Abstract
The impact of ongoing climate change on plant communities varies according to vegetation type and location across the globe. Snowbed flora count among the most sensitive vegetation due to their dependence on long-lasting snow patches. This is especially the case toward their rear distribution edge, where warming has already induced a marked decrease in snow deposition. Thus, analysing the dynamics of snowbed plant communities is crucial for understanding the ecological processes that condition their persistence under new environmental conditions. The Pyrenees represent the southern distribution limit of several eurosiberian snowbed species. We surveyed eight snowbeds based on permanent plots, where the presence of each taxon was recorded annually between 2012 and 2019. We analysed vegetation patterns between sites and plots, related them to environmental gradients, and assessed temporal trends of community dynamics. We detected important between-site differences regarding species composition. However, these differences were not supported by species' biogeographical patterns, which suggests that local abiotic factors filter species with distinct autecology. In parallel, temporal community turnover was observed through the expansion of widespread grassland species, which supports the hypothesis of colonisation of snowbeds by common alpine taxa. Such changes could be related to a decrease in snow cover over recent times, which releases extreme environmental constraints to plant growth. Therefore, it is crucial to characterise fine-scale ecological conditions to forecast plant community dynamics and provide reliable information for conserving snowbed vegetation across the Palearctic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Current global change induces profound modification to ecological processes (Walther 2010), influencing vegetation dynamics through species assemblage (Woodward and Diament 1991; Walther 2003). This has been widely studied over the past 30 years, especially the effect of land-use changes like the abandonment of traditional agriculture and habitat fragmentation on community turnover and succession (Fischer and Lindenmayer 2007; Thuiller et al. 2008). Understanding these processes is key to predicting an ecosystem's future and its possible extirpation (Lavergne et al. 2010) and is thus central to designing effective conservation actions (Guisan et al. 2006). It requires integrating large-scale biogeographical processes with community assembly rules (Callaway and Walker 1997; Götzenberger et al. 2012) to forecast future species turnover within communities and assess local colonisation/extinction dynamics (Buckley et al. 2021). Furthermore, modification of climatic conditions will impact not only global species distribution (Root et al. 2003) but also local assembly processes through the modification of microclimate (Ackerly et al. 2010), changes in competition output (Tylianakis et al. 2008), modification of phenology (Kudo and Hirao 2006; Petraglia et al. 2014). This can eventually disrupt plant-animal mutualisms such as pollination (Schweiger et al. 2010; Kudo 2020) or seed dispersion and stock (Ooi 2012; Alexander et al. 2018).
Among terrestrial ecosystems, mountains are expected to show important responses to ongoing climate changes (Dullinger et al. 2012; Rogora et al. 2018). Across the Western Palearctic, southernmost mountain ranges (e.g. the Pyrenees) are especially exposed to climate change due to both expected increased temperatures coupled with decreased precipitation (Engler et al. 2011), which are currently causing the glaciers to retreat (Chueca et al. 2007; Fernandes et al. 2018). The Pyrenees represent a key biogeographical barrier for the European flora, separating the temperate zone from the Mediterranean (Rica and Recoder 1990), and many species reach their southern distribution limit there (Hampe and Petit 2005; Pironon et al. 2017; Lenormand et al. 2019). Predictions at the Pyrenean massif scale imply earlier snowmelt in spring. At the same time, negative trends in snow duration and snowpack thickness have already been observed over the last four decades (Morán-Tejeda et al. 2017). The Pyrenean Climate Change Observatory (Climpy Project, https://opcc-ctp.org/fr/climpy) has reported an increase in global temperature and a diminution of rainfall among the Pyrenees (OPCC-CTP 2018), which is congruent with the general expected pattern for the Palearctic (Gobiet et al. 2014). Spring and summer temperatures rose by + 0.2 °C and + 0.4 °C per decade between 1951 and 2010. During the same period, the rainfall has decreased by 2.5% per decade. According to the projections, a halving of the snowpack thickness might be expected at 1800 m a.s.l. by 2050 in the central Pyrenees (OPCC-CTP 2018).
