Abstract
In seeds with impermeable coats, i.e., physical dormancy (PY), dormancy break may occur at room temperature during ex-situ storage or when seeds experiencing similar conditions when buried in the soil. Here, we tested the influence of initial seed moisture content and storage on dormancy break in the seeds of Adenanthera pavonina, Bauhinia racemosa, Cassia fistula, Dodonaea viscosa, and Delonix regia. Drying results in most seeds of these species becoming water-impermeable. We arbitrarily chose two moisture ranges, shallow (impermeable, high moisture content) and absolute (impermeable, low moisture content) PY, and stored the seeds at room temperature for 8.5 years. The moisture content at which the permeable to impermeable transition occurred and the range constituting shallow and absolute PY varied between species. Across species, the shallow PY group had a significantly higher number of nondormant seeds at the end of storage, whereas the absolute PY group did not show any germination, except c. 20% germination in A. pavonina and C. fistula. Thus, PY break in seeds stored at room temperature may occur after several years, but this largely depends on the initial seed moisture content.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability of seeds to develop an impermeable coat, i.e., physical dormancy (PY), has been known or inferred to occur in species of many-but not all- genera belonging to only 19 angiosperm families (Jaganathan 2022). The impermeable nature of the seed (or sometimes fruit) coat is due to one or more layer(s) of palisade layers of macrosclereid present in the coat, which restricts the entry of water to reach the embryo, which is required for germination (Baskin et al. 2000; Rolston 1978). For PY seeds to germinate, an entry pathway characterized by the irreversible opening of a small specialized seed structure called a ‘water-gap’, e.g., the lens in Fabaceae (Burrows et al. 2018; Geneve et al. 2018) or small plug adjacent to the hilum in the Sapindaceae (Turner et al. 2009) is required for the water to enter and hydrate the embryo. PY break may occur through a combination of factors and is thought to be a two-step process (Baskin and Baskin 2014; Taylor et al. 1991; Taylor 2005). Also, high summer temperatures with diurnal fluctuation of 15–20 °C prevalent on the soil surface in the tropics and Mediterranean ecosystems are sufficient to break dormancy (Quinlivan 1966, 1961; Quinlivan and Millington 1962). Further, PY break can also occur during erratic ecological events such as fire (Jaganathan 2015) or passage through the animal gut when herbivores ingest the seeds and the hydrochloric acid environment in the intestine erodes the coat (Jaganathan et al. 2016).
Under empirical conditions, PY can be broken after dipping the seeds in boiling water for a few seconds, dry heat, scarification, radiation, and liquid nitrogen exposure (Baskin and Baskin 2014). It has been demonstrated that PY seeds stored at room temperature also break dormancy, but the proportion of seeds breaking dormancy varies significantly. For instance, depending on the species, anywhere between 0xand 100% of the impermeable seeds become permeable to water during storage at room temperature within a few years (Egley 1979; Meisert 2002; Morrision et al. 1992; Cavanagh 1987; Galindez et al. 2010). Seed traits such as size (Liyanage and Ooi 2018; Rodrigues-Junior et al. 2018a) and coat thickness (Richard et al. 2018; Venier et al. 2012) have been linked to PY alleviation. Rodrigues-Junior et al. (2018b) examined these factors using Senna multijuga and found that large-sized seeds break dormancy relatively quickly compared to their small-sized counterparts, presumably due to the variation in potential internal pressure. The explanation that seed size and coat thickness variation are the likely factors behind the variation during dormancy break has some limitations. Given that these traits are affected by the maternal environment, selecting an arbitrary threshold cut-off point to separate large and small-sized seeds or thick and thin coats could be an artifact. Thus, despite the surge of studies offering novel insights into the PY-breaking mechanisms, there is no unequivocal answer to why only a proportion of impermeable seeds become permeable following a specific dormancy-breaking treatment.
A tight relationship exists between moisture content and the development of impermeability (Jaganathan 2016, 2022). Although the critical moisture content at which the seeds become impermeable varies between species and families, thus species-specific, this range occurs predominantly between 15 and 8% moisture content on a fresh weight basis (Mai-Hong et al. 2003; Ellis and Roberts 1982; Geneve 2009; Hyde 1954; Gladstones 1958; Jaganathan et al. 2017a, 2019; Hay et al. 2010). Furthermore, in several Fabaceae, the seed coat becomes impermeable when dried to a critical moisture content. However, the hilum remains open, and acts as a ‘one-way hygroscopic valve’, which allows the loss of water from internal tissues to the external environment under a low relative humidity environment, but when the seed is dried to lower moisture levels, the hilum closes leaving the seeds sealed permanently until the water gap opens and facilitates water entry (Hyde 1954; Rangaswamy and Nandakumar 1985).
