Abstract
Forest carbon storage inherently depends on the frequency and severity of characteristic disturbances and long-term changes in climate. The tropical forest of Lanjenchi, Taiwan is affected by the northeast monsoon wind, resulting in a varying vegetation structure depending on wind exposure. However, the northeast monsoon winds have been decreasing due to the climate change. We used four censuses over 22 years (1997, 2005, 2013, and 2019) to examine how tree density and aboveground biomass change under different levels of wind stress. We assessed tree density, aboveground biomass, aboveground woody productivity, and aboveground woody mortality from trees with diameter at breast height (DBH) ≥ 1 cm across 5.28 ha subdivided into 10 × 10 m quadrats. We tested for differences in tree density and aboveground biomass among three habitat types (windward, intermediate, and leeward), among small-diameter (1 cm ≤ DBH < 10 cm), medium-diameter (10 cm ≤ DBH < 17.2 cm) and large-diameter trees (DBH ≥ 17.2 cm). The 49,481 trees (density 9,272 ± 3612 trees ha−1) of 140 species held 129.37 ± 51.95 Mg ha−1 of aboveground biomass. From 1997 to 2019, tree density decreased and aboveground biomass was stable in the whole forest. However, changes in density and aboveground biomass were apparent among different habitat types and among different diameter classes. Specifically, in the windward habitat, aboveground biomass of small-diameter trees declined over time (from 35.34 Mg ha−1 to 26.29 Mg ha−1), and that of large-diameter trees increased (from 48.62 Mg ha−1 to 57.78 Mg ha−1). In the leeward habitat, large-diameter trees exhibited both high biomass productivity (1.04 Mg ha−1 year−1) and mortality (1.43 Mg ha−1 year−1). Although the overall state of the forest appears to possess multi-decadal stability, differences in dynamics among diameter classes and habitats may lead to forest changes if trees in those habitats continue to respond differentially to shifting magnitudes of monsoon wind speed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropical and subtropical forests represent the largest aboveground carbon pool in the world, accounting for half of the total global forest carbon (Pan et al. 2011). The aboveground biomass and productivity of these forests is important as a proxy for global carbon fluxes (IPCC 2007). Repeated tree measurements remains the “gold standard” for estimations of these forest carbon stocks and fluxes (Brienen et al. 2015; Rozendaal et al. 2022). Although remote sensing methods (Running et al. 2004) and computer simulations (Thornton et al. 2002) have benefits in terms of global extent and sampling frequency, these methods have inherently high uncertainties and must be calibrated with plot-based tree survey data (Hansen et al. 2019; Hill et al. 2013; Réjou‐Méchain et al. 2014).
Diameter is the key variable for estimating biomass as it has an allometric relationship with the aboveground biomass (Brown 1997; Brown et al. 1989; Chave et al. 2005). Small-diameter trees, although numerous, account for a small amount of aboveground biomass, while larger trees contribute most of the aboveground biomass. However, different tree size classes may respond differently to changing environmental conditions. Large-diameter trees dominate most carbon stocks in warmer, lower latitude forests (Bastin et al. 2015; Lutz et al. 2018, 2012); however, changing climate may lead to increases in large tree mortality with a concomitantly large aboveground biomass decrease (San-José et al. 2021). Small trees are abundant in tropical forests (King et al. 2006) and exhibit greater species diversity than larger trees (Memiaghe et al. 2016). However, small trees have often been overlooked because they contribute less to net primary production and biomass accumulation (Bastin et al. 2015; Fauset et al. 2012). Nevertheless, small trees are more sensitive to competition, disturbance, and biotic effects (Kamara and Kamruzzaman 2020), contributing more to the fluctuation of productivity and mortality than larger trees over time (Piponiot et al. 2022).
Topographic heterogeneity influences the distribution of aboveground biomass (Liu et al. 2020; Shen et al. 2018, 2016). Fine-scale habitat differentiation and varying topographic conditions shape the community composition, structure, and aboveground biomass on the local scale in tropical forests (Rodrigues et al. 2020). For instance, both McEwan et al. (2011) and Shen et al. (2018) showed that slope and elevation could predict aboveground biomass. Moreover, Lin et al. (2012) demonstrated that lower ridge habitats in the subtropical forest of China exhibited the highest aboveground biomass among four topographic habitats due to variations in the strengths of the association between species and their respective habitats. Therefore, forests with highly heterogeneous environmental conditions could exhibit significant variability in the distribution of aboveground biomass (Rodrigues et al. 2020) and also exhibit different responses to climate change among habitat types (Noguchi et al. 2022).
Changing climate, particularly changing maximum temperatures and drought could increase tree mortality and thereby rapidly decrease aboveground biomass (Feeley et al. 2007). Moreover, some research has found that increasing CO2 concentration may increase productivity (Pastore et al. 2019; Saban et al. 2019; Warren et al. 2015) and mortality rates (Ciais et al. 2014; Walker et al. 2021). Plant responses to increasing atmospheric CO2 concentration could increase the total amount of carbon stored in live organic matter (Ciais et al. 2014) and could increase photosynthetic efficiency, tree growth, and forest productivity (Luo 2007; Luo et al. 2009; Melillo et al. 1990, 2011). However, Bugmann and Bigler (2011) and Büntgen et al. (2019) found that the CO2 fertilization effect may reduce forests’ longevity, thus strongly reducing their carbon mitigation potential. Kubiske et al. (2019) found that trees grew faster in elevated atmospheric CO2 and that self-thinning resulted in communities carrying more stand basal area at equivalent densities.
Global warming and warming sea surface temperature are also contributing a decrease in the strength of the East Asia monsoon (determined by the pressure difference between the Siberian high and the Aleutian low; Loo et al. 2015; Wang and Lu 2017) wind, particularly in winter (Hung and Kao 2010). The southernmost portion of Taiwan is affected by the winter monsoon wind from the northeast side (i.e., the northeast monsoon wind; Chao et al. 2010; Hsieh et al. 1992; Ku et al. 2021). The northeast monsoon wind shapes the forest depending on whether the local wind exposure is windward, intermediate, or leeward (Ku et al. 2021) and there are strong habitat associations among these three habitats (Ku et al. 2021). Exposure to the northeast monsoon wind is associated with high tree density and species that have thick leaves (Su 1993), small leaf area (Su 1993), and high evapotranspiration rates (Chang 2008). However, the decreasing stress caused by the waning northeast monsoon wind may change those trees’ physiology and alter the interaction between trees from facilitation to competition (Bertness and Callaway 1994). Trees in high wind exposure habitats could then experience higher competition than those in the other habitats less affected by the northeast monsoon wind. Nonetheless, the potential impact of changing wind conditions on tree density and aboveground biomass remains unclear (e.g., Zimmerman et al. (2021)).
