal (23%), meat meal (21%), wheat meal (40.8%), fish oil (1%), soybean oil (1%), lysin (0.7%), methionine (0.5%), vitamin mix (1%) and mineral mix (1%); crude protein (37%), crude lipid (6%), crude fiber (6%), digestible phosphorus (1.25%) and moisture (7%) were thoroughly mixed while adding SEO and water gradually (Ghafarifarsani et al. 2022). The resulting mixture was pelletized using a meat grinder and dried in a dark place for 24 h.
Experimental design
Carp were purchased from the carp sales center in Hashtgerd (Karaj, Iran) and transferred to Mohammad Shahr (Karaj, Iran) for further testing. After isothermalization and adaptation of the juveniles to the new conditions and feeding on a basic diet in the form of pellets for two weeks, the fish were examined to ensure the health and natural structure of the body. After the initial bioassay, 360 completely healthy fish with an initial weight of 25.35 ± 0.13 g were kept in 18 fiberglass tanks (20 fish per tank) for 21 days in a completely randomized design with six treatment groups (T1: control treatment; T2: low concentration of toxin (25% LC50 pretilachlor herbicide); T3: high concentration of toxin (50% LC50 pretilachlor herbicide); T4: 1% SEO; T5: low concentration of toxin + 1% SEO; and T6: high concentration of toxin + 1% SEO). The 21 exposure period and 25% and 50% of LC50 treatment were chosen based on the OECD guide line for short term screening (OECD GUIDELINE FOR THE TESTING OF CHEMICALS 230 Adopted: 7 September 2009) and 1% of SEO was chosen based on previous studies in the literature in cyprinid diets (Ghafarifarsani et al. 2022). The oil was added to the basal diet every day, and the fish were fed (2% of fish body weight) twice a day.
During the experimental period, the physicochemical factors of reservoir water (temperature: 22.2 ± 0.7 °C (by thermometer, ZEAL, UK), pH: 7.6 ± 0.2 (by portable pH meter, Model AE-PH501), and dissolved oxygen: 6.1 ± 3 mg/l) (by portable oxygen meter: Oxyguard Polaris Dissolved Oxygen Meter, Dynamic Aqua Supply Ltd, Canada) were measured daily.
The fish were kept in a 12/12 h light/dark cycle. To maintain water quality and to remove waste products, uneaten foods were siphoned and water was completely renewed (100%) daily with the same concentration of the herbicide in each treatment.
Determination of lethal concentration (LC50) values of pretilachlor herbicide
Prior to the experiment, there was a need to determine the lethal range and acute concentration of the pretilachlor on the fish to specify the subacute test doses. To determine the LC50 value for pretilachlor herbicide on common carp based on the standard method of O.E.C.D (1994), 180 fish were assigned to six treatments in triplicate (10 fish in each replicate in 60-liter tanks). The lethal concentration test lasted 96 h and the number of deaths was counted at 24, 48, 72, and 96 h and recorded (Hedayati et al. 2015; Shahbazi Naserabad et al. 2017). The physicochemical properties of the water were controlled, and all conditions were maintained the same during the test period so that different doses of contamination were the only variable. Finally, the number of fish lost was recorded after 24, 48, 72, and 96 h. Then, the values of LC10, LC30, LC50, LC70, and LC90 were calculated for carp using Probit program version 0.16, (Table 1).
Blood sampling
In each treatment, six fish were randomly selected and anesthetized with clove powder (150 ppm) and the blood samples were taken from the caudal vein using a sterilized 2-mL syringe (Ghafarifarsani et al. (Ghafarifarsan et al. 2021) b).
To obtain serum to measure biochemical, immune, and antioxidant parameters, the blood samples were immediately transferred to tubes and allowed to coagulate at room temperature for 30 min (Ross et al. 2000; Ghafarifarsani et al. 2021 b).
Growth performance
After feeding the treatments with the specified feed for 21 days, at the end of the experiment, the number of fish losses, if any, during the study, the consumed feed, and the final weight of the fish were recorded. Then, growth indices were measured using the following equations:
Weight gain (WG) (g) = initial weight – final weight.
Weight gain (WG) (%) = (initial weight – final weight) × 100.
Specific growth rate (SGR) (%/d) = (ln final wt (g) – ln initial wt (g) / days) × 100.
Feed conversion rate (FCR) = total feed given (g) / weight gain (g).
Survival rate (SR) (%) = (final numbers / initial numbers) × 100.
Measurement of biochemical compounds
After drawing blood from the caudal vein, the blood was transferred into a 2-mL Eppendorf tube and centrifuged at a speed of 1000 × g at 4 °C for 5 min. The obtained serum was stored in a freezer at -20 °C until biochemical parameter measurements. Total protein (TP), albumin (ALB), glucose (GLU), cortisol (CORT), triglyceride (TRIG), cholesterol (CHOL), and lactate dehydrogenase (LDH) were measured using a biochemical analyzer (Roche Hitachi 911 Chemistry Analyzer, Japan), and the serum cortisol (CORT) levels were measured by a commercial ELISA kit (ZellBio, Germany). Finally, the serum globulin (GLO) was also calculated from the difference between total serum protein and albumin (Naiel et al. 2021).
Measurement of liver enzymes and antioxidants in blood serum
Antioxidant enzymes, including glutathione peroxidase (GPx), superoxide dismutase (SOD), catalase (CAT), and malondialdehyde (MDA), were determined using a commercial kit (Berlin, Germany, ®Zellbio) according to the manufacturer’s protocol (Hoseinifar et al. 2020b; Raissy et al. 2022).
The activity levels of liver enzymes including aspartate aminotransferase (AST), alkaline phosphatase (ALP), and alanine aminotransferase (ALT) were measured by spectrophotometry using commercial kits (Pars Azmun Co., Tehran, Iran) (Hoseini et al. 2012, 2018).
Assessment of parameters related to the mucosal immune system
In order to collect mucus samples, six fish were randomly sampled from each tank, anesthetized by clove powder separately, placed in polyethylene zipper bags containing 10 mL of 50 mM sodium chloride for two minutes, and then removed from the bags. The collected mucus samples were transferred to sterile 15-mL tubes and centrifuged with 1500 g for 10 min at 4 °C; the resulting supernatant was transferred to a 1.5 cc microtube for further analysis (Vali et al. 2020).
