Abstract
In this study, we investigated the effects of probiotic, acidifier and synbiotic supplementation on growth performance, mortality rate, intestinal gene expressions, fecal shedding, and organs colonization induced by Escherichia coli in broiler chickens. Six experimental groups were included; negative control group (NC), positive control group (PC), probiotic group (PR), acidifier group (AC), synbiotic group (SY) and colistin sulfate group (CS). Chickens in groups NC and PC were fed a basal diet, while chickens in groups PR, AC, SY, and CS were fed a basal diet containing probiotic, acidifier, synbiotic and colistin sulfate, respectively from the 1st day to the 28th day of age. At 7 days of age, all groups (not NC) were orally challenged with 0.5 ml (1.0 × 109 CFU/ml) E. coli O78. The dietary supplementation of acidifier and synbiotic were sufficient to quell the devastating effects of E. coli infection in broilers. Growth performances represented by body weight gain, feed intake and feed conversion ratio were significantly improved as well as, mortalities were prevented whilst the ileal pro-inflammatory gene expressions (IL-6, IL-8, IL-13, TLR-4, IFN-γ, LITAF, AvBD-2, and AvBD-9) were significantly downregulated and the anti-inflammatory cytokine (IL-10) was significantly increased. In addition, E. coli fecal shedding and organs colonization was significantly diminished. It was concluded that the addition of both acidifier and synbiotic to the diet of broilers infected with E. coli could modulate the intestinal inflammatory responses induced by E. coli infection and minimized the inflammation-induced damage which resulted in improvement in growth performance, prevention of mortalities and reduction of E. coli environmental contamination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Avian colibacillosis is caused by a group of pathogenic strains of E. coli called avian pathogenic Escherichia coli (APEC), especially serogroups O1, O2, and O78 (McPeake et al. 2005). It is a complicated disease of poultry that frequently causes extreme economic losses in the poultry production globally due to variable mortalities, egg production losses, carcasses condemnations and poor performances of the bird (Dho-Moulin and Fairbrother 1999). Strains of E. coli O78 can colonize the gut tissues and cause numerous extra-and intra intestinal diseases in different hosts (La Ragione et al. 1999, 2000; Adiri et al. 2003). In addition, several strains of E. coli are normal inhabitants in the gastrointestinal tract of birds, however, under certain conditions such as adverse environmental condition and/or a weakened immune system of the host (Barnes et al. 2008); it becomes pathogenic and causes intestinal disruption which leads to poor growth performance of chickens (Cao et al. 2013; Gao et al. 2013). It is well known that antibiotics are used to prevent and/or control of E. coli infection in animals, but overuse and/or misuse lead to the emergence and spread of multi-drug-resistant (MDR) mutants of E. coli among poultry farms (Awad et al. 2016). These MDR strains enter the food chain through contaminated meat and eggs and represent hazards to human health (Yang et al. 2004; Dheilly et al. 2013). Consequently, it is important to find possible alternatives to antibiotics to attenuate and control of avian colibacillosis which affects growth performance and health status of chickens. There are several alternative products including probiotics, acidifiers (organic acids), prebiotics, synbiotics, herbal products, enzymes, and immuno-modulators. The mechanism of action of these products in promoting the health of the digestive tract is not fully understood. A further comprehension of their role in the health of the gut will allow the evolution of new agents to control enteric diseases of poultry and understanding how these alternative treatments differ from conventional treatments against avian colibacillosis.
Probiotics are live microorganisms feed supplement that beneficially affect the host by enhancing the intestinal microbial equilibrium (Fuller 1999). Indeed, probiotics have been reported to improve immunity, intestinal morphology and prevent intestinal colonization by pathogenic bacteria, such as Clostridium perfringens and E. coli (Teo and Tan 2006; Zhang et al. 2016). Prebiotics typically refer to oligosaccharides that are non-digestible feed ingredients which have the ability to induce the growth or metabolic activity of some beneficial intestinal flora, improve the intestinal ecosystem and immunity and also, enhance the general status of the host (Gibson et al. 2004; Baurhoo et al. 2009). Synbiotics are a mixture of probiotic and prebiotic which have positive effects on the health of the gut, diet digestibility, and general performance of birds (Patterson and Burkholder 2003). Acidifiers are organic acids and their salts which improve the growth of birds by enhancing the nutrient absorption and preventing the damage of intestinal epithelial wall by reducing the colonization and the production of toxic components of pathogenic microbes (Langhout 2000; Adil et al. 2010).
The occurrence of inflammation appears as a leading and regular endpoint for regulating the intensity of the degradation of the gastrointestinal tract (Van Deun et al. 2008; Teirlynck et al. 2009). This can directly lead to damage to the gut integrity and, impede nutrients digestion and absorption (Olkowski et al. 2006). The final consequence of this cascade of calamities is lowering animal performance and significant economic loss due to disease. Accordingly, understanding the influence of alternative treatments on the inflammatory state and the health of the gastrointestinal tract explain the efficacy and benefits of these treatments. However, there is no thorough studies have been carried out on whether probiotic, acidifier and synbiotic can diminish intestinal inflammation against E. coli in broiler chickens yet. Thus, the effects of these additives on the intestinal gene expressions in one study have not been characterized. Therefore; the current study was conducted to determine the effects of dietary supplementation of probiotic, acidifier and synbiotic on growth performances, mortalities, intestinal inflammatory responses, and E. coli fecal shedding and organs colonization in broiler chickens challenged with E. coli.