The aim of this research is to understands how tree density and aboveground biomass change over time in the high wind exposure of Lanjenchi, and to examine the influence of tree size and habitat on these changes. Our aim was to answer the following questions: (1) How does aboveground biomass vary over time across different habitats? (2) Does tree size influence the variation of tree density and aboveground biomass across different habitats over time? (3) Does aboveground biomass decline due to decreases in tree density? (4) To what extent does the long-term northeast monsoon affect the gain and loss of aboveground biomass in the forest?
Methods
Study site
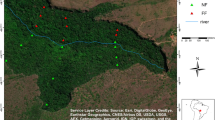
The Lanjenchi plot (120° 51′ 38″ E, 22° 03′ 23″ N) was established according to the protocols of the Smithsonian ForestGEO program (Davies et al. 2021). It is located on the eastern slope of Mt. Wanlite in the southernmost portion of Taiwan (Ku et al. 2021). The mean annual rainfall is 3,793 mm and the mean annual temperature is 22.3 °C (Ku et al. 2021). Elevation ranges from 280 to 340 m, and slope at the 10 × 10 m scale ranges from 10° to 30°. The wind velocity data from the weather station in Lanjenchi shows that sustained wind speed can be up to 20 m s−1 (hourly average from 2017 to 2020), with strong winds predominantly occurring during winter months and blowing in a northeast direction (Fig. 1). The most speciose families in the Lanjenchi plot were Fagaceae, Illiciaceae, and Lauraceae (Chao et al. 2010). The dissected topography and directionality of the northeast monsoon yield three principal habitats—windwards, leeward, and intermediate (Ku et al. 2021).
a Geographical location of the Lanjenchi forest. b Topographic map and habitat type for each 10 × 10 m quadrat within the 5.28 ha of Lanjenchi. c Windrose from the weather station at Lanjenchi. The three habitat types in b follow Ku et al. (2021). Red triangles indicate windward habitat; black circles represent intermediate habitat; white squares represent leeward habitat. The weather station was built in 2017, and each dot of the figure c represents the mean hourly wind speed (m s−1) data from 2017 to 2021. Red dots represent spring (February to April); yellow dots represent summer (May to July); orange dots represent autumn (August to October); blue dots represent winter (November to January)
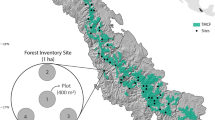
The Lanjenchi plot was initially established as a 3-ha plot in 1989 (Sun 1993) and was subsequently enlarged to 5.88-ha during 1997–2000 (Chao et al. 2007). We subdivided the original 5.88-ha plot into a 5.28 ha square plot composed of 528 subplots (10 m × 10 m). All stems with a diameter at breast height (DBH) ≥ 1 cm were measured, tagged, mapped, and identified to species.
Estimating aboveground biomass
This study used diameter data from four censuses conducted in 1997, 2005, 2013, and 2019. Aboveground biomass (AGB) was calculated from the allometric equation (Eq. 1) developed by Chave et al. (2014):
The equation includes wood density (ρ, g cm−3), tree diameter (D, cm), and tree height (H, m). Tree heights and wood densities were sampled in 2013 in each of the three habitat associations. Some trees were composed of multiple stems at breast height. For those multi-stemmed trees, aboveground biomass was calculated for each stem and then summed for aboveground tree biomass.
Wood density was derived from existing data and increment cores taken near the plot boundaries (Muller‐Landau 2004). We extracted a total of 195 core samples (at least three samples for each species) representing 33 out of the 140 species (the total species that appear from 1997 to 2019) using a 5.15 mm diameter increment borer. The minimum DBH criterion for the sampled trees was set at 6 cm to reduce the possibility of causing tree death. Each core was split into a 5 cm segment, and the radial position and length were recorded. Fresh volume was determined by the water displacement method within 24 h of collection. Dry mass was determined by oven-drying the sample to constant mass at 60 °C for 10 days and raising the temperature to 100 °C for two days. For the 93 remaining species, wood density was obtained from the Asian region of Global Wood Density Database (Chave et al. 2009; Zanne et al. 2009). We selected 23 species-level values, 69 genus-level values, and one family level value (Table S1). Densities for the remaining 14 species not listed in the Wood Density Database were calculated as the average of all the species’ wood densities in the specific 10 × 10 m quadrat (Table S1).
In 2013, we measured the diameter and height for the dominant species in each habitat type (197, 266, and 41 trees in the windward, intermediate, and leeward habitats, respectively). We fit four different candidate height-diameter allometric models. Two of the four models were log–log polynomial models with the order varying from 1 to 2. The remaining two models were asymptotic models, including a three-parameter Weibull (Feldpausch et al. 2012) and a two-parameter Michaelis–Menten model (Molto et al. 2013). Each model was fitted for each habitat type. The best fit models were selected according to the Akaike Information Criterion (AIC) (Sakamoto et al. 1986), the Root Mean Squared Error (RMSE) and the bias between the predicted and observed tree height. Windward habitat best fit to the log–log model with order 2 (Eq. 2); Intermediate habitat was best fit to the Weibull model (Eq. 3); and leeward habitat best fit to log–log model with order 1(Eq. 4) (Table S2; Figure S1).
where D is the tree diameter (cm), and H is the tree height (m) estimated by the tree diameter.
Diameter and habitation variations in aboveground biomass
Trees were classified into different diameter classes to represent their adaptability to the wind environment in different habitats. We classified trees as either small-diameter, medium-diameter, or large-diameter. The threshold for large-diameter trees was set as DBH ≥ 17.2 cm, following the large-diameter threshold of Lutz et al. (2018). Small-diameter trees were considered to have diameter 1 cm ≤ DBH < 10 cm, following the general convention for definition of small trees. Medium-diameter trees were those with diameter 10 cm ≤ DBH < 17.2 cm. Tree density and aboveground biomass for each diameter class was summed for each 10 × 10 m quadrat, and categorized according to the windward, intermediate, and leeward habitats as defined by Ku et al. (2021).