Immunological parameters were analyzed in samples of serum and mucus by using conventional techniques. Lysozyme activity was determined in serum and mucus samples according to the slightly modified method of Demers and Bayne (1997). In brief, 0.2 mg/ml of the bacterium (Micrococcus luteus) suspension was prepared with the sodium phosphate buffer (0.05 M, pH 6.2). Sixty µL of the sample was mixed with the bacterium suspension (2 ml) and incubated for three minutes; then the absorbance was read. One unit of lysozyme was considered a decrease of 0.001 per min in absorbance. Alternative complement activity (ACH50) was measured in samples of serum and mucus through the method developed by Ortuno et al. (2000), which is based on sheep red blood cells (SRBC) hemolysis. For the measurement of total immunoglobulin (total Ig), the samples were sedimented with a polyethene glycol solution (12.5%) (Sigma). The total Ig was then determined after calculating protein concentrations before and after sedimentation (Siwicki and Anderson 1993).
Protease activity in mucus was measured using the azocasein hydrolysis approach explained by Ross et al. (2000). Mucus alkaline phosphatase (ALP) activity and total protein (TP) level were determined by a commercial kit (Pars Azmun Co., Tehran, Iran).
Statistical analysis
The data were analyzed by one-way analysis of variance (ANOVA) using SPSS version 20 software. The significance level of statistical tests was considered less than 5%. Prior to the analysis of variance, normality of data distribution and homogeneity of variance of different experimental groups were assessed using Shapiro-Wilk and Levene’s tests, respectively. If the results of the analysis of variance were significant, Tukey’s post hoc test was used to compare the means of different treatments. The mean data were reported as Mean ± standard error (SE).
Results
At the end of the experimental period, the final weight showed a significant difference between the control and the experimental groups (P < 0.05). The highest mean weight was obtained in the treatment, T4 (34.73 ± 1.12 g) and the lowest mean weight was obtained in the treatment, T2 (29.65 ± 1.30 g).
In the present study, the feed conversion ratio (FCR) showed a significant difference between control and experimental treatments (P < 0.05), with the lowest and highest values in the treatments, T4 and T3, respectively (Table 2). The valuses of daily growth rate were significantly higher in control and the treatment, T4 compared to other experimental groups (Table 2, P < 0.05). There were no significant differences in growth rate between control and fish of T4 (Table 2, P > 0.05).
The total protein content and globulin level significantly increased with the addition of SEO (T4) (3.42 ± 0.03) compared to the control (3.19 ± 0.02), with highest levels in the treatments, T5 and T6 respectively (Table 3, P < 0.05).
The levels of TRIG, CORT, GLU, CHOL and LDH parameters decreased with the addition of SEO (T4) compared to the control group and exhibited a significant difference with the control (Table 3, P < 0.05). The CORT had the highest level (92.09 ± 1.27) in T3 (high concentration of pretilachlor herbicide) and the lowest level (68.50 ± 1.36) in T4 (Table 3, P < 0.05). The trend of GLU changes was similar to CORT (Table 3, P < 0.05).
The addition of SEO to the common carp diet caused a significant decrease in ALP and AST enzyme activity compared to the control group (Table 4, P < 0.05). The activity level of these enzymes increased in treatments, T2, T3, and T6 had a significant difference from the control group (Table 4, P < 0.05). The highest level of ALP activity (26.14 ± 0.41) was observed in T3 and the lowest value (21.44 ± 0.42) was in T4 (Table 3, P < 0.05).
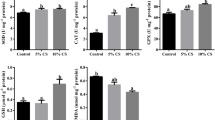
The levels of CAT, SOD, and GPx increased in the T4, which was different from the control group (Table 5, P < 0.05). However, the MDA content decreased in T4, while it increased in the treatments, T2 and T3 (Table 5, P < 0.05).
Except for T5 treatment, all treatments showed a significant difference in serum lysozyme activity compared to the control group (Table 6, P < 0.05). The lysozyme levels significantly decreased in the treatments,T2, T3, and T6 compared to the control, while its levels significantly increased in T4 compared to other treatments (Table 6, P < 0.05).
Serum complement (ACH50) showed a significant increase in the treatments, T4 and T5 compared to the control, with the highest levels in T4 (54.14 ± 2.9) (Table 6, P < 0.05).
The results showed the highest mucosal lysozyme activity in the treatment T4 (29.32 ± 0.64), which was significantly different from other treatments and the control group (27.07 ± 0.60) (Fig. 1, P < 0.05). The treatments T2 and T3, which received the low and high concentrations of pretilachlor herbicide, respectively, showed a decrease in lysozyme level, which was significantly different from the control group (Fig. 1, P < 0.05).
Effect of dietary supplementation with Satureja hortensis and/or exposure to sub-lethal Pretilachlor toxicity (25% and 50% LC50; mg/l) for 21 days on the mucus immunological indices of Cyprinus carpio
*Abbreviations: Total Ig, total immunoglobulin; ACH50, alternative complement activity; ALP, alkaline phosphatase
Data are expressed as the mean ± SE. Different letters above bars indicate the significant difference among the treatments (P < 0.05; Tukey test)
The highest level of mucosal immunoglobulin observed in T4 (11.22 ± 0.53 mg/l), which showed a significant increase compared to control group (Fig. 1, P < 0.05).
The protease level increased in T4 (20.30 ± 0.84) compared to the control (19.15 ± 0.84), while its level decreased in the other treatments (Fig. 1, P < 0.05).