Material and methods
Experimental birds
Day-old Hubbard broiler chicks (n = 250) were purchased from a commercial hatchery. On arrival 16 chicks were randomly selected, sacrificed and cultured for E. coli. All chicks were negative for E. coli isolation. Chicks were reared in cleaned and disinfected pens and kept under complete observation for 4 weeks (experimental period). All birds were offered unmedicated broiler ration and had free access to water and feed. Diets were formulated to meet the NRC (1994) recommendation for broiler chickens (Table 1). The temperature was adjusted to 32 °C in the first week and gradually decreased to 25 °C at the end of the experiment. Experimental procedures were performed and conducted under the recommendations of the Mansoura University Animal Care and Use Committee.
Escherichia coli challenge strain
The E. coli O78 strain was isolated in our laboratory from a field case of avian colibacillosis and had been fully identified, classified and serotyped as previously performed by (Edwards and Ewing 1972; Quinn et al. 1994). E. coli characterized by resistance to 20 μg/ml of nalidixic acid. Fresh E. coli O78 inoculums adjusted to contain 1.0 × 109 CFU/ml as described by (Wang et al. 2016). At 7 days of age, each bird in the experimentally infected groups was orally inoculated with 0.5 ml (1.0 × 109 CFU/ml) E. coli O78 isolate by using a polyethylene tube attached to a syringe. Birds in the negative control group were orally inoculated with the same volume of sterile PBS on day 7.
Experimental design and diet
A total of 234, one-day-old chicks randomly allocated into six treatment groups with 3 replicates for each group (13 birds × 3 replicates). The negative control group (NC) consisted of non-challenged birds fed a basal diet. The positive control group (PC) consisted of challenged birds fed a basal diet. Probiotic group (PR) consisted of challenged birds fed a basal diet supplemented with 2.5 × 109 cfu Pediococcus acidilactici/Kg of diet (Bactocell®, Lallemand Animal Nutrition, France). Acidifier group (AC) consisted of challenged birds fed a basal diet supplemented with a mixture of organic acids and their salt at 0.6 g/Kg of diet (Galliacid®, Jefo, Company, Saint- Hyacinthe Canada). Synbiotic group (SY) consisted of challenged birds fed a basal diet supplemented with probiotic strain Enterococcus faecium and prebiotic fructo-oligosaccharides at 1 g/Kg of diet (Biomin®IMBO, Biomin, GmbH, Herzogenburg, Austria). Colistin sulfate group (CS) consisted of challenged birds fed a basal diet supplemented with 20 mg colistin sulfate/Kg of diet. All groups had similar initial body weights.
Growth performance
Birds in each replicate were weighed individually to evaluate the body weight and average body weight gain (BWG), also feed intake was determined for each replicate to calculate feed conversion ratio (FCR) at 7, 14, and 21 days post-challenge.
Clinical signs, mortalities and gross lesions
In every group, the birds were observed twice daily throughout the experimental period for monitoring and recording clinical signs and mortalities. Dead and sacrificed birds were collected, necropsied, and samples were collected to detect the colonization of E. coli in internal organs, and record any gross lesions.
Ileum sample collection
Six birds were randomly selected and sacrificed from each group for ileum sample collection at 2 days post-challenge. Samples from ileum were sterile collected, washed in phosphate buffer saline (PBS), snap frozen in liquid nitrogen and stored at −80 °C for quantification of gene expression.
Total RNA extraction and reverse transcription
Total RNA was extracted from ileum tissues using Trizol reagent following the manufacturer’s instructions (Direct-zolTM RNA MiniPrep, catalog No. R2050). The amount of extracted RNA was quantified and qualified using NanoDrop® ND-1000 Spectrophotometer. The cDNA of each sample was synthesized following the manufacturer’s protocol (SensiFastTM cDNA synthesis kit, Bioline, catalog No. Bio- 65,053). The reaction mixture was carried out in a total volume 20 μl consisted of total RNA up to 1 μg, 4 μl 5x Trans Amp buffer, 1 μl reverse transcriptase and DNase free-water up to 20 μl. The final reaction mixture was placed in a thermal cycler and the following program was carried out; primer annealing at 25 °C for 10 min, reverse transcription at 42 °C for 15 min followed by inactivation at 85 °C for 5 min. The samples were held at 4 °C.
Quantitative real time PCR
Relative quantification of mRNA levels of IL-6, IL-8, IL-10, IL-13, TLR-4, IFN-γ, LITAF, AvBD-2 and AvBD-9 were performed by real-time PCR using SYBR Green PCR Master Mix (2x SensiFastTM SYBR, Bioline, catalog No. Bio-98,002). Primer sequences were shown in (Table 2). The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a constitutive control for normalization. The reaction mixture was carried out in a total volume 20 μl consisted of 10 μl 2x SensiFast SYBR, 3 μl cDNA, 5.4 μl H2O (deionized distilled water), 0.8 μl of each primer. The PCR cycling conditions were as follows: 95 °C for 10 min followed by 45 cycles of 94 °C for 15 s, annealing temperatures as shown in Table 1 for 20 s, and 72 °C for 20 s. At the end of the amplification phase, a melting curve analysis was performed to confirm the specificity of the PCR product. The relative expression of the gene in each sample versus a control in comparison to GAPDH gene and calculated according to the 2-ΔΔCt method (Pfaffl 2001).