Aboveground biomass change
We calculated the total annual aboveground biomass change for each 10 × 10 m quadrat, each habitat, and each diameter class according to Eq. 5.
where AGBi is the aboveground biomass of each tree i, t1 is the census time, and the t0 is the previous census time. Biomass productivity was the sum of aboveground biomass change from surviving trees and newly recruited trees. Biomass mortality was the sum of aboveground biomass change for the trees that died between censuses. Net aboveground biomass change was the sum of aboveground biomass change.
Data analysis
We use the generalized estimating equation (GEE) test (Liang and Zeger 1986; Zeger and Liang 1986) with Tukey’s HSD post-hoc comparison to examine differences in tree density and aboveground biomass across the three diameter classes within each habitat (windward, intermediate, and leeward) during the four censuses. The data for tree density and aboveground biomass were obtained from 10 × 10 m quadrats and transformed using the square root function to fit the Gaussian distribution for the GEE test. The GEE method is suitable for analyzing data with repeated measurements and temporal autocorrelation. To account for the correlation structure between observations within the plots, we used the autoregressive AR1 correlation structure in the GEE analysis. We used ANOVA to assess the difference in net aboveground biomass, biomass productivity and mortality across different tree diameter classes and habitats using yearly data. Analyses were performed in the R statistical software package v4.2.1 (R Core Team 2022). The GEE analysis were operated by geelm function in the geepack package (Højsgaard et al. 2005). The tree height model used the modelHD function in BIOMASS package (Réjou‐Méchain et al. 2017). Aboveground biomass was fitted using the computeAGB function under the BIOMASS package (Réjou‐Méchain et al. 2017).
Results
Aboveground biomass
In 2019, the 5.28 ha Lanjenchi plot had a tree density of 9272 ± 3612 trees ha−1 (mean ± SD) and aboveground biomass of 129.37 ± 51.95 Mg ha−1 (Table 1). The windward habitat had the highest densities (11,383 ± 3692 trees ha−1) and the lowest aboveground biomass (113.77 ± 32.83 Mg ha−1; Table 1). The leeward habitat had fewer trees (7128 ± 2687 trees ha−1) and higher aboveground biomass (132.89 ± 64.20 Mg ha−1) (Table 1; Fig. 2). Small-diameter trees comprised 87.5% of trees and 16.4% of aboveground biomass. Large-diameter trees accounted for 4.8% of trees and 62.2% of the total aboveground biomass (Fig. 2).
Density (trees ha−1) and aboveground biomass (Mg ha−1) among small-, medium-, and large-diameter trees in each habitat and Lanjenchi overall for the four censuses. Data points represent the mean, interquartile range (from 25 to 75th percentile), and the 5th to 95th percentile, with variation derived from the 528, 10 × 10 m quadrats. The small letter on each point indicates a significant difference between the census years using Tukey’s HSD post-hoc comparison
The windward habitat was dominated by small trees, which accounted for 35.4% of all trees in 2019 (Fig. 2a). Large-diameter trees comprised 1.9% of trees in intermediate habitats; intermediate habitats contained the highest number of large-diameter trees among the habitats (Fig. 2a). However, the large-diameter trees in the leeward habitat held higher aboveground biomass than the large-diameter trees in the intermediate habitat (Fig. 2b), with the large-diameter trees in the leeward habitat contributing 24.9% of the aboveground biomass in the entire plot (Fig. 2b).
Aboveground biomass and density change over time
Overall, density and the aboveground biomass were stable over the 22 years from 1997 to 2019 (Table 1). Notably, there were no changes in the aboveground biomass observed in the three habitats (Table 1).
However, the distribution of density and biomass changed when considered by diameter class and habitat type. Tree density decreased in windward habitats (14,505 trees ha−1 to 11,383 trees ha−1) and increased in leeward habitats (6378 trees ha−1 to 7128 trees ha−1; Table 1). In the windward habitat, the aboveground biomass of small-diameter trees decreased, while that of large-diameter trees increased from 1997 to 2019 (Fig. 2). However, in the leeward habitat, only small trees increased in density and aboveground biomass. In the intermediate habitat, there was no change among the diameter classes over time (Fig. 2b).
Dynamic of aboveground biomass flux
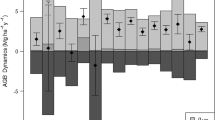
Considered by biomass, the mortality of small-diameter trees was greater than their productivity (Fig. 3; Figure S2), resulting in a net loss of aboveground biomass among the three habitats. Small-diameter trees in the windward habitat decreased the most. However, for medium- and large-diameter trees, the mortality biomass was lower than the productivity biomass, yielding an increase among the three habitats over time (Fig. 3).
Changes in aboveground biomass from each interval between small-, medium-, and large-diameter trees in windward, intermediate, and leeward habitats. Each boxplot represents the annualized change among the 22 year census in a net biomass, b biomass productivity, c biomass mortality. The small letter on each bar represent significant difference among different habitat and tree size classes using Tukey’s HSD post-hoc comparison
Discussion
This study provides an examination of the dynamics of tree density and aboveground biomass among different tree diameter classes and different wind exposures in a northeast monsoon wind-affected forest in Taiwan. Aboveground biomass in this forest is low and tree density is high compared to other global forests (Lutz et al. 2018). Over 22 years, even in the presence of repeated, strong wind effects, aboveground biomass has remained stable. However, the dynamics of the forest differed among wind exposures and tree diameters. Diameter-class distributions in the windward habitat exhibited a greater change, with a decrease in small-diameter trees and an increase in large-diameter trees. Large-diameter trees in leeward habitats showed both high productivity and mortality over time. Although the Lanjenchi forest as a whole exhibited stability, these fluctuations in diameter classes among habitat types revealed a dynamic equilibrium (sensu Lutz et al. (2014)). However, under different environmental conditions, the trees in the study forest showed unequal demographic changes, indicating a more nuanced level of dynamics than overall trends would imply.
Forest aboveground biomass structure in size classes among habitats
The distribution of topographic factors and nutrients can influence the species and tree size distribution in forest ecosystems (Lin et al. 2012; McEwan et al. 2011; Xu et al. 2015). Trees in windward habitats are exposed to the strong northeast monsoon wind. To adapt to the long-term wind stress, trees alter the morphology and physiology (Chang 2008; Su 1993) with a result in high wood density, thick leaves, and short tree height to prevent water loss and withstand damage (Telewski 1995, 2012) (Figure S1), resulting in a forest with numerous small trees. A high density of small trees with flexible wood is also a common strategy for trees in windward habitat to resist uprooting. Consequently, the topographic difference can lead to a distribution of smaller and larger trees that varies across different habitat in this wind-affected forests.