Discussion
Special attention has recently been paid to the use of immunostimulants as dietary supplements capable of improving nonspecific defense and resistance to pathogens and toxins (Antache et al. 2014; Jahanjoo et al. 2018; Yousefi et al. 2020; Farag et al. 2021; Ghafarifarsani et al. 2021c, 2022; Owolabi and Abdulkareem 2021; Raissy et al. 2022; Rudiansyah et al. 2022). Due to the medicinal importance and benefits of summer savory, this study aimed to investigate the possible protective effect of essential oil of this plant on various biological parameters of common carp exposed to the pretilachlor herbicide. Besides measuring the trend of changes in biochemical factors, antioxidant enzymes and immunological factors of serum and mucus can be considered a suitable tool for predicting and determining the health of a living organism, these factors can also be used to determine drug safety (Subramanian et al. 2007; Mauri et al. 2011; Harikrishnan et al. 2011; Yonar and Sakin 2011; Hedayati et al. 2019; Bisht et al. 2020; Hoseinifar et al. 2020; a,b Vazirzadeh et al. 2020; Vali et al. 2020; Farag et al. 2021; Hamed et al. 2021).
Our findings here showed that the use of SEO was efficient to improve the health and growth and strengthen the immune system of common carp. The highest survival rate, final weight, daily growth rate, and specific growth rate were observed in the SEO-containing treatments. Accordingly, all growth parameters were reduced in the treatments T2 and T3, which contained low and high concentrations of pretilachlor herbicide, compared to the control group. However, the growth parameters showed better conditions in the treatments T5 and T6, which were co-administered with toxin and SEO compared to T2 and T3 Treatments. This was while the best growth performance conditions were recorded in T4 treatment (recipient of 1%SEO without any toxin challenge). Mohamadi Saei et al. (2016) investigated the effects of diets containing different levels of Myrtus communis and Satureja khuzestanica extracts on the growth, survival, and nutritional indices of rainbow trout. They reported that the highest feed conversion ratio was observed in fish fed with diets containing M. communis and S. khuzestanica extracts, which is in accordance with the results of the present study.
Studies have shown that immunostimulants or antioxidant compounds can improve animal growth by eliminating inflammatory markers and restoring the integrity of the gastrointestinal wall (Niewold 2014; Celi et al. 2019; Yousefi et al. 2021b) investigated the effect of different levels of Origanum majorana extract (from the Lamiaceae family) on growth, hematological, immunological, and biochemical parameters of common carp (Cyprinus carpio), and observed that there are significant effects of marjoram extract on fish growth performance. Numerous studies have also shown that the Labiatae family (which includes several plants such as Thymus vulgaris and Origanum vulgare) have growth-promoting, antioxidant and immunostimulatory properties in fishes (Zheng et al. 2009; Diler et al. 2017; Zargar et al. 2019; Abdel-Latif et al. 2020;,b).
In the present study, the use of SEO significantly reduced the level of TRIG and CHOL in the common carp compared to the control group. The extracts of some plants decrease the synthesis of cellular CHOL by increasing the level of Cholesterol 7α-hydroxylase activity in liver cells (Asgary et al. 2000). Measuring blood GLU levels is a common factor in assessing stress levels in fishes under environmental, nutritional, and manipulation stresses (Prasad and Charles 2010). The present study showed that the SEO-containing treatments reduced the GLU levels in fish. At the end of the experimental period, the total protein content of SEO-treated fish increased significantly compared to the control group. Elevated serum albumin and globulin levels are known to boost immunity in fish. Serum globulin and albumin levels in the SEO-containing treatments increased compared to the control. Asadi et al. (2012) reported that the use of Nasturtium nasturtium extract in rainbow trout increased globulin levels in the blood. There are not any consensus absolute TRIG, CORT, GLU, CHOL and LDH concentrations in different ages and different populations or lines in common carp in the literature, but the reduction in TRIG, CORT, GLU, CHOL and LDH in our study has brought the levels of these parameters to the optimum levels. Therefore, the fish with SEO were in better general health condition and consequently the SEO fish had better growth and immune system performance.
The serum AST, ALT, and ALP are important enzymes for exploring tissue and muscle damage, especially liver tissue (Paris-Palacios et al. 2000; Orisakwe et al. 2003). Therefore, they are major stress indicators in monitoring toxicological changes in the environment (Brusle and Anadon 2017; Abdel-Latif et al.2020a). In the present study, the pretilachlor herbicide increased all three enzymes compared to the control group, but the addition of SEO to the treatments decreased the levels of these “ enzymes” in the blood. This suggests that SEO has protective effects on liver tissue damage caused by pretilachlor herbicide. A similar result was observed in the study of feeding rainbow trout (Hoseini and Yousefi 2019) and common carp (Ghafarifarsani et al. 2021,b) with thyme extract. In general, the addition of pretilachlor herbicide to the experimental treatments caused damage to liver tissue; as a result, the activity of these enzymes increased at the serum level, and our findings suggest the positive effect of SEO on the liver maintenance of fish exposed to toxins due to its antioxidant activity. Numerous studies have shown that the chemical composition of summer savory contains high amounts of carvacrol along with other phenolic compounds, flavonoids, triterpenoids, steroids, and tannins (Farsam et al. 2004). Therefore, it has been stated that savory has effective antioxidant properties.
The MDA content is evaluated as an indicator of lipid peroxidation in fish blood plasma (Yousefi et al. 2021b), which increased in pretilachlor herbicide treatments in the present study and decreased with the addition of SEO to the treatments. In line with the decrease in MDA content, the activity of SOD and GPX antioxidant enzymes in SEO-containing treatments increased compared to the control and treatments containing pretilachlor herbicide. The benzene ring and electron resonance in phenolic compounds of savory can trap free radicals and prevent the continuation of chain reactions and the production of free radicals (Farahi et al. 2012; Roby et al. 2013). Similarly, such results were reported for MDA and other antioxidant enzymes in the study of the effect of marjoram (from the Labiatae family) on common carp (Yousefi et al. 2021b), Shirazi thyme (Zataria multiflora) on rainbow trout (Mirghaed et al. 2020), and oregano (Origanum vulgare) on common carp (Abdel-Latif et al. 2020#x002C;b).
The lysozyme is one of the most important components of the fish’s innate immune system, which destroys the bacterial wall, activates complement, and increases phagocytic activity (Sakai 1999; Saurabh and Sahoo 2008). Increased serum lysozyme activity indicates an improvement in the immune status of fish and its increase helps the immune system of fish perform better against infectious and pathogenic agents (Ringø et al. 2012). Here, the lysozyme levels decreased in treatments containing pretilachlor herbicide and showed a significant difference compared to the control group, but lysozyme levels increased with the addition of SEO which seems to be due to the stimulatory ability of active ingredients of SEO (gamma-Terpinene and carvacrol). Therefore, this practice shows that SEO increases the level of immunity and resistance of common carp exposed to the pretilachlor herbicide. These results were consistent with the study of Khansari et al. (2013) who evaluated the effect of Khuzestani savory on the parameters of immunity and hematology in the common carp.