E. coli fecal shedding
Cloacal swabs were collected from all birds in each group at 7, 14, and 21 days post-challenge. A sterile cotton swab was inserted into the cloaca of each bird and spread out gently to collect the sample. The swab was transferred to a 9 ml tube containing nutrient broth and incubated at 37 °C overnight. A loopful of broth was then streaked on eosin methylene blue (EMB) agar (Oxoid, Basingstoke, UK) containing 20 μg/ml of nalidixic acid and incubated for 18–24 h at 37 °C. Typical colonies were subcultured onto MacConkey agar (Oxoid, Basingstoke, UK) containing 20 μg/ml of nalidixic acid and incubated at 37 °C for 24 h. Suspected colonies were characterized morphologically, biochemically, and serologically as reported by (Edwards and Ewing 1972; Quinn et al. 1994).
Re-isolation of E. coli from internal organs
To re-isolate the challenge E. coli from internal organs, six chickens per group (2 chickens per replicate) were randomly selected and sacrificed at 7, 14, and 21 d post-challenge. In these birds, swabs from liver, spleen, and cecum were plated on eosin methylene blue (EMB) agar (Oxoid, Basingstoke, UK) containing 20 μg/ml of nalidixic acid and incubated for 18–24 h at 37 °C. Typical colonies were subcultured onto MacConkey agar (Oxoid, Basingstoke, UK) containing 20 μg/ml of nalidixic acid and incubated at 37 °C for 24 h. Suspected colonies were characterized morphologically, biochemically, and serologically as reported by (Edwards and Ewing 1972; Quinn et al. 1994).
Statistical analysis
The obtained data were analyzed by using the statistical program SPSS software version 17.0 (SPSS Inc., Chicago, IL) through one-way analysis of variance (ANOVA). The results of growth performance, mortality rate and E. coli fecal shedding and organs colonization were further compared via Tukey’s test. Duncan’s multiple range test was used to compare the means of ileal gene expression levels. Comparisons were statistically significant at P < 0.05.
Results
Growth performance
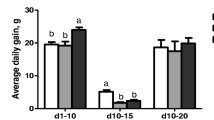
Body weight gains (BWG) and feed intake (FI) of the birds in PC (positive control) group were significantly (p < 0.05) lower than that of the birds in other groups during 7, 14 and 21 days post-challenge (Table 3). Moreover, at 7 and 14 days post-challenge, the birds in the CS (Colistin sulfate) group had a significant (p < 0.05) less BWG than the birds in NC (negative control) group and AC (acidifier) group, while there was no significant difference (p > 0.05) among the BWG of CS, PR (probiotic) and SY (synbiotic) groups. Overall, AC group birds had greater BWG than SY, PR, and CS groups. On the other hand, there was no significant difference (p > 0.05) among the FI of NC, AC, SY, PR and CS groups at 7, 14 and 21 days post-challenge. Birds in the NC, AC, SY, PR and CS groups had a significant (p < 0.05) better feed conversion ratio (FCR) than those in PC group from 7 to 21 days post-challenge. The FCR was nearly similar between the NC and AC groups at 7 and 14 days post-challenge while, it became equal at 21 days post-challenge. The NC and AC groups had the lowest FCR.
Clinical signs and mortality rate
The signs recorded in the experimental birds were ruffled feathers, inappetence, respiratory signs, sitting on the hocks and diarrhea, during 7 and 14 days post-challenge. Birds in PR, AC, SY, and CS had less clinical signs than in E. coli challenged ones. No mortalities were recorded in NC, AC, SY and PR groups throughout the experimental period (Table 3). The number of dead birds was reduced from 4 in the PC group to 2 in CS group during the 7 days post-challenge. Moreover, at 14 days post-challenge, the mortality was found only in the PC group (one bird). Necropsy of the dead birds revealed pericarditis and enteritis.
Relative expression of ileal genes
The upregulation in mRNA expressions of ileal IFN-γ, LITAF, AVBD-2, and AvBD-9 caused by E. coli challenge was significantly (p < 0.05) downregulated by dietary supplementation of probiotic, acidifier, synbiotic and antibiotic. In addition, probiotic, acidifier and synbiotic treated birds showed significant (p < 0.05) lower ileal Toll-like receptor 4 (TLR-4), IL-8 and IL-13 expressions compared with challenged ones, while there were no significant differences (p > 0.05) between antibiotic-treated and PC birds (Fig. 1). E. coli challenge downregulated the ileal IL-10 expression, but the administration of acidifier and synbiotic into challenged birds significantly (p < 0.05) negated the reduction in IL-10, while administration of probiotic and antibiotic non-significance (p > 0.05) prevent the reduction of it. An increasing trend of ileal IL-6 expression was found with E. coli challenge. The effect of the addition of probiotic and antibiotic was non-significant (p > 0.05) on ileal IL-6 expression, while acidifier and synbiotic had a significant effect (p < 0.05).