Moreover, different tree sizes have different sensitivity to environmental conditions. Larger trees are found in areas with high soil fertility compared to smaller trees (Sellan et al. 2019). Some research has found that the low convexity and less disturbance can contribute more to large tree survival than other topographic factors (McEwan et al. 2011; Rozendaal et al. 2022). Moreover, high levels of nitrogen could also contribute to large tree survival. At Lanjenchi, Chen et al. (1997) found that the soil fertility exhibits a gradient from the windward habitat to the leeward habitat. Soil in the windward habitat has less available N, exchangeable K, and cation exchange capacity (CEC) than in the leeward habitat. The large trees are associated with high soil fertility in the leeward habitat, and small trees appear to survive in the lower soil fertility of the windward habitat.
Here, large-diameter trees had 45.8% more biomass than the category of small trees (16.4% biomass, 8,116 trees), emphasizing their importance to carbon dynamics. The productivity of large-diameter trees is still higher than their mortality, making them an important ongoing carbon sink as well as an important ecological constituent of this tropical old-growth forest (Chave et al. 2003; Clark and Clark 1996; Lutz et al. 2018, 2013; Meakem et al. 2018).
Although large-diameter trees can contribute more aboveground biomass than small-diameter trees, small-diameter trees contribute disproportionately to carbon cycling and species diversity (Memiaghe et al. 2016). These small trees (1 cm ≤ DBH < 10 cm) are often omitted in forest inventories obscuring important dynamics. The mortality of small trees in the Lanjenchi plot was 24% of the total mortality of aboveground biomass (Figure S2) and decreased with decreasing density. Although small-diameter trees contribute less aboveground biomass to the total, they may lose a larger proportion of biomass due to changes in competition, resource, or magnitude of disturbances.
Drivers of aboveground biomass change
Trees of different sizes exhibited divergent patterns of mortality and productivity within different habitats. Specifically, in windward habitat, small-diameter trees had high biomass mortality (0.79 Mg ha−1 year−1) and large-diameter trees showed high biomass productivity (0.75 Mg ha−1 year−1), whereas in the leeward habitat, large-diameter trees exhibited both high mortality and productivity. Several mechanisms could contribute to these dynamics. CO2 fertilization has the potential to increase forest productivity (Luo 2007; Luo et al. 2009; Melillo et al. 1990, 2011), but it also increases mortality (Büntgen et al. 2019; Bugmann & Bigler 2011). Additionally, the concentration of nitrogen in the soil affects CO2 fertilization and can promote the forest productivity (Saban et al. 2019). However, in the nitrogen-limited windward habitat, competition for nutrients and water is high. This is consistent with Kamara and Kamruzzaman (2020) and Kubiske et al. (2019), who found that smaller trees undergo high frequency self-thinning, and that large-diameter trees contribute more to increases in aboveground biomass than small trees. In contrast, the leeward habitat has more nutrients and water than the windward habitat, perhaps contributing to the high mortality and high productivity of larger trees.
However, the changing environmental conditions may affect the relationship of the tree mortality in different tree sizes among habitats (McDowell et al. 2022; Rau et al. 2022; Ukkola et al. 2020) Based on the stress gradient hypothesis, the relationship between species may shift from facilitation to competition because of neighborhood habitat amelioration (Bertness and Callaway 1994). Winter wind speed measured by the meteorological stations along the Chinese coastline (Xu et al. 2006) and in Taiwan (Hung and Kao 2010) has decreased over the past five decades. The wind speed data from the Central Weather Bureau of Taiwan showed that the magnitude of the winter monsoon decreased from 1950 to 2013 at the Hen-Chun station, near Lanjenchi (Hung and Kao 2010; Wang and Lu 2017). We speculate that the interaction between trees of different habitats may be due to the decreasing northeast monsoon wind speed. The reduction of wind speed may decrease the vapor pressure deficit (Yuan et al. 2019). It could cause a lower evaporation rate and increase the intercellular gas exchange rate (Zhang et al. 2022). The stomata of trees, which were closed by the strong northeast monsoon wind, open as the wind weakens. This could provide the plants with a higher photosynthetic rate (Avecilla et al. 2015; Jin et al. 2014), thus promoting vegetation growth (Zhang et al. 2022). However, these factors may be secondary to the decreased mechanical mortality (from uprooting or crushing) due to decreasing wind speeds (Lutz and Halpern 2006).
Limitation of examining the long-term aboveground biomass under different habitats.
The Chave et al. (2014) allometric equations have limitations when it comes to accurately estimating aboveground biomass for trees smaller than 5 cm in diameter. However, small trees contribute a relatively smaller proportion to the overall aboveground biomass of the forest. Nevertheless, in other studies comparing various survey data, the equation proposed by Chave et al. (2014) is still employed for estimating aboveground biomass of trees ≤ 5 cm (Lutz et al. 2018, 2021; Muscarella et al. 2020). To ensure comparability with these studies, we utilized the same Chave’s model but integrated a local tree height model. This approach sought to overcome the limitations of estimating aboveground biomass for trees smaller than 5 cm in diameter and aimed to enhance the accuracy of aboveground biomass estimation by improving the representation of tree height within the specific habitat context.
The habitat classification follows Ku et al. (2021), who utilized the Multivariate Regression Tree (MRT) method developed by De’Ath (2002). MRT employs a resampling technique to verify the relationship between vegetation and topographic data for habitat classification as using vegetation data alone may lead to a tautological explanation of forest structure. Habitat classification derived from the topographic data itself are straightforward, even though the process includes some consideration of vegetation data. There still remains a possibility of spatial autocorrelation affecting the results. However, habitat classification has proven to be a valid approach to analysis in large forest dynamics plots with this caveat (Chave et al. 2003). The division into three habitat types and the concomitantly large number of quadrats in each type minimizes errors of this nature. This approach provides a less subjective and more objective method for habitat classification, as the habitat can be delineated based on specific topographic indices.