The complement system is a collection of more than 35 types of serum proteins that are very closely related and controlled by each other and other molecules of the immune system (Sunyer et al. 1997). The most important task of this system is to destroy microorganisms through phagocytic processes, inflammatory reactions, clearance of immune complexes, induction, and improvement of antibody responses (Mauri et al. 2011). In the present study, the highest values of complement system factors were observed in the treatment containing SEO and showed a significant difference from the control group. Changes in serum complement are very important in protecting the ability of the nonspecific immune system of fish, and high levels of complement indicate the health of the fish (Yano 1992). Other studies have shown the hat consumption of peppermint stimulated the activity of complement system components in rainbow trout (Adel et al. 2015). The ACH50 activity and total Ig level as indicators of immune status may be suppressed in fish exposed to toxins (Wang et al. 2014; Sharifian et al. 2015). Here, the total Ig level decreased in fish exposed to pretilachlor herbicide compared to the control group but increased in the SEO-containing group. Hoseini and Yousefi (2019) investigated the effect of thyme (Thymus vulgaris) extract on rainbow trout and reported a significant increase in the lysozyme, ACH50, and total Ig levels.
It has been reported that the efficacy of the herbal extract on biological performance in aquatic animals can be sex (Ghosal et al. 2021), and age (Mulero et al. 1998) dependent which is not investigated in our study and it could be a topic of interest for future investigations.
Conclusions
To conclude, the results of this study demonstrated that the addition of summer savory (Satureja hortensis) essential oil to the diets of common carp in exposure to stressful conditions and toxin-induced contamination improved digestion and absorption of nutrients, promoted better growth, increased antioxidant capacity and boosted the immune system.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Abdel-Latif HM, Abdel-Tawwab M, Khafaga AF, Dawood MA (2020) Dietary oregano essential oil improved the growth performance via enhancing the intestinal morphometry and hepato-renal functions of common carp (Cyprinus carpio L.) fingerlings. Aquaculture 526:735432
Abdel-Latif HM, Abdel-Tawwab M, Khafaga AF, Dawood MA (2020) Dietary origanum essential oil improved antioxidative status, immune-related genes, and resistance of common carp (Cyprinus carpio L.) to Aeromonas hydrophila infection. Fish Shellfish Immunol 104:1–7
Abdel-Tawwab M, El-Araby DA (2021) Immune and antioxidative effects of dietary licorice (Glycyrrhiza glabra L.) on performance of Nile tilapia, Oreochromis niloticus (L.) and its susceptibility to Aeromonas hydrophila infection. Aquaculture 530:735828
Abdel-Tawwab M, Ahmad MH, Seden ME, Sakr SF (2010) Use of green tea, Camellia sinensis L., in practical diet for growth and protection of Nile tilapia, Oreochromis niloticus (L.), against Aeromonas hydrophila infection. J World Aquac Soc 41:203–213
Abdel-Latif HM, Dawood MA, Alagawany M, Faggio C, Nowosad J, Kucharczyk D (2022) Health benefits and potential applications of fucoidan (FCD) extracted from brown seaweeds in aquaculture: An updated review. Fish & Shellfish Immunology
Adel M, Pourgholam R, Zorriehzahra SJ, Ghiasi M (2015) The effect of different level of Mentha piperita on some of the hematological, biochemical and immune parameters of Oncorhynchus mykiss. Iran Fish Sci J 24(1):37–46
Agrahari S, Pandey KC, Gopal K (2007) Biochemical alteration induced by monocrotophos in the blood plasma of fish, Channa punctatus (Bloch). Pestic Biochem Physiol 88(3):268–272
Akbarzadeh M (2003) Medicinal Plants of Labiatae Family in the summer rangelands of Vaz region in Mazandaran Province. Iran J Med Arom Plants Res 19(1):37–46
Alagawany M, Farag MR, Abdelnour SA, Elnesr SS (2021) A review on the beneficial effect of thymol on health and production of fish. Rev Aquac 13(1):632–641
Alishahi M, Soltani M, Mesbah M, Rad AE (2011) Effects of dietary Silybum marianum extract on immune parameters of the common carp (Cyprinus carpio). J Vet Res 66(3):255–286
Antache A, Cristea V, Grecu I, Dediu L, Cretu M, Bocioc E, Petrea SM (2014) Effects of Dietary Supplementation at Nile tilapia with Thymus vulgaris, Trigonela foenum graecum and Azadirachta indica on Welfare Status Bull Univ Agric Sci Vet Med Cluj-Napoca. Anim Sci Biotechnol 71(2):115–122
Asadi MS, Mirvaghefei AR, Nematollahi MA, Banaee M, Ahmadi K (2012) Effects of Watercress (Nasturtium nasturtium) extract on selected immunological parameters of rainbow trout (Oncorhynchus mykiss). Open Vet J 2(1):32–39
Asgary S, Naderi GH, Sarrafzadegan N, Mohammadifard N, Mostafavi S, Vakili R (2000) Antihypertensive and antihyperlipidemic effects of Achillea wilhelmsii. Drugs Exp Clin Res 26(3):89–94
Awad E, Awaad A (2017) Role of medicinal plants on growth performance and immune status in fish. Fish Shellfish Immunol 67:40–54
Bisht M, Kumar A, Shah TK (2020) Effect of Moringa oleifera leaf powder on skin mucosal immune responses and growth performance of guppy, Poecilia reticulata (Peter, 1860). Aquacult Res 51(12):4984–4990
Blahová J et al (2013) Oxidative stress responses in zebrafish Danio rerio after subchronic exposure to atrazine. Food Chem Toxicol 61:82–85