Jaganathan (2016) proposed that depending on the moisture content attained by the seeds during development and post-dispersal conditions, two levels of PY could exist, namely (i) shallow PY, wherein seeds have higher moisture content and possibly reverse to a permeable state when RH increases the moisture content of the seeds; and (ii) absolute PY, where seeds are in a completely dry state with low moisture content and reversal is impossible, regardless of the external RH. Plausibly, the requirements for dormancy break vary between shallow and absolute PY. For example, in Lupinus varius, seeds with moisture content above 10% became permeable in moist soil, but the drier counterparts with c. 8% moisture content required seasonal temperature fluctuations to break dormancy (Quinlivan 1968). More recently, Tangney et al. (2019) also found that the drier seeds tolerate much higher temperatures than those with high moisture content, indicating that the moisture content controls dormancy break. Seeds persisting in the soil seed banks may dry to different moisture content, and therefore, the dormancy-breaking requirement might change based on the moisture content at the time of dormancy break (Magalhães et al. 2021; Jaganathan 2022). Further, seeds buried deep in the soil may experience constant temperature at different moisture levels, which would be a critical factor during dormancy break.
We hypothesized that seeds with high vs. low initial moisture content follow a different pattern of dormancy loss when stored at room temperature, which is similar to the conditions experienced by seeds under ecological conditions when buried at 3–5 cm depths. This hypothesis was tested in seeds of four Fabaceae and one Sapindaceae species. More specifically, our explicit aims of this study were to understand (1) the critical moisture content of the seeds at which impermeability is induced; (2) if seeds dried to different moisture levels have shallow and absolute PY; and (3) what are the effects of room temperature storage on dormancy break of seeds with shallow vs. absolute PY.
Materials and methods
Seed materials
Pods of Adenanthera pavonina, Bauhinia racemosa, Cassia fistula, Dodonaea viscosa, and Delonix regia were directly collected from plants in the Western Ghats in Coimbatore, Tamil Nadu, India (11° 100N, 76° 740E) between January and June of 2014. Before collection, plastic film was spread on the ground under the tree to ensure that only pods attached to the trees at the time of shaking were collected. For B. racemosa, C. fistula, and D. viscosa, collections were made from 23 trees within 11 km radius, but for D. regia, pods were collected from 41 trees with a maximum collection site spanning 82 km. Pods of A. pavonina were collected from 18 trees from one standing population within a 4 km radius. The pods were gently opened with a scalpel or struck gently against a wooden table and then opened by hand to collect seeds. To minimize changes to physiological maturity, the seeds of each species were cleaned, visually inspected, pooled into one lot, and used in the experiments immediately. Seeds were stored in closed plastic containers under ambient conditions (25–28 °C, 50–60% RH) for three days before long-term storage experiments began.
Moisture content and imbibition
We used the standard oven drying method at 103 °C for 17 h for moisture content measurement and expressed the final results on a fresh weight basis (ISTA 2009, 2023). Three replicates of 25 seeds were used for each species. Imbibition tests were conducted on 90 mm Petri dishes containing Whatman no. 1 filter paper moistened with distilled water for 35 days. For each species, four replicates of 25 seeds were incubated at 20/25 °C with 40 μm m−2 s−1, 400–700 nm, cool white fluorescent light provided for 12 h during the warm phase of the cycle. Seeds germinating during the imbibition test were removed from the Petri dishes, and water was added whenever the filter paper began to dry. Germination following other treatments was conducted by placing seeds on 90 mm Petri dishes containing Whatman no. 1 filter paper moistened with distilled water. Germination tests were terminated after 35 days or when all the seeds had germinated. A seed was considered to be germinated if the radicle emerged to 2 mm.