Conclusion
The monsoon-influenced forest in Taiwan has a high tree density and a low aboveground biomass compared with the other tropical forests, but like many old-growth forests, overall density and biomass appear relatively stable. Different tree diameter classes exhibited different dynamics over 22 years. Large-diameter trees accumulated more aboveground biomass than medium- and small-diameter trees, which could reflect a dominance of asymmetrical competition in this forest. Over the past 22 years, tree density has decreased while aboveground biomass has remained relatively stable. However, the aboveground biomass dynamics of trees in different diameter classes depended on their exposure to monsoon winds. Large-diameter trees had high biomass mortality and productivity in the intermediate and leeward habitats. Windward habitats had a high variation of aboveground biomass change in both small- and large-diameter trees. Small trees apparently have high frequently of self-thinning in the windward habitat. So even though the density of small trees decreased with a concomitant decrease in aboveground biomass, large-diameter trees compensated for that decline in the windward habitat. Overall, aboveground biomass was in a state of dynamic equilibrium over time. Due to the heterogeneous environmental conditions and the influence of the northeast monsoon wind, the various changes of aboveground biomass in different diameter classes and habitats kept the aboveground biomass stable over 22 years. However, if large-diameter trees continue to mainly persist in the windward habitat, this could cause a divergence in aboveground biomass between the windward and leeward habitats over time.
Data accessibility statement
Raw data for each census belongs to the funding unit and can be obtained from the site principal investigator on reasonable request. The information of the principal investigator in the Nanjenshan site can be found on the ForestGEO website (https://forestgeo.si.edu/sites/asia/nanjenshan).
Data Availability
Data are available upon reasonable request from the corresponding author.
References
Avecilla F, Panebianco JE, Buschiazzo DE (2015) Variable effects of saltation and soil properties on wind erosion of different textured soils. Aeolian Res 18:145–153. https://doi.org/10.1016/j.aeolia.2015.07.005
Büntgen U, Krusic PJ, Piermattei A, Coomes DA, Esper J, Myglan VS, Kirdyanov AV, Camarero JJ, Crivellaro A, Körner C (2019) Limited capacity of tree growth to mitigate the global greenhouse effect under predicted warming. Nat Commun 10:1–6. https://doi.org/10.1038/s41467-019-10174-4
Bastin J-F, Barbier N, Réjou-Méchain M, Fayolle A, Gourlet-Fleury S, Maniatis D, De Haulleville T, Baya F, Beeckman H, Beina D (2015) Seeing Central African forests through their largest trees. Sci Rep 5:1–8. https://doi.org/10.1038/srep13156
Bertness MD, Callaway R (1994) Positive interactions in communities. Trends Ecol Evol 9:191–193. https://doi.org/10.1016/0169-5347(94)90088-4
Brienen RJ, Phillips OL, Feldpausch TR, Gloor E, Baker TR, Lloyd J, Lopez-Gonzalez G, Monteagudo-Mendoza A, Malhi Y, Lewis SL (2015) Long-term decline of the Amazon carbon sink. Nature 519:344–348. https://doi.org/10.1038/nature14283
Brown S (1997) Estimating biomass and biomass change of tropical forests: a primer. Food & Agriculture Org, Rome
Brown S, Gillespie AJ, Lugo AE (1989) Biomass estimation methods for tropical forests with applications to forest inventory data. For Sci 35:881–902. https://doi.org/10.1093/forestscience/35.4.881
Bugmann H, Bigler C (2011) Will the CO2 fertilization effect in forests be offset by reduced tree longevity? Oecologia 165:533–544. https://doi.org/10.1007/s00442-010-1837-4
Chang C-Y (2008) Effects of wind speed and other environmental factors on sap flux of three species in a subtropical rainforest of Nanjenshan. National Taiwan University, Taipei, p 87
Chao W-C, Chao K-J, Song G-ZM, Hsieh C-F (2007) Species composition and structure of the lowland subtropical rainforest at Lanjenchi, Southern Taiwan. Taiwania 52:253–269. https://doi.org/10.6165/tai.2007.52(3).253
Chao W-C, Song G-ZM, Chao K-J, Liao C-C, Fan S-W, Wu S-H, Hsieh T-H, Sun I-F, Kuo Y-L, Hsieh C-F (2010) Lowland rainforests in southern Taiwan and Lanyu, at the northern border of Paleotrophics and under the influence of monsoon wind. Plant Ecol 210:1–17. https://doi.org/10.1007/s11258-009-9694-0
Chave J, Andalo C, Brown S, Cairns MA, Chambers JQ, Eamus D, Folster H, Fromard F, Higuchi N, Kira T, Lescure JP, Nelson BW, Ogawa H, Puig H, Riera B, Yamakura T (2005) Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145:87–99. https://doi.org/10.1007/s00442-005-0100-x
Chave J, Condit R, Lao S, Caspersen JP, Foster RB, Hubbell SP (2003) Spatial and temporal variation of biomass in a tropical forest: results from a large census plot in Panama. J Ecol 91:240–252. https://doi.org/10.1046/j.1365-2745.2003.00757.x
Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE (2009) Towards a worldwide wood economics spectrum. Ecol Lett 12:351–366. https://doi.org/10.1111/j.1461-0248.2009.01285.x
Chave J, Réjou-Méchain M, Burquez A, Chidumayo E, Colgan MS, Delitti WB, Duque A, Eid T, Fearnside PM, Goodman RC, Henry M, Martinez-Yrizar A, Mugasha WA, Muller-Landau HC, Mencuccini M, Nelson BW, Ngomanda A, Nogueira EM, Ortiz-Malavassi E, Pelissier R, Ploton P, Ryan CM, Saldarriaga JG, Vieilledent G (2014) Improved allometric models to estimate the aboveground biomass of tropical trees. Glob Chang Biol 20:3177–3190. https://doi.org/10.1111/gcb.12629
Chen Z-S, Hsieh C-F, Jiang F-Y, Hsieh T-H, Sun I-F (1997) Relations of soil properties to topography and vegetation in a subtropical rain forest in southern Taiwan. Plant Ecol 132:229–241. https://doi.org/10.1023/A:1009762704553