Brusle J, Anadon GG (2017) The structure and function of fish liver. Fish morphology. Routledge, pp 77–93
Celi P, Verlhac V, Calvo EP, Schmeisser J, Kluenter AM (2019) Biomarkers of gastrointestinal functionality in animal nutrition and health. Anim Feed Sci Technol 250:9–31
Dar OI, Aslam R, Sharma S, Jia AQ, Kaur A, Faggio C (2022) Biomolecular alterations in the early life stages of four food fish following acute exposure of Triclosan. Environ Toxicol Pharmacol 91:103820
Demers NE, Bayne CJ (1997) The immediate effects of stress on hormones and plasma lysozyme in rainbow trout. Dev Comp Immunol 21(4):363–373
Diler O, Gormez O, Diler I, Metin SEÇİL (2017) Effect of oregano (Origanum onites L.) essential oil on growth, lysozyme and antioxidant activity and resistance against Lactococcus garvieae in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac Nutr 23(4):844–851
Dügenci SK, Arda N, Candan A (2003) Some medicinal plants as immunostimulant for fish. J Ethnopharmacol 88(1):99–106
Farag MR, Alagawany M, Khalil SR, Moustafa AA, Mahmoud HK, Abdel-Latif HM (2021) Astragalus membranaceus polysaccharides modulate growth, hemato-biochemical indices, hepatic antioxidants, and expression of HSP70 and apoptosis-related genes in Oreochromis niloticus exposed to sub-lethal thallium toxicity. Fish Shellfish Immunol 118:251–260
Farag RS, Badei AZMA, Hewedi FM, El-Baroty GSA (1989) Antioxidant activity of some spice essential oils on linoleic acid oxidation in aqueous media. J Am Oil Chem Soc 66(6):792–799
Farahi A, Kasiri M, Sudagar M, Soleimani Iraei M, Zorriehzahra SMJ (2012) Effect of dietary supplementation of Melissa officinalis and Aloe vera on hematological traits, lipid oxidation of carcass and performance in rainbow trout (Oncorhynchus mykiss). Online J Anim Feed Res 1:1–5
Farsam H, Amanlou M, Radpour MR, Salehinia AN, Shafiee A (2004) Composition of the essential oils of wild and cultivated Satureja khuzistanica Jamzad from Iran. Flavour Fragr J 19(4):308–310
Frankic T, Voljc M, Salobir J, Rezar V (2009) Use of herbs and spices and their extracts in animal nutrition. Acta Agric Slov 94(2):95–102
Galina J, Yin G, Ardo L, Jeney Z (2009) The use of immunostimulating herbs in fish. An overview of research. Fish Physiol Biochem 35(4):669–676
Ghafarifarsan H, Hoseinifar SH, Adorian TJ, Ferrigolo FRG, Raissy M, Van Doan H (2021a) The effects of combined inclusion of Malvae sylvestris, Origanum vulgare, and Allium hirtifolium boiss for common carp (Cyprinus carpio) diet: Growth performance, antioxidant defense, and immunological parameters. Fish Shellfish Immunol 119:670–677
Ghafarifarsani H, Hoseinifar SH, Aftabgard M, Van Doan H (2022) The improving role of savory (Satureja hortensis) essential oil for Caspian Roach (Rutilus caspicus) fry: Growth, haematological, immunological, and antioxidant parameters and resistance to salinity stress. Aquaculture 548:737653
Ghafarifarsani H, Kachuei R, Imani A (2021b) Dietary supplementation of garden thyme essential oil ameliorated the deteriorative effects of aflatoxin B1 on growth performance and intestinal inflammatory status of rainbow trout (Oncorhynchus mykiss). Aquaculture 531:735928
Ghafarifarsani H, Rashidian G, Bagheri T, Hoseinifar SH, Van Doan H (2021c) Study on growth enhancement and the protective effects of dietary prebiotic inulin on immunity responses of rainbow trout (Oncorhynchus mykiss) fry infected with Aeromonas hydrophila. Ann Anim Sci 21(2):543–559
Gharaei A, Karimi M, Mirdar J, Miri M, Faggio C (2020) Population growth of Brachionus calyciflorus affected by deltamethrin and imidacloprid insecticides. Iran J Fish Sci 19(2):588–601
Ghosal I, Mukherjee D, Chakraborty SB (2021) The effects of four plant extracts on growth, sex reversal, immunological and haemato-biochemical parameters in Nile tilapia, Oreochmomis niloticus (Linnaeus, 1758). Aquacult Res 52(2):559–576
Greathead H (2003) Plants and plant extracts for improving animal productivity. Proc Nutr Soc 62(2):279–290
Hamed HS, Ismal SM, Faggio C (2021) Effect of allicin on antioxidant defense system, and immune response after carbofuran exposure in Nile tilapia, Oreochromis niloticus. Comp Biochem Physiol Part C: Toxicol Pharmacol 240:108919
Harikrishnan R, Balasundaram C, Heo MS (2011) Impact of plant products on innate and adaptive immune system of cultured finfish and shellfish. Aquaculture 317(1–4):1–15
Hedayati SA, Farsani HG, Naserabad SS, Hoseinifar SH, Van Doan H (2019) Protective effect of dietary vitamin E on immunological and biochemical induction through silver nanoparticles (AgNPs) inclusion in diet and silver salt (AgNO3) exposure on Zebrafish (Danio rerio). Comp Biochem Physiol C Toxicol Pharmacol 222:100–107
Hedayati SAA, Ghafari Farsani H, Shahbazi Naserabad S, Gerami MH (2015) Acute toxicity and behavioral changes associated with diazinon in Rutilus rutilus caspicus and Hypophthalmicthys molitrix. Iran J Toxicol 9(30):1354–1359
Hernández-Contreras Á, Hernández MD (2020) Application of aromatic plants and their extracts in aquaculture. Feed Additives. Academic Press
Hoseini SM, Yousefi M (2019) Beneficial effects of thyme (Thymus vulgaris) extract on oxytetracycline-induced stress response, immunosuppression, oxidative stress and enzymatic changes in rainbow trout (Oncorhynchus mykiss). Aquac Nutr 25(2):298–309