Drying of seeds
Preliminary experiments were conducted on fresh seeds to determine the drying duration required to decrease seed moisture levels (data not shown). Based on these results, fresh seeds were dried above silica gel in a ratio of 1:3 for 12, 24, 48, and 96 h in separate airtight containers. For drying, batches of seeds were placed in airtight plastic containers held above the silica gel. At the end of each drying period, four replicates of 25 seeds were tested for imbibition, and three replicates of 15 seeds were tested for moisture content. Seeds that remained impermeable for 55 days were mechanically scarified and placed on moist filter paper for 30 days to observe imbibition and germination. Three hundred seeds of each species were dried for 12 or 24 h to represent a shallow dormant state (group I), and another group containing 300 seeds per species was dried for 96 h, which is in an absolute dormant state (group II). Throughout, we refer to the fresh, nontreated seeds as the control..
Room temperature storage
Impermeable seeds with different moisture contents (groups I and II) were stored at room temperature in plastic trays (not airtight) for 8.5 years. In November 2022, 245 seeds from groups I and II per species were retrieved to test the moisture content and imbibition. Our earlier studies indicated that seeds do not dry further because of high RH maintaining equilibrium moisture content. Four replicates of 50 seeds from each group were used for imbibition/germination, and three replicates of 45 seeds were used for moisture content determination. Seeds that did not absorb water for 55 days were mechanically scarified and tested for imbibition.
Statistical analysis
The data were explored for violation of assumptions (normality, heterogeneity, independence, sphericity, interactions) prior to analysis. Data were analysed in Rstudio (R Core Team 2022) by fitting response variables (moisture content, germination) to a linear mixed-effects model with an appropriate link (Gaussian, binomial) with predictors (legume, absolute and shallow dormancy) as fixed effects and block as a random effect in package lme4 (Bates et al. 2014). All models were evaluated through visual assessment of the residuals, and Akaike information criterion (AIC) were used for model selection. Paired comparisons between treatments were estimated using the Tukey method for p value adjustment, and the degrees of freedom method used was Kenward-Roger (Hu and Spilke 2009; Lenth et al. 2019). Estimated marginal means and significance letters were extracted from the models using package emmeans (Lenth et al. 2019).
Results and discussion
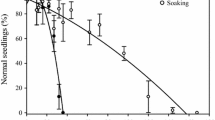
The average 100 seed mass ranged between 0.2 g in D. viscosa and 30.3 g in A. pavonina. The moisture content of the seeds at the time of collection varied significantly between species. Cassia fistula had 12.65% moisture content, but the moisture content of the other four species was less than 10% (Fig. 1). None of the D. viscosa control seeds germinated when tested for germination, but 50% of C. fistula seeds imbibed and germinated within 30 days. Seeds of D. regia, A. pavonina and B. racemosa had 8, 12 and 13% germination, respectively. We conclude that the high moisture content (12.65%) and 50% germination of C. fistula seeds at the time of collection indicates that the seeds had not dried enough to develop impermeability. On the contrary, the relatively low seed moisture content of the other four species was correlated with their impermeability and low germination percentage. In the non-germinated seeds, there was no evidence of water absorption as determined by weighing seeds and comparing the mass of control seeds with that of seeds of moist filter paper for 30 days (data not shown). This confirms the presence of PY in the seeds of these species and PY in some of the species had been reported in earlier studies conducted (Jaganathan and Liu 2014, 2015; Jaganathan et al. 2017b, 2018). As such, these results agree with the previous findings available on Fabaceae (Hay et al. 2010), Geraniaceae (Gama-Arachchige et al. 2011) and Nelumbonaceae (Jaganathan et al. 2017a) that moisture content plays a significant role in the development of PY. However, similar results are not available for Sapindaceae to compare the results. Yet, we presume that the moisture content of D. viscosa (7.4%) was low enough to promote the development of water-impermeability.
The moisture content of Adenanthera pavonina, Bauhinia racemosa, Cassia fistula, Delonix regia, and Dodonaea viscosa, control, shallow, and absolute PY seeds. The error bars represent the standard deviation of the mean. Different lowercase letters represent the moisture content differed significantly between treatments (P > 0.05)
The moisture content declined rapidly when control seeds were dried in silica gel. Such a decline in moisture content during drying above silica gel is a common phenomenon for PY species and other species that do not produce PY (Christiansen and Moore 1959; Jayasuriya et al. 2007; Jaganathan et al. 2019; Baskin and Baskin 2014; Roberts 1973). We chose the moisture range at which all seeds become impermeable as shallow PY and at least 2% lower moisture content range as absolute PY (Fig. 1). Thus, control seeds for all species had higher moisture content than absolute and shallow PY. Further, all absolute PY seeds had significantly lower moisture content than shallow PY seeds from all species. Seeds of all five species became 100% impermeable when dried to shallow MC range. This result further supports the assertion that seed-to-seed variation might have existed at the time of collection, leading to a few seeds being permeable to water while others were impermeable. When dried, the latter group also became impermeable. A decline in the germination percent of species producing impermeable seed coats during drying was reported before (Heatherly et al. 1995; Hay et al. 2010). To prove that seeds were impermeable and not dead, we subjected both shallow and absolute PY groups of all species to mechanical scarification, which resulted in 98–100% germination across species (data not shown), indicating that the seeds were viable.