mRNA levels of ileal IL-6, IL-8, IL-10, IL-13, TLR-4, IFN-γ, LITAF, AvBD-2 and AvBD-9 expressions in NC, PC, PR, AC, SY and CS groups. Small alphabetic letters show significance when (P < 0.05). NC = Negative control group. PC = Positive control group. PR = Probiotic group. AC = Acidifier group. SY = Synbiotic group. CS = Colistin sulfate group
Recovery of E. coli from cloacal swabs
The overall fecal shedding of E. coli in the positive control group was significantly (p < 0.05) higher than NC, PR, AC, and SY groups and was non-significantly higher than CS group. At 7, 14 days post-challenge, the addition of probiotic, acidifier and synbiotic to the diet of infected birds significantly (p < 0.05) reduced the fecal shedding of E. coli, while infected birds treated with colistin sulfate had non-significantly lower E. coli fecal shedding than PC birds. At 21 days post-challenge, acidifier, and synbiotic treated birds had significantly (p < 0.05) lower E. coli fecal shedding than PC birds, while there were no significance (p > 0.05) between probiotic and colistin sulfate treated and control positive birds (Table 4). E. coli was isolated from more than 50% of the birds in the positive control group at 7 and 14 days post-challenge. E. coli fecal shedding was not recovered from cloacal swabs of the bird of the negative control group along the course of this study.
Re-isolation of E. coli from internal organs
The recovery of the challenge E. coli from visceral organs of different groups is listed in (Table 5). The re-isolation of the challenge organism from liver, spleen, and cecum of birds in NC group was negative during the experimental period. The rate of recovery from liver, spleen, and cecum was significantly (p < 0.05) higher in PC group than in other groups at 7, 14 and 21 days post-challenge. At 21 days post-challenge, E. coli could not be re-isolated from the liver of birds in PR, AC, SY and CS groups. In addition, it could not be recovered from the spleen of birds in AC and SY groups at 14 and 21 days post-challenge. Moreover, no E. coli isolated from the cecum of birds in AC group from 14 to 21 days post-challenge.
Discussion
The non-antibiotic feed additives like probiotic, prebiotic, synbiotic and acidifier have received a concern as health and growth promoters. So, they are a promising future for the health care industry. The role of these additives in enhancing and supporting the growth performance and feed utilization efficiency in poultry was previously studied (Alimohamadi et al. 2014; Al-Sultan et al. 2016). The breeding of chickens in an unclean environment suppresses their growth performance due to intestinal distortion induced by bacterial infection (Cao et al. 2013; Gao et al. 2013). The results of the present study confirmed the negative effects of E. coli on growth performance of the birds including, BWG, FI, and FCR. While, the diet supplemented with probiotic, acidifier, synbiotic and antibiotics significantly improved the growth performance of the infected birds. In addition, the FI and FCR in PR, AC and SY groups were numerically better than in the CS group. Our results were in accordance with previous studies (Teo and Tan 2006; Yang et al. 2008; Cao et al. 2013; Zhang et al. 2016). In contrast, consumption of probiotic, prebiotic and synbiotic had no significant effect on BWG, FI, and FCR of chickens (Salehimanesh et al. 2016; Wang et al. 2016; Gadde et al. 2017). The improvement of growth parameters by these additives could be attributed to the improvement of digestibility, enhancement of intestinal microflora population, healthy intestine, and augmentation in the number of beneficial microorganisms which inhibit colonization of pathogenic bacteria (Guo et al. 2004). So, applications of these additives at the early stage of broilers life provide optimal effects due to immature gastro-intestinal function (Uni 1999).
In the current study, broiler chickens fed diets contain probiotic, acidifier, and synbiotic prior to challenge did significantly reduce the number of mortalities resulting from infection with E. coli. Similarly, administration of competitive exclusion culture to neonatal pigs at 12 and 24 h after birth significantly reduced mortality caused by enterotoxigenic E. coli when compared with control ones (Genovese et al. 2000). While in another study, there was no significant difference in mortalities between birds fed prebiotic + E. coli challenged and E. coli challenged only (Huff et al. 2006). Based on mortality, probiotic, acidifier and synbiotic treatments were more effective than antibiotic treatment in protecting birds from E. coli infection.