Ciais P, Sabine C, Bala G, Bopp L, Brovkin V, Canadell J, Chhabra A, DeFries R, Galloway J and Heimann M (2014) Carbon and other biogeochemical cycles. Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, pp 465–570
Clark DB, Clark DA (1996) Abundance, growth and mortality of very large trees in neotropical lowland rain forest. For Ecol Manage 80:235–244. https://doi.org/10.1016/0378-1127(95)03607-5
Davies SJ, Abiem I, Salim KA, Aguilar S, Allen D, Alonso A, Anderson-Teixeira K, Andrade A, Arellano G, Ashton PS (2021) ForestGEO: Understanding forest diversity and dynamics through a global observatory network. Biol Conserv 253:108907. https://doi.org/10.1016/j.biocon.2020.108907
De’Ath G (2002) Multivariate regression trees: a new technique for modeling species–environment relationships. Ecology 83:1105–1117. https://doi.org/10.1890/0012-9658(2002)083[1105:MRTANT]2.0.CO;2
Fauset S, Baker TR, Lewis SL, Feldpausch TR, Affum-Baffoe K, Foli EG, Hamer KC, Swaine MD (2012) Drought-induced shifts in the floristic and functional composition of tropical forests in Ghana. Ecol Lett 15:1120–1129. https://doi.org/10.1111/j.1461-0248.2012.01834.x
Feeley KJ, Davies SJ, Ashton PS, Bunyavejchewin S, Nur Supardi M, Kassim AR, Tan S, Chave J (2007) The role of gap phase processes in the biomass dynamics of tropical forests. Proc Biol Sci 274:2857–2864. https://doi.org/10.1098/rspb.2007.0954
Feldpausch TR, Lloyd J, Lewis SL, Brienen RJ, Gloor M, Monteagudo Mendoza A, Lopez-Gonzalez G, Banin L, Abu Salim K, Affum-Baffoe K (2012) Tree height integrated into pantropical forest biomass estimates. Biogeosciences 9:3381–3403. https://doi.org/10.5194/bg-9-3381-2012
Højsgaard S, Halekoh U, Yan J (2005) The R package geepack for generalized estimating equations. J Stat Softw 15:1–11. https://doi.org/10.18637/jss.v015.i02
Hansen MC, Potapov P, Tyukavina A (2019) Comment on “Tropical forests are a net carbon source based on aboveground measurements of gain and loss.” Science 363:3629. https://doi.org/10.1126/science.aar3629
Hill TC, Williams M, Bloom AA, Mitchard ET, Ryan CM (2013) Are inventory based and remotely sensed above-ground biomass estimates consistent? PLoS ONE 8:e74170
Hsieh C-F, Chen Z-S, Sun I, Hsieh T-H, Zheng Y-B, Wang K-H, Su M-H, Jiang F-Y (1992) The subtropical rain forest in Nanjenshan Area, Kenting National Park (Report No. RES085). Kenting National Park, Construction and Planning Agency, Ministry of the Interior, p 269
Hung C-W, Kao P-K (2010) Weakening of the winter monsoon and abrupt increase of winter rainfalls over northern Taiwan and southern China in the early 1980s. J Clim 23:2357–2367. https://doi.org/10.1175/2009JCLI3182.1
IPCC (2007) Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. In: Core Writing Team P, R.K and Reisinger A (eds) (ed). Cambridge Univ. Press, IPCC, Geneva, Switzerland, p 104
Jin D, Gao Q, Wang Y, Xu L (2014) Impacts of reduced wind speed on physiology and ecosystem carbon flux of a semi-arid steppe ecosystem. Sci Cold Arid Reg 6:556–565
Kamara M, Kamruzzaman M (2020) Self-thinning process, dynamics of aboveground biomass, and stand structure in overcrowded mangrove Kandelia obovata stand. Reg Stud Mar Sci 38:101375. https://doi.org/10.1016/j.rsma.2020.101375
King DA, Davies SJ, Noor NSM (2006) Growth and mortality are related to adult tree size in a Malaysian mixed dipterocarp forest. For Ecol Manage 223:152–158. https://doi.org/10.1016/j.foreco.2005.10.066
Ku C-C, Song G-ZM, Chao K-J, Chao W-C (2021) Species-habitat associations of tree species under the northeast monsoon wind-affected tropical forest at Lanjenchi Forest Dynamics Plot. Taiwan Taiwania 66:39–47. https://doi.org/10.6165/tai.2021.66.39
Kubiske ME, Woodall CW, Kern CC (2019) Increasing atmospheric CO2 concentration stand development in trembling aspen forests: are outdated density management guidelines in need of revision for all species? J for 117:38–45. https://doi.org/10.1093/jofore/fvy058
Liang K-Y, Zeger SL (1986) Longitudinal data analysis using generalized linear models. Biometrika 73:13–22
Lin D, Lai J, Muller-Landau HC, Mi X, Ma K (2012) Topographic variation in aboveground biomass in a subtropical evergreen broad-leaved forest in China. PLoS ONE 7:e48244. https://doi.org/10.1371/journal.pone.0048244
Liu L, Zeng F, Song T, Wang K, Du H (2020) Stand structure and abiotic factors modulate karst forest biomass in Southwest China. Forests 11:443. https://doi.org/10.3390/f11040443
Loo YY, Billa L, Singh A (2015) Effect of climate change on seasonal monsoon in Asia and its impact on the variability of monsoon rainfall in Southeast Asia. Geosci Front 6:817–823. https://doi.org/10.1016/j.gsf.2014.02.009
Luo Y (2007) Terrestrial carbon–cycle feedback to climate warming. Annu Rev Ecol Evol Syst 38:683–712. https://doi.org/10.1146/annurev.ecolsys.383091206.095808
Luo Y, Sherry R, Zhou X, Wan S (2009) Terrestrial carbon-cycle feedback to climate warming: experimental evidence on plant regulation and impacts of biofuel feedstock harvest. Glob Change Biol Bioenerg 1:62–74. https://doi.org/10.1111/j.1757-1707.2008.01005.x
Lutz JA, Furniss TJ, Johnson DJ, Davies SJ, Allen D, Alonso A, Anderson-Teixeira KJ, Andrade A, Baltzer J, Becker KM (2018) Global importance of large-diameter trees. Global Ecol Biogeogr 27:849–864. https://doi.org/10.1111/geb.12747
Lutz JA, Halpern CB (2006) Tree mortality during early forest development: a long-term study of rates, causes, and consequences. Ecol Monogr 76:257–275
Lutz JA, Larson AJ, Freund JA, Swanson ME, Bible KJ (2013) The importance of large-diameter trees to forest structural heterogeneity. PLoS ONE 8:e82784. https://doi.org/10.1371/journal.pone.0082784