Across species, the shallow PY group broke dormancy more than the absolute PY counterpart after 8.5 years of storage (Fig. 2). In particular, 73% of C. fistula seeds from shallow PY became permeable, but only 14% of the absolute PY group germinated when retrieved after 8.5 years at room temperature (df = 24; t ratio = − 8.18; P < 0.001). Similarly, 63% and 14% of shallow and absolute A. pavonina seeds became permeable when tested after 8.5 years, respectively (df = 24; t ratio = − 7.03; P < 0.001). In contrast, the absolute PY group of D. regia did not break dormancy even after 8.5 years of storage at room temperature, although the shallow group had 11% of the seeds imbibing water (P > NS; Fig. 2). Likewise, the percentage of D. viscosa seeds becoming nondormant significantly differed between shallow and absolute groups (stored for 8.5 years) but remained less than 25% (df = 24; t ratio = − 2.95; P < 0.05). Previous studies conducted on room temperature storage of PY species also showed only a small proportion of seeds came out of dormancy, but this depends mainly on the duration of storage. For example, 60% of Mimosa foliolos a germinated after 13 years of storage at room temperature (Nativel et al. 2015), but four years at 15 °C and 15% RH was sufficient for seeds of Collaea argentina to reach 60% germination (Galindez et al. 2010). Morrison et al. (1992) showed that several Fabaceae species maintained PY during 3.5 years of laboratory storage, with a few species showing an increase in the number of seeds with impermeable coats at the end of storage. We presume a seed lot would have a mixture of shallow and absolute PY, and most shallow PY seeds would become nondormant after a few years. Thus, the proportion of dormancy break depends also on initial moisture content.
Germination percentage of Adenanthera pavonina, Bauhinia racemosa, Cassia fistula, Delonix regia, and Dodonaea viscosa shallow and absolute PY seeds before and after 8.5 years of storage at room temperature. The error bars represent the standard deviation of the mean. Different lowercase letters represent the moisture content differed significantly between treatments (P > 0.05)
The ideal dormancy-breaking conditions in species with PY is understood to be a two-step process (Taylor 2005; Jayasuriya et al. 2009). Firstly, specific environmental cues, including constant or fluctuating temperatures depending on the species, make seeds sensitive to dormancy break, then a second step involves a set of different constant or fluctuating temperatures which ruptures the water gap, thus making the seeds water permeable (Gama-Arachchige et al. 2012; Geneve et al. 2018). Dormancy break occurring after a particular seasonal temperature increases species fitness because the germination is synchronized with the growing season (Jayasuriya et al. 2008; Jaganathan and Liu 2014; Jaganathan et al. 2017b; Jaganathan 2022; Van Assche et al. 2003). When PY is broken after other ecological cues (e.g., fire) the optimal germination conditions may not occur immediately. Yet, dormancy-broken seeds readily survive the post-dormancy-breaking environment and transition into seedlings once the soil conditions support germination and emergence. However, seeds buried at depths may not experience temperature fluctuations or high temperatures during the fire, similar to those on the soil surface. These seeds experience constant temperature close to the room temperature tested here because the soil is a good insulator of heat; at soil depths > 5 cm, the temperature remains constant between 20 and 30 °C (Taylor 1984; Silva Dias et al. 2019; Liyanage and Ooi 2017). Based on the results of the present study, we propose that such temperature may break dormancy in a very small proportion of PY seeds. Seeds stored at depths and retrieved by animals or tillage to the surface may have a higher number of seeds released from dormancy cf. non-stored/fresh seeds, similar to those described in several legumes in Western Australia (Harrison et al. 2021).