TLR-4 is a receptor for lipopolysaccharides (LPS) that is present in the cell wall of Gram-negative bacteria such as E. coli, which lead to an intense excess of synthesis and production of pro-inflammatory cytokines through initiation and activation of downstream signaling cascades such as nuclear factor-kB (NF-kB) (Li and Verma 2002). In addition, exposure of intestine to LPS resulted in excessive production of pro-inflammatory cytokine tumor necrosis factor alpha (TNF-α), which stimulated the secretion of IL-8 and the expression of TLR-4 from intestinal epithelial cell leading to amplification of intestinal inflammation (Bai et al. 2004; Hausmann et al. 2002). Although inflammation was important for protecting tissues after infection, the uncontrolled inflammatory reaction would lead to tissue damage along with high nutrient consumption (Dinarello 2000; Dobrovolskaia and Vogel 2002; Hakansson and Molin 2011). Following E. coli infection, we observed an increase in ileal TLR-4, IL-8 and IL-13 expressions, which elucidated that the intestinal inflammatory responses induced by E. coli, might be attributed to TLR-4/ NF-kB signaling pathway. This was similar to some recent studies (Li et al. 2015; Wang et al. 2016). The addition of probiotic, acidifier and synbiotic to the diet reduced the expression of ileal TLR-4, IL-8 and IL-13 expressions in challenged birds. These findings suggested that dietary supplementation of probiotic, acidifier and synbiotic lighten the inflammation of ileum and lessen the ileal pro-inflammatory cytokines produced by E. coli infection through the repression of TLR-4/ NF-kB signaling pathway (Wang et al. 2016). In the same manner, the higher intestinal TLR4 and IL-8 expressions induced by E. coli infection in broiler chickens were alleviated by dietary supplementation of probiotic and prebiotic (Wang et al. 2016). Moreover, probiotic and prebiotic minimized the higher intestinal TLR-4 expression in pigs infected with E. coli (Badia et al. 2012) and suppressed the expression of pro-inflammatory cytokines in the intestine of mice due to pathogen invasion (Jawhara et al. 2012). Many studies showed that systemic and intestinal immunological response during inflammatory challenge could be altered by probiotics supplementation (Bai et al. 2004; Thomas and Versalovic 2010; Yang et al. 2015). The pivotal anti-inflammatory cytokine (IL-10) was the result of macrophage activation, which maintained the immune balance by inhibiting the exaggerated pro-inflammatory cytokine productions (Corwin 2000), thus the elevation of ileal IL-10 expression in infected broilers fed acidifier and synbiotic might have also resulted from the reduction of intestinal inflammation induced by E. coli challenge. These results were in agreement with Wang et al. (2016) recorded increases in ileal IL-10 expressions in broilers fed mannan-oligosaccharide and challenged with E. coli when compared to E. coli challenged only ones. Moreover, probiotics induced an immune response in broiler chickens infected with E. coli K88 (Zhang et al. 2016). On the other hand, a previous study reported that the immune response was not significantly affected by inclusion of probiotics in the diet of broilers challenged with E. coli K88 (Cao et al. 2013). The lymphoid cells, especially intestinal intraepithelial lymphocytes (IELs) were the site of LITAF expression which upregulated the TNF-α gene expression. Similarly, The expression of LITAF elevated when in vitro stimulation of macrophages with Salmonella and E. coli endotoxins (Hong et al. 2006). AvBD-9 and AvBD-2 played an essential role in innate host defense which significantly increased in the digestive tract of chickens following foodborne pathogens infection as S. Typhimurium and E. coli (van Dijk et al. 2007; Akbari et al. 2008). In this trial, all dietary supplementation significantly decreased the ileal IFN-γ, LITAF, AVBD-2, and AvBD-9 expression in the challenging broilers. Acidifier, synbiotic and probiotic would modulate the inflammatory activities resulted from the E. coli challenge and played a role in the restoration of cytokine balance to minimize inflammation-induced damage. These finding could explain the downregulation of proinflammatory genes recorded in AC, SY and PR groups in descending arrangement.
The numbers of birds shedding E. coli in all experimental challenged groups remained E. coli positive during the entire course of our study. Clearly, the overall fecal shedding of E. coli was significantly affected by all treatments except antibiotic treatment with the superiority of acidifier followed by synbiotic, then probiotic. The results concurred with a previous study, where the fecal shedding of E. coli O78 was significantly higher in challenged birds than probiotic treated infected one (La Ragione et al. 2001). Moreover, the neonatal pigs fed competitive exclusion and challenged with E. coli showed a significant decrease in fecal shedding of E. coli (Genovese et al. 2000). In other studies, the fecal shedding of S. Enteritidis significantly diminished by using acidifier, prebiotic and probiotic in broilers infected with S. Enteritidis (Knap et al. 2011; Attia et al. 2012). Thus, the reduction of E. coli fecal shedding could distinctly minimize the environmental contamination and the horizontal transmission of E. coli within and between chicken flocks (Arafat et al. 2017).
Collectively, the rate of E. coli recovery from internal organs was affected by supplementation of diets with additives, where chickens that were fed probiotic, acidifier, synbiotic and antibiotic followed by E. coli infection had significantly lower rate of E. coli re-isolation than chickens infected with E. coli only. These results were consistent with another study that concluded that acidifier, prebiotic, probiotic and antibiotic groups had significantly lower S. Enteritidis re-isolation from liver, spleen, and cecum than positive control ones (Attia et al. 2012). Furthermore, there was a marked reduction in the colonization of broiler ceca by E. coli when competitive exclusion was used (Hofacre et al. 2002; Nuotio et al. 2013). Moreover, colonization of E. coli O78 in the cecum and deep tissues significantly decreased in day-old chicks provided by probiotics and infected with E. coli O78 (La Ragione et al. 2001). Higgins et al. (2008) found that birds administered probiotic then challenged with S. Enteritidis had a marked reduction in S. Enteritidis colonization. While in another report, the levels of E. coli colonization in the ceca are equal in birds administered probiotic + E. coli challenge and birds infected with E. coli only (La Ragione et al. 2004).