Hoseini SM, Hosseini SA, Soudagar M (2012) Dietary tryptophan changes serum stress markers, enzyme activity, and ions concentration of wild common carp Cyprinus carpio exposed to ambient copper. Fish Physiol Biochem 38(5):1419–1426
Hoseinifar SH et al (2020) Dietary supplementation of lemon verbena (Aloysia citrodora) improved immunity, immune-related genes expression and antioxidant enzymes in rainbow trout (Oncorrhyncus mykiss). Fish Shellfish Immunol 99:379–385
Hoseinifar SH, Shakouri M, Yousefi S, Van Doan H, Shafiei S, Yousefi M, Mazandarani M, Mozanzadeh MT, Faggio C (2020) Humoral and skin mucosal immune parameters, intestinal immune related genes expression and antioxidant defense in rainbow trout (Oncorhynchus mykiss) fed olive (Olea europea L.) waste. Fish Shellfish Immunol 100:171–178
Hoseinifar SH, Zou HK, Van Doan H, Kolangi Miandare H, Hoseini SM (2018) Evaluation of some intestinal cytokines genes expression and serum innate immune parameters in common carp (Cyprinus carpio) fed dietary loquat (Eriobotrya japonica) leaf extract. Aquacult Res 49(1):120–127
Jahanjoo V, Yahyavi M, Akrami R, Bahri AH (2018) Influence of Adding Garlic (Allium sativum), Ginger (Zingiber officinale), Thyme (Thymus vulgaris) and Their Combination on the Growth Performance, HaematoImmunological Parameters and Disease Resistance to Photobacterium damselae in Sobaity Sea Bream (Sparidentex hasta) Fry. Turk J Fish Aquat Sci 18(4):633–645
Jiang J, Chen Y, Yu R, Zhao X, Wang Q, Cai L (2016) Pretilachlor has the potential to induce endocrine disruption, oxidative stress, apoptosis and immunotoxicity during zebrafish embryo development. Environ Toxicol Pharmacol 42:125–134
Kapoorchali MF, Fatemi SR, Vosoghy G, Matinfar M, Sharifian M (2009) Increasing in growth of Rutilus frisii kutum larvae with using slurry (fermented organic manure) in Yosefpoor propagation and rearing center (Iran). J Fish Aquat Sci 4(1):22–31
Khansari A, Yavari V, Alishahi M, Mousavi SM, Ghorbanpoor M, Bastami KD, Azizi S (2013) Effects of Oliviera decumbens and Satureja khuzestanica extract on some immunological and haematological parameters of Cyprinus carpio. Comp Clin Pathol 22(3):339–342
Kumar J, Priyadharshini M, Madhavi M, Begum SS, Ali AJ, Musthafa MS, Faggio C (2022) Impact of Hygrophila auriculata supplementary diets on the growth, survival, biochemical and haematological parameters in fingerlings of freshwater fish Cirrhinus mrigala (Hamilton, 1822). Comp Biochem Physiol Part A: Mol Integr Physiol 263:111097
Kumari K (2020) Pesticides toxicity in fishes: A review. J Entomol Zool Stud 8(5):1640–1642
Lumsangkul C, Linh NV, Chaiwan F, Abdel-Tawwab M, Dawood MA, Faggio C, Jaturasitha S, Van Doan H (2022) Dietary treatment of Nile tilapia (Oreochromis niloticus) with aquatic fern (Azolla caroliniana) improves growth performance, immunological response, and disease resistance against Streptococcus agalactiae cultured in bio-floc system. Aquac Rep 24:101114
Magnadottir B (2006) Innate immunity of fish (overview). Fish Shellfish Immunol 20:137–151
Mauri I et al (2011) Changes in complement responses in Gilthead seabream (Sparus aurata) and European seabass (Dicentrarchus labrax) under crowding stress, plus viral and bacterial challenges. Fish Shellfish Immunol 30(1):182–188
Merola C, Fabrello J, Matozzo V, Faggio C, Iannetta A, Tinelli A, Crescenzo G, Amorena M, Perugini M (2022) Dinitroaniline herbicide pendimethalin affects development and induces biochemical and histological alterations in zebrafish early-life stages. Sci Total Environ 828:154414
Mirghaed AT, Hoseini SM, Hoseinifar SH, Van Doan H (2020) Effects of dietary thyme (Zataria multiflora) extract on antioxidant and immunological responses and immune-related gene expression of rainbow trout (Oncorhynchus mykiss) juveniles. Fish Shellfish Immunol 106:502–509
Mohamadi Saei M, Beiranvand K, Khalesi MK, Mehrabi F (2016) Effects of dietary savory and myrtle essential oils on growth, survival, nutritional indices, serum biochemistry, and Hematology of farmed rainbow trout, Oncorhynchus mykiss, fry. J World Aquac Soc 47(6):779–785
Mohammadi G, Rashidian G, Hoseinifar SH, Naserabad SS, Van Doan H (2020) Ginger (Zingiber officinale) extract affects growth performance, body composition, haematology, serum and mucosal immune parameters in common carp (Cyprinus carpio). Fish Shellfish Immunol 99:267–273
Mulero V, Esteban M, Munoz J, Meseguer J (1998) Dietary intake of levamisole enhances the immune response and disease resistance of the marine teleost gilthead seabream (Sparus aurataL.). Fish Shellfish Immunol 8(1):49–62
Myszka K, Olejnik A, Majcher M, Sobieszczańska N, Grygier A, Powierska-Czarny J, Rudzińska M (2019) Green pepper essential oil as a biopreservative agent for fish-based products: Antimicrobial and antivirulence activities against Pseudomonas aeruginosa KM01. LWT 108:6–13
Naiel MA, Negm SS, Abd El-hameed SA, Abdel-Latif HM (2021) Dietary organic selenium improves growth, serum biochemical indices, immune responses, antioxidative capacity, and modulates transcription of stress-related genes in Nile tilapia reared under sub-optimal temperature. J Therm Biol 99:102999
Niewold TA (2014) Gut health, intestinal immunity and performance. In: Australian Poultry Science Symposium 72–77
Siwicki A, Anderson D (1993) An easy spectrophotometric assay for determining total protein and immunoglobulin levels in fish sera: correlation to fish health. Tech Fish Immunol 3:23–30