Jaganathan and Harrison (2023) proposed a strong relationship between temperature and duration for PY break and hypothesized that the moisture content might influence this relationship. Their model suggested that increasing temperature decreases the duration required to break dormancy. Thus, the seeds may need up to several years at room temperature to allow dormancy break, but seeds will only need a few months of a higher temperature, c. 40–60 °C to precondition, followed by a few months of a certain temperature range to open the water gap. However, dormancy break occurs in a few seconds when seeds experience high temperatures resulting from fire. We found that a long time is required for a PY break to occur at room temperature. In particular, only a proportion of seeds held at room temperature germinated after 8.5 years, and the initial moisture content significantly affected the percentage of seeds coming out of dormancy. We presume seeds buried at depths of c. 5 cm experience a constant temperature and break dormancy in a small proportion. The successful germination and seedling establishment depend on water availability and post-dormancy-breaking conditions. However, our study did not shed any light on how dormancy break occurs in seeds stored at room temperature. Most dormancy-breaking cues open the ‘water gap’ in PY species. However, the passage of seeds through the animal gut may have cracks in the seed coat due to acid scarification. While we speculate that PY is broken in seeds held at room temperature via the opening of the water gap, further confirmatory studies using scanning electron microscopy and dye tracking are needed.
References
Baskin CC, Baskin JM (2014) Seeds: ecology, biogeography, and evolution of dormancy and germination. Elsevier, Amsterdam
Baskin JM, Baskin CC, Li X (2000) Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Species Biol 15:139–152
Bates D, Mächler M, Bolker B, Walker S (2014) Fitting linear mixed-effects models using lme4. Computer program, arXiv preprint arXiv:1406.5823.
Burrows GE, Alden R, Robinson WA (2018) The lens in focus–lens structure in seeds of 51 Australian Acacia species and its implications for imbibition and germination. Austral J Bot 66:398–413
Cavanagh H (1987) Germination of hard-seeded species (Order Fabales). In: Langkamp P (ed) Germination of Australian native plant seed. Inkata Press, Melbourne
Christiansen MN, Moore R (1959) Seed coat structural differences that influence water uptake and seed quality in hard seed cotton. Agronomy J 51:582–584
Egley G (1979) Seed coat impermeability and germination of showy crotalaria (Crotalaria spectabilis) seeds. Weed Sci 27:355–361
Ellis RH, Roberts EH (1982) Desiccation, rehydration, germination, imbibition injury and longevity of pea seeds (Pisum sativum). Seed Sci Technol 10:501–508
Galindez G, Ortega-Baes P, Seal C, Daws M, Scopel AL, Pritchard H (2010) Physical seed dormancy in Collaea argentina (Fabaceae) and Abutilon pauciflorum (Malvaceae) after 4 years storage. Seed Sci Technol 38:777–782
Gama-Arachchige N, Baskin J, Geneve R, Baskin C (2011) Acquisition of physical dormancy and ontogeny of the micropyle–water-gap complex in developing seeds of Geranium carolinianum (Geraniaceae). Ann Bot 108:51–64
Gama-Arachchige N, Baskin J, Geneve R, Baskin C (2012) The autumn effect: timing of physical dormancy break in seeds of two winter annual species of Geraniaceae by a stepwise process. Ann Bot 110:637–651
Geneve RL (2009) Physical seed dormancy in selected Caesalpinioid legumes from eastern North America. Propag Ornamental Plants 9:129–134
Geneve RL, Baskin CC, Baskin JM, Jayasuriya KG, Gama-Arachchige NS (2018) Functional morpho-anatomy of water-gap complexes in physically dormant seed. Seed Sci Res 28:186–191
Gladstones JS (1958) The influence of temperature and humidity in storage on seed viability and hard-seededness in the west Australian, Blue Lupin, Lupinus digitatus Forsk. Aust J Agric Res 9:171–181
Harrison RJ, Howieson JG, Yates RJ, Nutt BJ (2021) Long-term storage of forage legumes greatly alters the hard seed breakdown pattern in situ. Grass Forage Sci 76:72–81
Hay FR, Smith RD, Ellis RH, Butler LH (2010) Developmental changes in the germinability, desiccation tolerance, hardseededness, and longevity of individual seeds of Trifolium ambiguum. Ann Bot 105:1035–1052
Heatherly LG, Kenty MM, Kilen TC (1995) Effects of storage environment and duration on impermeable seed coat in soybean. Field Crop Res 40:57–62
Hu X, Spilke J (2009) Comparison of various spatial models for the analysis of cultivar trials. N Z J Agric Res 52:277–287
Hyde EOC (1954) The function of the hilum in some Papilionaceae in relation to the ripening of the seed and the permeability of the testa. Ann Bot 18:241–256
ISTA (2009) International rules for seed testing. Chapter 9, Moisture Content Determination. Switzerland: International Seed Testing Association.