It was concluded that the addition of both acidifier and synbiotic to the diet of broilers infected with E. coli could modulate the intestinal inflammatory responses induced by E. coli infection and minimized the inflammation-induced damage which resulted in improvement in growth performance, prevention of mortalities and reduction of E. coli environmental contamination. Therefore, these products might be promising alternatives for antibiotic in poultry production.
References
Adil S, Tufail B, Gulam AB, Masood S, Manzoor R (2010) Effect of dietary supplementation of organic acids on performance, intestinal histomorphology, and serum biochemistry of broiler chicken. Vet Med Int 7 pages. https://doi.org/10.4061/2010/479485
Adiri RS, Gophna U, Ron EZ (2003) Multilocus sequence typing (MLST) of Escherichia coli O78 strains. FEMS Microbiol Lett 222:199–203
Akbari MR, Haghighi HR, Chambers JR, Brisbin JL, Read R, Sharif S (2008) Expression of antimicrobial peptides in cecal tonsils of chickens treated with probiotics and infected with Salmonella enterica serovar typhimurium. Clin Vaccine Immunol 15:1689–1693
Alimohamadi K, Taherpour K, Ghasemi KHA, Fatahnia F (2014) Comparative effects of using black seed (Nigella sativa), cumin seed (Cuminum cyminum), probiotic or prebiotic on growth performance, blood haematology and serum biochemistry of broiler chicks. J Anim Physiol Anim Nutr 98:538–546
Al-Sultan S, Abdel-Raheem S, El-Ghareeb W, Mohamed M (2016) Comparative effects of using prebiotic, probiotic, synbiotic and acidifier on growth performance, intestinal microbiology and histomorphology of broiler chicks. Jpn J Vet Res 64:S187–S195
Arafat N, Eladl A, Mahgoub H, El-Shafei R (2017) Effect of infectious bursal disease (IBD) vaccine on Salmonella Enteritidis infected chickens. Vaccine 35:3682–3689
Attia YA, Ellakany HF, Abd El-Hamid AE, Fulvia B, Ghazaly SA (2012) Control of Salmonella enteritidis infection in male layer chickens by acetic acid and/or prebiotics, probiotics and antibiotics. Arch Geflugelkd 76:239–245
Awad A, Arafat N, Elhadidy M (2016) Genetic elements associated with antimicrobial resistance among avian pathogenic Escherichia coli. Ann Clin Microbiol Antimicrob 15:299
Badia R, Lizardo R, Martinez P, Badiola I, Brufau J (2012) The influence of dietary locust bean gum and live yeast on some digestive immunological parameters of piglets experimentally challenged with Escherichia coli. J Anim Sci 90:260–262
Bai AP, Ouyang Q, Zhang W, Wang CH, Li SF (2004) Probiotics inhibit TNFalpha- induced interleukin-8 secretion of HT29 cells. World J Gastroenterol 10:455–457
Barnes J, Nolan LK, Vaillancourt JP (2008) Colibacillosis. In: Saif YM, Fadly JR, Glisson JR, LR MD, Nolan LK, Swayne DE (eds) Diseases of poultry, 12th edn. Blackwell Publishing, Iowa State Press, Ames, pp 691–737
Baurhoo BA, Ferket PR, Zhao X (2009) Effect of diets containing different concentrations of mannanoligosaccharide or antibiotics on growth performance, intestinal development, cecal and litter microbial populations, and carcass parameters of broilers. Poult Sci 88:2262–2272
Cao GT, Zeng XF, Chen AG, Zhou L, Zhang L, Xiao YP, Yang C (2013) Effects of a probiotic, Enterococcus faecium, on growth performance, intestinal morphology, immune response, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult Sci 92:2949–2955
Corwin EJ (2000) Understanding cytokines part I: physiology and mechanisms of action. Biol Res Nurs 2:30–40
Dheilly A, Le Devende L, Mourand G, Jouy E, Kempf I (2013) Antimicrobial resistance selection in avian pathogenic E. coli during treatment. Vet Microbiol 166:655–658
Dho-Moulin M, Fairbrother JM (1999) Avian pathogenic Escherichia coli (APEC). Vet Res 30:299–316
Dinarello CA (2000) Proinflammatory cytokines. Chest 118:503–508
Dobrovolskaia MA, Vogel SN (2002) Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect 4:903–914
Ebers KL, Zhang CY, Zhang MZ, Bailey RH, Zhang S (2009) Transcriptional profiling avian beta-defensins in chicken oviduct epithelial cells before and after infection with Salmonella enterica serovar Enteritidis. BMC Microbiol 9:153
Edwards PR, Ewing WH (1972) Identification of Enterobacteriaceae. Burgess Pub. Co, Minneapolis
Fuller R (1999) Probiotics for farm animals. In: Tannock GW (ed) Probiotics: a critical review. Horizon Scientific Press, New York, pp 15–22
Gadde U, Oh S, Lee Y, Davis E, Zimmerman N, Rehberger T, Lillehoj H (2017) Dietary Bacillus subtilis-based direct-fed microbials alleviate LPS-induced intestinal immunological stress and improve intestinal barrier gene expression in commercial broiler chickens. Res Vet Sci 114:236–243