OECD (1994) OECD Guidelines for the Testing of Chemicals: Organization for Economic
Orisakwe OE, Afonne OJ, Chude MA, Obi E, Dioka CE (2003) Sub-chronic toxicity studies of the aqueous extract of Boerhavia diffusa leaves. J Health Sci 49(6):444–447
Owolabi OD, Abdulkareem SI (2021) Carica papaya and Mangifera indica modulate haematological, biochemical and histological alterations in atrazine-intoxicated fish, Clarias gariepinus (Burchell 1822). J Basic Appl Zool 82(1):1–18
Pandey G, Madhuri S, Mandloi AK (2012) Medicinal plants useful in fish diseases. Plant Arch 12(1):1–4
Paris-Palacios S, Biagianti-Risbourg S, Vernet G (2000) Biochemical and (ultra) structural hepatic perturbations of Brachydanio rerio (Teleostei, Cyprinidae) exposed to two sublethal concentrations of copper sulfate. Aquat Toxicol 50(1–2):109–124
Prasad G, Charles S (2010) Haematology and leucocyte enzyme cytochemistry of a threatened yellow catfish Horabagrus brachysoma (Gunther 1864). Fish Physiol Biochem 36(3):435–443
Puangkaew J, Kiron V, Satoh S, Watanabe T (2005) Antioxidant defense of rainbow trout (Oncorhynchus mykiss) in relation to dietary n-3 highly unsaturated fatty acids and vitamin E contents. Comp Biochem Physiol C Toxicol Pharmacol 140(2):187–196
Raissy M, Ghafarifarsani H, Hoseinifar SH, El-Haroun ER, Naserabad SS, Van Doan H (2022) The effect of dietary combined herbs extracts (oak acorn, coriander, and common mallow) on growth, digestive enzymes, antioxidant and immune response, and resistance against Aeromonas hydrophila infection in common carp, Cyprinus carpio. Aquaculture 546:737287
Rashidian G, Lazado CC, Mahboub HH, Mohammadi-Aloucheh R, Prokić MD, Nada HS, Faggio C (2021) Chemically and green synthesized ZnO nanoparticles alter key immunological molecules in common carp (Cyprinus carpio) skin mucus. Int J Mol Sci 22(6):3270
Rashidian G, Mahboub HH, Fahim A, Hefny AA, Prokić MD, Rainis S, Boldaji JT, Faggio C (2022a) Mooseer (Allium hirtifolium) boosts growth, general health status, and resistance of rainbow trout (Oncorhynchus mykiss) against Streptococcus iniae infection. Fish Shellfish Immunol 120:360–368
Rashidian G, Shahin K, Elshopakey GE, Mahboub HH, Fahim A, Elabd H, Prokić MD, Faggio C (2022b) The Dietary Effects of Nutmeg (Myristica fragrans) Extract on Growth, Hematological Parameters, Immunity, Antioxidant Status, and Disease Resistance of Common Carp (Cyprinus carpio) against Aeromonas hydrophila. J Mar Sci Eng 10(3):325
Reverter M, Bontemps N, Lecchini D, Banaigs B, Sasal P (2014) Use of plant extracts in fish aquaculture as an alternative to chemotherapy: current status and future perspectives. Aquaculture 433:50–61
Ringø E, Olsen RE, Vecino JG, Wadsworth S, Song SK (2012) Use of immunostimulants and nucleotides in aquaculture: a review. J Mar Sci Res Dev 2(1):104
Roby MHH, Sarhan MA, Selim KAH, Khalel KI (2013) Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind Crops Prod 43:827–831
Ross NW, Firth KJ, Wang A, Burka JF, Johnson SC (2000) Changes in hydrolytic enzyme activities of naive Atlantic salmon Salmo salar skin mucus due to infection with the salmon louse Lepeophtheirus salmonis and cortisol implantation. Dis Aquat Org 41(1):43–51
Rudiansyah M, Abdelbasset WK, Jasim SA, Mohammadi G, Dharmarajlu SM, Nasirin C, Jalil AT, Opulencia MJC, Abid MK, Naserabad SS(2022) Beneficial alterations in growth performance, blood biochemicals, immune responses, and antioxidant capacity of common carp (Cyprinus carpio) fed a blend of Thymus vulgaris, Origanum majorana, and Satureja hortensis extracts. Aquaculture 738254.
Saha S, Chukwuka AV, Mukherjee D, Patnaik L, Nayak S, Dhara K, Saha NC, Faggio C (2021) Chronic Effects of Diazinon® Exposures Using Integrated Biomarker Responses in Freshwater Walking Catfish, Clarias batrachus. Appl Sci 11(22):10902
Sakai M (1999) Current research status of fish immunostimulants. Aquaculture 172(1–2):63–92
Saurabh S, Sahoo PK (2008) Lysozyme: an important defence molecule of fish innate immune system. Aquacult Res 39(3):223–239
Shahbazi Naserabad S, Mirvaghefi A, Rashidiyan G, Rostamian N, Ghafari Farsani H (2017) Investigating the Agent of Temperature into Acute Toxicity (LC50 96 h) of Edifenphos in Rutilus Frisii Kutum (Kamensky, 1901). Iran J Toxicol 11(2):39–44
Sharifian I, Rastiannasab A, Gandomkar H (2015) Effects of diazinon on some immunological components of common carp, Cyprinus carpio juveniles. Comp Clin Pathol 24(6):1339–1341
Silambarasan N, Hemalatha S (2015) Protective Role of Vitamin-C Against Malathion Toxicity On Certain Biochemical Parameters In Liver Of Fresh Water Fish. Int J Modn Res Revs 3(11):1058–1061
Soni R, Verma SK (2018) Acute toxicity and behavioural responses in Clarias batrachus (Linnaeus) exposed to herbicide pretilachlor. Heliyon 4(12):e01090
Soni R, Verma SK (2020) Impact of herbicide pretilachlor on reproductive physiology of walking catfish, Clarias batrachus (Linnaeus). Fish Physiol Biochem 46(6):2065–2072
Srivastava P, Singh A, Pandey AK (2016) Pesticides toxicity in fishes: biochemical, physiological and genotoxic aspects. Biochem Cell Arch 16(2):199–218
Stara A, Pagano M, Albano M, Savoca S, Di Bella G, Albergamo A, Koutkova Z, Sandova M, Velisek J, Fabrello J, Faggio C (2021) Effects of long-term exposure of Mytilus galloprovincialis to thiacloprid: A multibiomarker approach. Environ Pollut 289:117892