Lutz JA, Larson AJ, Furniss TJ, Donato DC, Freund JA, Swanson ME, Bible KJ, Chen J, Franklin JF (2014) Spatially nonrandom tree mortality and ingrowth maintain equilibrium pattern in an old-growth Pseudotsuga-Tsuga forest. Ecology 95:2047–2054. https://doi.org/10.1890/14-0157.1
Lutz JA, Larson AJ, Swanson ME, Freund JA (2012) Ecological importance of large-diameter trees in a temperate mixed-conifer forest. PLoS ONE 7:e36131. https://doi.org/10.1371/journal.pone.0036131
Lutz JA, Struckman S, Furniss TJ, Birch JD, Yocom LL, McAvoy DJ (2021) Large-diameter trees, snags, and deadwood in southern Utah, USA. Ecol Process 10:1–12. https://doi.org/10.1186/s13717-020-00275-0
McDowell NG, Sapes G, Pivovaroff A, Adams HD, Allen CD, Anderegg WR, Arend M, Breshears DD, Brodribb T, Choat B (2022) Mechanisms of woody-plant mortality under rising drought, CO2 and vapour pressure deficit. Nat Rev Earth Environ 3:294–308
McEwan RW, Lin Y-C, Sun I, Hsieh C-F, Su S-H, Chang L-W, Song G-ZM, Wang H-H, Hwong J-L, Lin K-C (2011) Topographic and biotic regulation of aboveground carbon storage in subtropical broad-leaved forests of Taiwan. For Ecol Manage 262:1817–1825. https://doi.org/10.1016/j.foreco.2011.07.028
Meakem V, Tepley AJ, Gonzalez-Akre EB, Herrmann V, Muller-Landau HC, Wright SJ, Hubbell SP, Condit R, Anderson-Teixeira KJ (2018) Role of tree size in moist tropical forest carbon cycling and water deficit responses. New Phytol 219:947–958. https://doi.org/10.1111/nph.14633
Melillo J, Callaghan T, Woodward F, Salati E and Sinha S (1990) Effects on ecosystems. Climate change: The IPCC scientific assessment, pp 283–310
Melillo JM, Butler S, Johnson J, Mohan J, Steudler P, Lux H, Burrows E, Bowles F, Smith R, Scott L (2011) Soil warming, carbon–nitrogen interactions, and forest carbon budgets. Proc Natl Acad Sci USA 108:9508–9512. https://doi.org/10.1073/pnas.1018189108
Memiaghe HR, Lutz JA, Korte L, Alonso A, Kenfack D (2016) Ecological importance of small-diameter trees to the structure, diversity and biomass of a tropical evergreen forest at Rabi. Gabon Plos One 11:e0154988. https://doi.org/10.1371/journal.pone.0154988
Molto Q, Rossi V, Blanc L (2013) Error propagation in biomass estimation in tropical forests. Methods Ecol Evol 4:175–183. https://doi.org/10.1111/j.2041-210x.2012.00266.x
Muller-Landau HC (2004) Interspecific and inter-site variation in wood specific gravity of tropical trees. Biotropica 36:20–32
Muscarella R, Kolyaie S, Morton DC, Zimmerman JK, Uriarte M (2020) Effects of topography on tropical forest structure depend on climate context. J Ecol 108:145–159. https://doi.org/10.1111/1365-2745.13261
Noguchi M, Hoshizaki K, Matsushita M, Sugiura D, Yagihashi T, Saitoh T, Itabashi T, Kazuhide O, Shibata M, Hoshino D (2022) Aboveground biomass increments over 26 years (1993–2019) in an old-growth cool-temperate forest in northern Japan. J Plant Res 135:69–79. https://doi.org/10.1007/s10265-021-01358-5
Pan Y, Birdsey RA, Fang J, Houghton R, Kauppi PE, Kurz WA, Phillips OL, Shvidenko A, Lewis SL, Canadell JG, Ciais P, Jackson RB, Pacala SW, McGuire AD, Piao S, Rautiainen A, Sitch S, Hayes D (2011) A large and persistent carbon sink in the world’s forests. Science 333:988–993. https://doi.org/10.1126/science.1201609
Pastore MA, Lee TD, Hobbie SE, Reich PB (2019) Strong photosynthetic acclimation and enhanced water-use efficiency in grassland functional groups persist over 21 years of CO2 enrichment, independent of nitrogen supply. Global Change Biol 25:3031–3044. https://doi.org/10.1111/gcb.14714
Piponiot C, Anderson-Teixeira KJ, Davies SJ, Allen D, Bourg NA, Burslem DF, Cárdenas D, Chang-Yang CH, Chuyong G, Cordell S (2022) Distribution of biomass dynamics in relation to tree size in forests across the world. New Phytol. https://doi.org/10.1111/nph.17995
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Réjou-Méchain M, Muller-Landau HC, Detto M, Thomas SC, Le Toan T, Saatchi SS, Barreto-Silva JS, Bourg NA, Bunyavejchewin S, Butt N (2014) Local spatial structure of forest biomass and its consequences for remote sensing of carbon stocks. Biogeosciences 11:6827–6840. https://doi.org/10.5194/bg-11-6827-2014
Réjou-Méchain M, Tanguy A, Piponiot C, Chave J, Hérault B (2017) biomass: an r package for estimating above-ground biomass and its uncertainty in tropical forests. Methods Ecol Evol 8:1163–1167. https://doi.org/10.1111/2041-210X.12753
Rau E, Gardiner BA, Fischer FJ, Maréchaux I, Joetzjer E, Sun I, Chave J (2022) Wind speed controls forest structure in a subtropical forest exposed to cyclones: a case study using an individual-based model. Front for Glob Change 5:753100
Rodrigues AC, Villa PM, Ali A, Ferreira-Júnior W, Neri AV (2020) Fine-scale habitat differentiation shapes the composition, structure and aboveground biomass but not species richness of a tropical Atlantic forest. J for Res 31:1599–1611. https://doi.org/10.1007/s11676-019-00994-x
Rozendaal DM, Suarez DR, De Sy V, Avitabile V, Carter S, Yao CA, Alvarez-Davila E, Anderson-Teixeira K, Araujo-Murakami A, Arroyo L (2022) Aboveground forest biomass varies across continents, ecological zones and successional stages: refined IPCC default values for tropical and subtropical forests. Environ Res Lett 17:014047. https://doi.org/10.1088/1748-9326/ac45b3
Running SW, Nemani RR, Heinsch FA, Zhao M, Reeves M, Hashimoto H (2004) A continuous satellite-derived measure of global terrestrial primary production. Bioscience 54:547–560. https://doi.org/10.1641/0006-3568(2004)054[0547:ACSMOG]2.0.CO;2
Saban JM, Chapman MA, Taylor G (2019) FACE facts hold for multiple generations; Evidence from natural CO2 springs. Global Change Biol 25:1–11. https://doi.org/10.1111/gcb.14437
Sakamoto Y, Ishiguro M, Kitagawa G (1986) Akaike information criterion statistics. D. Reidel, Dordrecht 81: 26853. Doi: https://doi.org/10.1080/01621459.1988.10478680.