Gao Y, Han F, Huang X, Rong Y, Yi H, Wang Y (2013) Changes in gut microbial populations, intestinal morphology, expression of tight junction proteins, and cytokine production between two pig breeds after challenge with Escherichia coli K88: a comparative study. J Anim Sci 91:5614–5625
Genovese JK, Anderson R, Harvey R, Nisbet D (2000) Competitive exclusion treatment reduces the mortality and fecal shedding associated with enterotoxigenic Escherichia coli infection in nursery-raised neonatal pigs. Can J Vet Res 64:204–207
Gibson GR, Probert HM, Van Loo J, Rastall RA, Roberfroid MB (2004) Dietary modulation of the colonic microbiota: updating the concept of prebiotics. Nutr Res Rev 17:259–275
Guo FC, Williams BA, Kwakkel RP, Li HS, Li XP, Luo JY, Li WK, Verstegen MWA (2004) Effects of mushroom and herb polysaccharides, as alternatives for an antibiotic, on the cecal microbial ecosystem in broiler chickens. Poult Sci 83:175–182
Hakansson A, Molin G (2011) Gut microbiota and inflammation. Nutrients 3:637–682
Hausmann M, Kiessling S, Mestermann S, Webb G, Spöttl T, Andus T, Schölmerich J, Herfarth H, Ray K, Falk W (2002) Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology 122:1987–2000
Higgins SE, Higgins JP, Wolfenden AD, Henderson SN, Torres-Rodriguez A, Tellez G, Hargis B (2008) Evaluation of a Lactobacillus-based probiotic culture for the reduction of Salmonella Enteritidis in neonatal broiler chicks. Poult Sci 87:27–31
Hofacre CL, Johnson AC, Kelly BJ, Froyman R (2002) Effect of a commercial competitive exclusion culture on reduction of colonization of an antibiotic-resistant pathogenic Escherichia coli in day-old broiler chickens. Avian Dis 46:198–202
Hong YH, Lillehoj HS, Lee SH, Park DW, Lillehoj EP (2006) Molecular cloning and characterization of chicken lipopolysaccharide-induced TNF-α factor (LITAF). Dev Comp Immunol 30:919–929
Huff GR, Huff WE, Rath NC, Tellez G (2006) Limited treatment with β-1,3/1,6-glucan improves production values of broiler chickens challenged with Escherichia coli. Poult Sci 85:613–618
Jawhara S, Habib K, Maggiotto F, Pignede G, Vandekerckove P, Maes E, Dubuquoy L, Fontaine T, Guérardel Y, Poulain D (2012) Modulation of intestinal inflammation by yeasts and cell wall extracts: strain dependence and unexpected anti-inflammatory role of glucan fractions. PLoS One 7:e40648
Knap I, Kehlet B, Bennedsen M, Mathis G, Hofacre C, Lumpkins B, Jensen M, Raun M, Lay A (2011) Bacillus subtilis (DSM17299) significantly reduces Salmonella in broilers. Poult Sci 90:1690–1694
La Ragione RM, Collighan RJ, Woodward MJ (1999) Non-curliation of Escherichia coli O78:K80 isolates associated with IS1 insertion in csgB and reduced persistence in poultry infection. FEMS Microbiol Lett 174:247–253
La Ragione RM, Cooley WA, Woodward MJ (2000) Adherence of avian Escherichia coli O78:K80 to tissue culture, tracheal and gut explants; the role of fimbriae and flagella. J Med Microbiol 49:327–338
La Ragione RM, Casula G, Cutting MS, Woodward MJ (2001) Bacillus subtilis spores competitively exclude Escherichia coli O78:K80 in poultry. Vet Microbiol 79:133–142
La Ragione RM, Narbad A, Gasson MJ, Woodward MJ (2004) In vivo characterization of Lactobacillus johnsonii FI9785 for use as a defined competitive exclusion agent against bacterial pathogens in poultry. Lett Appl Microbiol 38:197–205
Lamont SJ, Pinard-van der Laan MH, Cahaner A, van der Poel JJ, Parmentier HK (2003) Selection for disease resistance: direct selection on the immune response. In: Muir WM, Aggrey SE (eds) Poultry genetics, breeding and biotechnology. CAB International, pp 399–418
Langhout P (2000) New additives for broiler chickens. World Poult 16:22–27
Li Q, Verma IM (2002) NF-κB regulation in the immune system. Nat Rev Immunol 2:725–734
Li Y, Zhang H, Chen YP, Yang MX, Zhang LL, Lu ZX, Zhou YM, Wang T (2015) Bacillus amyloliquefaciens supplementation alleviates immunological stress and intestinal damage in lipopolysaccharide-challenged broilers. Ani Feed Sci Technol 208:119–131
Liu H, Zhang M, Han H, Yuan J, Li Z (2010) Comparison of the expression of cytokine genes in the bursal tissues of the chickens following challenge with infectious bursal disease viruses of varying virulence. Virol J 7:364
Lu H, Sunday A, Layi A, Kolapo A (2014) Anti-inflammatory effects of non-antibiotic alternatives in Coccidia challenged broiler chickens. J Poul Sci 51:14–21