ISTA (2023) International rules for seed testing. Wallisellen, Switzerland
Jaganathan GK (2015) Are wildfires an adapted ecological cue breaking physical dormancy in the Mediterranean basin? Seed Sci Res 25:120–126
Jaganathan GK (2016) Influence of maternal environment in developing different levels of physical dormancy and its ecological significance. Plant Ecol 217:71–79
Jaganathan GK (2022) Unravelling the paradox in physically dormant species: elucidating the onset of dormancy after dispersal and dormancy-cycling. Ann Bot 130:121–129
Jaganathan GK, Harrison RJ (2023) Decoding the decisive role of seed moisture content in physical dormancy break: filling the missing links. Plant Biol: https://doi.org/10.1111/plb.13602
Jaganathan GK, Liu B (2014) Seasonal influence on dormancy alleviation in Dodonaea viscosa (Sapindaceae) seeds. Seed Sci Res 24:229–237
Jaganathan GK, Liu B (2015) Role of seed sowing time and microclimate on germination and seedling establishment of Dodonaea viscosa (Sapindaceae) in a seasonal dry tropical environment—an insight into restoration efforts. Botany 93:23–29
Jaganathan GK, Yule K, Liu B (2016) On the evolutionary and ecological value of breaking physical dormancy by endozoochory. Perspect Plant Ecol Evol Syst 22:11–22
Jaganathan GK, Song D, Liu W, Han Y, Liu B (2017a) Relationship between seed moisture content and acquisition of impermeability in Nelumbo nucifera (Nelumbonaceae). Acta Botanica Brasilica 31:639–644
Jaganathan GK, Wu GR, Han YY, Liu B (2017b) Role of the lens in controlling physical dormancy break and germination of Delonix regia (Fabaceae: Caesalpinioideae). Plant Biol 19:53–60
Jaganathan GK, Yule KJ, Biddick M (2018) Determination of the water gap and the germination ecology of Adenanthera pavonina (Fabaceae, Mimosoideae); the adaptive role of physical dormancy in mimetic seeds. AoB Plants 10:ply048
Jaganathan GK, Li J, Biddick M, Han K, Song D, Yang Y, Han Y, Liu B (2019) Mechanisms underpinning the onset of seed coat impermeability and dormancy-break in Astragalus adsurgens. Scientific Rep 9:1–10
Jayasuriya K, Baskin JM, Geneve RL, Baskin CC (2007) Seed development in Ipomoea lacunosa (Convolvulaceae), with particular reference to anatomy of the water gap. Ann Bot 100:459–470
Jayasuriya KG, Baskin JM, Baskin CC (2008) Cycling of sensitivity to physical dormancy-break in seeds of Ipomoea lacunosa (Convolvulaceae) and ecological significance. Ann Bot 101:341–352
Jayasuriya KG, Baskin JM, Geneve RL, Baskin CC (2009) Sensitivity cycling and mechanism of physical dormancy break in seeds of Ipomoea hederacea (Convolvulaceae). Int J Plant Sci 170:429–443
Lenth R, Singmann H, Love J, Buerkner P, Herve M (2019) Emmeans: Estimated marginal means, aka least-squares means (R package version 1.5. 1.)[Computer software].