San-José M, Werden L, Peterson CJ, Oviedo-Brenes F, Zahawi RA (2021) Large tree mortality leads to major aboveground biomass decline in a tropical forest reserve. Oecologia 197:795–806. https://doi.org/10.1007/s00442-021-05048-w
Sellan G, Thompson J, Majalap N, Brearley FQ (2019) Soil characteristics influence species composition and forest structure differentially among tree size classes in a Bornean heath forest. Plant Soil 438:173–185. https://doi.org/10.1007/s11104-019-04000-5
Shen A, Wu C, Jiang B, Deng J, Yuan W, Wang K, He S, Zhu E, Lin Y, Wu C (2018) Spatiotemporal variations of aboveground biomass under different terrain conditions. Forests 9:778. https://doi.org/10.3390/f9120778
Shen Y, Yu S, Lian J, Shen H, Cao H, Lu H, Ye W (2016) Tree aboveground carbon storage correlates with environmental gradients and functional diversity in a tropical forest. Sci Rep 6:1–10. https://doi.org/10.1038/srep25304
Su M-H (1993) The leaf structure of canopy of Nanjenshan subtropical rain forest. National Taiwan University, Taipei, p 82
Sun I-F (1993) The species composition and forest structure of a subtropcial rain forest at southern Taiwan. Dissertation. University of California, Berkeley. 410 pp
Telewski FW (1995) Wind-induced physiological and developmental responses in tress. Cambridge University Press, Cambridge
Telewski FW (2012) Is windswept tree growth negative thigmotropism? Plant Sci 184:20–28. https://doi.org/10.1016/j.plantsci.2011.12.001
Thornton PE, Law BE, Gholz HL, Clark KL, Falge E, Ellsworth DS, Goldstein AH, Monson RK, Hollinger D, Falk M (2002) Modeling and measuring the effects of disturbance history and climate on carbon and water budgets in evergreen needleleaf forests. Agric for Meteorol 113:185–222. https://doi.org/10.1016/S0168-1923(02)00108-9
Ukkola AM, De Kauwe MG, Roderick ML, Abramowitz G, Pitman AJ (2020) Robust future changes in meteorological drought in CMIP6 projections despite uncertainty in precipitation. Geophys Res Lett 47:e2020GL087820
Walker AP, De Kauwe MG, Bastos A, Belmecheri S, Georgiou K, Keeling RF, McMahon SM, Medlyn BE, Moore DJ, Norby RJ (2021) Integrating the evidence for a terrestrial carbon sink caused by increasing atmospheric CO2. New Phytol 229:2413–2445. https://doi.org/10.1111/nph.16866
Wang L, Lu M-M (2017) The East Asian winter monsoon. In: Chang C-P, Kuo H-C, Lau N-C, Johnson RH, Wang B, Wheeler MC (eds) The global monsoon system: research and forecast. World Scientific, Singapore, pp 51–61
Warren JM, Jensen AM, Medlyn BE, Norby RJ, Tissue DT (2015) Carbon dioxide stimulation of photosynthesis in Liquidambar styraciflua is not sustained during a 12-year field experiment. AoB Plants. https://doi.org/10.1093/aobpla/plu074
Xu M, Chang CP, Fu C, Qi Y, Robock A, Robinson D, Zhang HM (2006) Steady decline of east Asian monsoon winds, 1969–2000: Evidence from direct ground measurements of wind speed. J Geophys Res Atmos. https://doi.org/10.1029/2006JD007337
Xu Y, Franklin SB, Wang Q, Shi Z, Luo Y, Lu Z, Zhang J, Qiao X, Jiang M (2015) Topographic and biotic factors determine forest biomass spatial distribution in a subtropical mountain moist forest. For Ecol Manage 357:95–103. https://doi.org/10.1016/j.foreco.2015.08.010
Yuan W, Zheng Y, Piao S, Ciais P, Lombardozzi D, Wang Y, Ryu Y, Chen G, Dong W, Hu Z (2019) Increased atmospheric vapor pressure deficit reduces global vegetation growth. Sci Adv 5:eaax1396. https://doi.org/10.1126/sciadv.aax1396
Zanne AE, Lopez-Gonzalez G, Coomes DA, Ilic J, Jansen S, Lewis SL, Miller RB, Swenson NG, Wiemann MC, Chave J (2009) Data from: towards a worldwide wood economics spectrum [Dataset]. Dryad. https://doi.org/10.5061/dryad.234
Zeger SL, Liang K-Y (1986) Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42:121–130
Zhang T, Xu X, Jiang H, Qiao S, Guan M, Huang Y, Gong R (2022) Widespread decline in winds promoted the growth of vegetation. Sci Total Environ 825:153682. https://doi.org/10.1016/j.scitotenv.2022.153682
Zimmerman JK, Wood TE, González G, Ramirez A, Silver WL, Uriarte M, Willig MR, Waide RB, Lugo AE (2021) Disturbance and resilience in the Luquillo experimental forest. Biol Conserv 253:108891. https://doi.org/10.1016/j.biocon.2020.108891
Acknowledgements
We would like to express our sincere appreciation to all the volunteers, workers, and research assistants who dedicated their time, effort, and expertise to the tropical forest from 1991 to 2019. We are also grateful to Chieh-Yu Liao and Wen-Ling Shen for their careful review of this study. Furthermore, we would like to acknowledge the financial support provided from Forestry Bureau of Taiwan (tfbp-1010223, tfbc-1070204) and the logistic assistance provided by Kenting National Park. Additionally, Chen-Chia Ku and Kuo-Jung Chao were supported by the Innovation and Development Center of Sustainable Agriculture from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan. We would also like to express our sincere appreciation to Jianwu Tang, our co-author, who unfortunately passed away before this paper could be published. He made significant contributions to Nanjenshan, and his invaluable insights and expertise to our work will not be forgotten.
Funding
This work was supported by the Forestry Bureau of Taiwan (Grant numbers tfbc-1070204 and tfbp-1010223). The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by CCK and WCC. The first draft of the manuscript was written by CCK and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by Peter le Roux.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ku, CC., Tang, J., Chao, WC. et al. Large-diameter trees buffer monsoonal changes to tree biomass over two decades. Plant Ecol 224, 1037–1048 (2023). https://doi.org/10.1007/s11258-023-01360-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-023-01360-y