McPeake W, Smuth A, Ball J (2005) Characterization of avian pathogenic Escherichia coli (APEC) associated with colisepticemia compared to faecal isolates from healthy birds. Vet Microbiol 110:245–253
NRC (1994) Nutrient requirements of poultry. 9th rev. ed. Natl. Acad. Press, Washington, DC
Nuotio L, Schneitz C, Nilsson O (2013) Effect of competitive exclusion in reducing the occurrence of Escherichia coli producing extended-spectrum β-lactamases in the ceca of broiler chicks. Poult Sci 92:250–254
Olkowski AA, Wojnarowicz C, Chirino-Trejo M, Drew D (2006) Responses of broiler chickens orally challenged with Clostridium perfringens isolated from field cases of necrotic enteritis. Res Vet Sci 81:99–108
Patterson JA, Burkholder KM (2003) Application of prebiotics and probiotics in poultry production. Poult Sci 82:627–631
Pfaffl MW (2001) A new mathematical model for relative quantification in real time RT-PCR. Nucleic Acids Res 29:2002–2007
Quinn PJ, Carter ME, Markey B, Carter GR (1994) Brucella species. In: Quinn PJ, Carter ME, Markey BK, Carter GR (eds) Clinical veterinary microbiology. Wolfe Publishing, London, pp 261–267
Salehimanesh A, Mohammadi M, Roostaei-Ali Mehr M (2016) Effect of dietary probiotic, prebiotic and synbiotic supplementation on performance, immune responses, intestinal morphology and bacterial populations in broilers. J Anim Physiol Anim Nutr 100:694–700
Sunkara L, Achanta M, Schreiber N, Bommineni Y, Dai G, Jiang W, Lamont S, Lillehoj H, Beker A, Teeter R, Zhang G (2011) Butyrate enhances disease resistance of chickens by inducing antimicrobial host defense peptide gene expression. PLoS One 6:e27225
Teirlynck E, Bjerrum L, Eeckhaut V, Huygebaert G, Pasmans F, Haesebrouck F, Dewulf J, Ducatelle R, Van Immerseel F (2009) The cereal type in feed influences gut wall morphology and intestinal immune cell infiltration in broiler chickens. Brit J Nutr 102:1453–1461
Teo L, Tan M (2006) Effect of Bacillus subtilis PB6 (CloSTAT) on broilers infected with a pathogenic strain of Escherichia coli. J Appl Poult Res 15:229–235
Thomas CM, Versalovic J (2010) Probiotics-host communication: modulation of signaling pathways in the intestine. Gut Microbes 1:148–163
Uni Z (1999) Functional development of the small intestine in domestic birds: cellular and molecular aspects. Avian Poult Biol Rev 10:167–179
van Deun K, Pasmans F, Ducatelle R, Flahou B, Vissenberg K, Martel A, van den Broeck W, Van Immerseel F, Haesebrouck F (2008) Colonization strategy of Campylobacter jejuni results in persistent infection of the chicken gut. Vet Microbiol 130:285–297
van Dijk A, Veldhuizen EJ, Kalkhove SI, Tjeerdsmavan Bokhoven JL, Romijn RA, Haagsman HP (2007) The β-defensin gallinacin-6 is expressed in the chicken digestive tract and has antimicrobial activity against food-borne pathogens. Antimicrob Agents Chemother 51:912–922
Wang W, Li Z, Han Q, Guo Y, Zhang B, D'inca R (2016) Dietary live yeast and mannan-oligosaccharide supplementation attenuate intestinal inflammation and barrier dysfunction induced by Escherichia coli in broilers. Brit J Nutr 116:1878–1888
Yang H, Chen S, White DG, Zhao S, McDermott P, Walker R, Meng J (2004) Characterization of multiple-antimicrobial-resistant Escherichia coli isolates from diseased chickens and swine in Chin. J Clin Microbiol 42:3483–3489
Yang Y, Jii PA, Kocher A, Mikkelsen LL, Choct M (2008) Effects of mannanoligosaccharide and fructooligosaccharide on the response of broilers to pathogenic Escherichia coli challenge. Brit Poul Sci 49:550–559
Yang F, Wang A, Zeng X, Hou C, Liu H, Qiao S (2015) Lactobacillus reuteri I5007 modulates tight junction protein expression in IPEC-J2 cells with LPS stimulation and in newborn piglets under normal conditions. BMC Microbiol 15:32
Zhang L, Zhang L, Zhan X, Zeng X, Zhou L, Cao G, Chen A, Yang C (2016) Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. J Anim Sci Biotechnol 7:3
Acknowledgements
Authors wish to thank departments of Poultry diseases, Animal Husbandry and Wealth Development, Physiology and Hygiene and Zoonoses for their help, encouragement, and support during the course of this study.
Funding
This work did not get any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ateya, A.I., Arafat, N., Saleh, R.M. et al. Intestinal gene expressions in broiler chickens infected with Escherichia coli and dietary supplemented with probiotic, acidifier and synbiotic. Vet Res Commun 43, 131–142 (2019). https://doi.org/10.1007/s11259-019-09753-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-019-09753-z