Subramanian S, MacKinnon SL, Ross NW (2007) A comparative study on innate immune parameters in the epidermal mucus of various fish species. Comp Biochem Physiol Biochem Mol Biol 148(3):256–263
Suchiang P(2021) A Review on Toxicity of Pesticides in Catfishes: Reproductive, Haematological and Biochemical Aspects.Annu Rev Res Biol47–59
Sunyer JO, Tort L, Lambris JD (1997) Diversity of the third form of complement, C3, in fish: functional characterization of five forms of C3 in the diploid fish Sparus aurata. Biochem J 326(3):877–881
Taherian SMR, Hosseini SA, Jafari A, Etminan A, Birjandi M (2019) Acute toxicity of Zinc Oxide nanoparticles from Satureja hortensis on Rainbow Trout (Oncorhynchus mykiss). Turk J Fish Aquat Sci 20(6):481–489
Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M (2006) Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf 64(2):178–189
Vali S, Majidiyan N, Azadikhah D, Varcheh M, Tresnakova N, Faggio C (2022) Effects of Diazinon on the Survival, Blood Parameters, Gills, and Liver of Grass Carp (Ctenopharyngodon idella Valenciennes, 1844; Teleostei: Cyprinidae). Water 14(9):1357
Vali S, Mohammadi G, Tavabe KR, Moghadas F, Naserabad SS (2020) The effects of silver nanoparticles (Ag-NPs) sublethal concentrations on common carp (Cyprinus carpio): Bioaccumulation, hematology, serum biochemistry and immunology, antioxidant enzymes, and skin mucosal responses. Ecotoxicol Environ Saf 194:110353
Van Doan H, Hoseinifar S, Sringarm K, Jaturasitha S, Yuangsoi B, Dawood MA, Faggio C (2019) Effects of Assam tea extract on growth, skin mucus, serum immunity and disease resistance of Nile tilapia (Oreochromis niloticus) against Streptococcus agalactiae. Fish & Shellfish Immunol 93:428–435
Vazirzadeh A, Marhamati A, Rabiee R, Faggio C (2020) Immunomodulation, antioxidant enhancement and immune genes up-regulation in rainbow trout (Oncorhynchus mykiss) fed on seaweeds included diets. Fish Shellfish Immunol 106:852–858
Wang T, Long X, Cheng Y, Liu Z, Yan S (2014) The potential toxicity of copper nanoparticles and copper sulphate on juvenile Epinephelus coioides. Aquat Toxicol 152:96–104
Windisch W, Schedle K, Plitzner C, Kroismayr A (2008) Use of phytogenic products as feed additives for swine and poultry. Anim Sci J 86(suppl14):140–148
Wu G et al (2007) Immunological and biochemical parameters in carp (Cyprinus carpio) after Qompsell feed ingredients for long-term administration. Aquacult Res 38(3):246–255
Yano T (1992) Assays of hemolytic complement activity. Techniques in Fish Immunology. SOS Publications, Fair Haven, NJ, pp 131–141
Yonar ME, Sakin F (2011) Ameliorative effect of lycopene on antioxidant status in Cyprinus carpio during pyrethroid deltamethrin exposure. Pestic Biochem Physiol 99(3):226–231
Yousefi M, Farsani MN, Ghafarifarsani H, Hoseinifar SH, Van Doan H (2021a) The effects of dietary supplementation of mistletoe (Viscum album) extract on the growth performance, antioxidant, and innate, immune responses of rainbow trout (Oncorhynchus mykiss). Aquaculture 536:736385
Yousefi M, Ghafarifarsani H, Hoseinifar SH, Rashidian G, Van Doan H (2021b) Effects of dietary marjoram, Origanum majorana extract on growth performance, hematological, antioxidant, humoral and mucosal immune responses, and resistance of common carp, Cyprinus carpio against Aeromonas hydrophila. Fish Shellfish Immunol 108:127–133
Yousefi M, Vatnikov YA, Kulikov EV, Plushikov VG, Drukovsky SG, Hoseinifar SH, Van Doan H (2020) The protective effects of dietary garlic on common carp (Cyprinus carpio) exposed to ambient ammonia toxicity. Aquaculture 526:735400
Zargar A, Rahimi-Afzal Z, Soltani E, Taheri Mirghaed A, Ebrahimzadeh‐Mousavi HA, Soltani M, Yuosefi P (2019) Growth performance, immune response and disease resistance of rainbow trout (Oncorhynchus mykiss) fed Thymus vulgaris essential oils. Aquacult Res 50(11):3097–3106
Zheng ZL, Tan JY, Liu HY, Zhou XH, Xiang X, Wang KY (2009) Evaluation of oregano essential oil (Origanum heracleoticum L.) on growth, antioxidant effect and resistance against Aeromonas hydrophila in channel catfish (Ictalurus punctatus). Aquaculture 292(3–4):214–218
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization, Turki Jalil; Methodology, Field Study, Sampling, Shahbazi Naserabad., Abdelbasset and Turki Jalil; Software, Widjaja and Altimari; Validation, Turki Jalil, Widjaja; Data curation, Aravindhan, Attia Thijail; Writing original draft preparation, Shahbazi Naserabad, Abdelbasset; Writing-review and editing, Turki Jalil, Fakri Mustafa, Widjaja; Supervision, Turki Jalil and Widjaja; Project administration, Turki Jalil. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
There is no conflict of interest to declare.
Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All experiments were performed following the protocol approved by the committee of ethics of the faculty of sciences of the University of Tehran (357; 8 November 2000).
Consent to participate
Not applicable.
Consent for publication
All authors give consent for publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jalil, A.T., Abdelbasset, W.K., Shichiyakh, R.A. et al. Protective effects of summer savory (Satureja hortensis) oil on growth, biochemical, and immune system performance of common carp exposed to pretilachlor herbicide. Vet Res Commun 46, 1063–1074 (2022). https://doi.org/10.1007/s11259-022-09970-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-022-09970-z