Liyanage GS, Ooi MK (2017) Do dormancy-breaking temperature thresholds change as seeds age in the soil seed bank? Seed Sci Res 27:1–11
Liyanage GS, Ooi MK (2018) Seed size-mediated dormancy thresholds: a case for the selective pressure of fire on physically dormant species. Biol J Linn Soc 123:135–143
Magalhães CR, Garcia QS, Oliveira DM (2021) Post-dispersion humidity condition alters the surface of the testa and the proportion of seeds with physical dormancy in Erythrina speciosa. Seed Sci Res 31:149–156
Mai-Hong T, Hong TD, Hien NT, Ellis RH (2003) Onset of germinability, desiccation tolerance and hardseededness in developing seeds of Peltophorum pterocarpum (DC) K. Heyne (Caesalpinioideae). Seed Sci Res 13:323–327
Meisert A (2002) Physical dormancy in Geraniaceae seeds. Seed Sci Res 12:121–128
Morrision DA, Auld TD, Rish S, Porter C, McClay K (1992) Patterns of testa-imposed seed dormancy in native Australian legumes. Ann Bot 70:157–163
Morrison DA, Auld TD, Rish S, Porter C, McCalay K (1992) Patterns of testa-imposed seed dormancy in native Australian legumes. Ann Bot 70:157–163
Nativel N, Buisson E, Silveira FAO (2015) Seed storage-mediated dormancy alleviation in Fabaceae from campo rupestre. Acta Botanica Brasilica 29:445–447
Quinlivan B (1961) The effect of constant and fluctuating temperatures on the permeability of the hard seeds of some legume species. Aust J Agric Res 12:1009–1022
Quinlivan B (1966) The relationship between temperature fluctuations and the softening of hard seeds of some legume species. Crop Pasture Sci 17:625–631
Quinlivan B (1968) The softening of hard seeds of san-plain lupin (Lupinus varius). Aust J Agric Res 19:507–515
Quinlivan BJ, Millington AJ (1962) The effect of a Mediterranean summer environment on the permeability of hard seeds of subterranean clover. Aust J Agric Res 13:377–387
R Core Team (2022) R: A language and environment for statistical computing, reference index version 4.1. 3. Vienna, Austria: R Foundation for Statistical Computing.
Rangaswamy N, Nandakumar L (1985) Correlative studies on seed coat structure, chemical composition, and impermeability in the legume Rhynchosia minima. Bot Gaz 146:501–509
Richard GA, Zabala JM, Cerino MC, Marinoni LR, Beutel ME, Pensiero JF (2018) Variability in hardseededness and seed coat thickness of three populations of Desmanthus virgatus (Fabaceae, Mimosoideae). Grass Forage Sci 73:938–946
Roberts E (1973) Predicting the storage life of seeds. Seed Sci Tech 1:499–514
Rodrigues-Junior AG, Baskin CC, Baskin JM, Garcia QS (2018a) Sensitivity cycling in physically dormant seeds of the Neotropical tree Senna multijuga (Fabaceae). Plant Biol 20:698–706
Rodrigues-Junior AG, Mello ACM, Baskin CC, Baskin JM, Oliveira DM, Garcia QS (2018b) Why large seeds with physical dormancy become nondormant earlier than small ones. PLoS ONE 13:e0202038
Rolston MP (1978) Water impermeable seed dormancy. Bot Rev 44:365–396
Silva Dias L, Pires Pereira I, Soveral Dias A (2019) Seed germination in Cistus ladanifer: Heat shock, physical dormancy, soil temperatures and significance to natural regeneration. Plants 8:63
Tangney R, Merritt DJ, Fontaine JB, Miller BP (2019) Seed moisture content as a primary trait regulating the lethal temperature thresholds of seeds. J Ecol 107:1093–1105
Taylor G (1984) Effect of burial on the softening of hard seeds of subterranean clover. Aust J Agric Res 35:201–210
Taylor GB (2005) Hardseededness in Mediterranean annual pasture legumes in Australia: a review. Aust J Agric Res 56:645–661
Taylor G, Maller R, Rossiter R (1991) A model describing the influence of hard seededness on the persistance of an annual forage legume, in a ley farming system, in a mediterranean-type environment. Agr Ecosyst Environ 37:275–301
Turner S, Cook A, Baskin J, Baskin C, Tuckett R, Steadman K, Dixon K (2009) Identification and characterization of the water gap in the physically dormant seeds of Dodonaea petiolaris: a first report for Sapindaceae. Ann Bot 104:833–844
Van Assche JA, Debucquoy KL, Rommens WA (2003) Seasonal cycles in the germination capacity of buried seeds of some Leguminosae (Fabaceae). New Phytol 158:315–323
Venier P, Funes G, García CC (2012) Physical dormancy and histological features of seeds of five Acacia species (Fabaceae) from xerophytic forests in central Argentina. Flora 207:39–46
Acknowledgements
We thank Benedict Arthur for the statistical support.
Funding
None.
Author information
Authors and Affiliations
Contributions
GKJ- carried out the experiment, wrote, revised and edited the manuscript. RJH- performed statistical analyses, prepared figures and contributed to writing.
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Communicated by Thomas Abeli.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jaganathan, G.K., Harrison, R.J. Physical dormancy alleviation at room temperature storage is influenced by the initial moisture content of the seeds. Plant Ecol 225, 491–497 (2024). https://doi.org/10.1007/s11258-024-01406-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-024-01406-9