Mountain vegetation is structured along elevational gradients, and climate change is expected to induce a broad-scale upslope migration under a warming effect (Walther et al. 2005; Lenoir et al. 2008; Pauli et al. 2012). Low elevation thermophilous species are assumed to reach higher vegetation belts where cold-adapted species are currently restricted (Gottfried et al. 2012). At the community scale, shifts in species composition are expected due to modifications of the mechanism of assemblage that may be influenced by changing microclimate conditions and topography (Spasojevic et al. 2013). Earlier snowmelt dates and shorter snowpack duration at high elevations (López-Moreno and García-Ruiz 2004; Schöb et al. 2009) will lengthen the growing season and impact the outcome of plant competition (Hülber et al. 2011). Among the different vegetation types found in the Palearctic mountains, those depending on snow cover and duration, such as snowbed communities, are considered to be among the most sensitive to climate change (Matteodo et al. 2016).
Snowbed vegetation is composed of species able to cope with long periods of snow cover, extending from October to June. Snow is the main factor controlling vegetation (Galen and Stanton 1995), and the length of the snow period prevents non-adapted plants from germinating and growing. Nevertheless, it provides thermal insulation where the winter air temperature is negative (Körner 2003; Lluent 2007). Snowbed specialists start to grow when the snow has not yet melted (Björk and Molau 2007), and snowmelt provides constant moisture and soil nutrients at the beginning of the growth period (Körner 2003). All these abiotic factors have triggered the evolution of particular species (so-called chionophilous species), adapted to complete their life cycle during a growing season of two to three months (Schöb et al. 2009; Körner et al. 2019). Moreover, those snowbed communities can show a heterogeneous pattern of vegetation explained by topography, which also defines the accumulation and persistence of snow in specific places (Graae et al. 2018).
How will assembly processes evolve over climate modification in snowbed communities? Changes in snow persistence directly influence the composition of snowbed vegetation, in particular regarding the competition outputs. The low competitiveness of chionophilous species (Kudo 1999; Schöb et al. 2009) and a shorter and shifting blooming period within snowbed (Petraglia et al. 2014; Kudo 2020) are expected to promote modification of turnover rates over time. Seed production and seed banks could also be impacted (Ninot et al. 2013). Then, future competition between alpine generalist and chionophilous species might affect their respective persistence, with an expected disadvantage for the latter (Heegaard and Vandvik 2004; Hülber et al. 2011).
This work aims to explore the dynamics of Pyrenean snowbed plant communities and address the mechanisms driving temporal changes through correlative approaches. First, we analyse the composition of plant communities in 8 snowbed sites and relate these patterns to climate characteristics. Second, we analyse the temporal dynamics of such communities based on three approaches; among all plots, we investigate which community has significantly changed over the study period; at the plot scale, we quantify how communities have gradually changed from their original composition, year after year; for each species, we investigate the overall change in frequency within communities to detect species-specific trends. We expect to see a combination of biogeographical and environmental (including climate) factors explaining the evolution of the floristic composition of the vegetation.
Materials and method
Study area
The Pyrenean range, with an axial west–east orientation ranging from the Atlantic to the Mediterranean coast over more than 450 km, is the natural frontier between Spain and France, separating the Iberian Peninsula from the rest of Europe. The central part of the Pyrenees has a maximum width of about 150 km and hosts the highest peaks, with more than 200 peaks exceeding 3000 m a.s.l. (Aneto is the highest peak with 3404 m a.s.l.). The northern slope is rather steep and sharp, whereas the southern one spreads out over extended areas where elevation decreases gradually.
The Pyrenees can be split into two geological zones: an axial zone made of ancient rocks, mainly granites and gneiss, corresponding roughly to the highest part of the range, and a peripheral zone of Jurassic and Cretaceous sedimentary rocks (i.e. mostly limestone) surrounding this axial zone.
The region faces a plurality of climate conditions: most of the northern slope and the westernmost part of the southern slope have an oceanic climate sensu Rivas-Martínez (1996), the easternmost part of the range has a Mediterranean climate, and the south-central part of the Pyrenees faces a more continental climate sometimes called inland climate (Izard 1985). The alpine belt is found mainly above 2400 m on southern slopes and above 2200 m on northern slopes (Ninot et al. 2007). In this belt, rocks are mainly acidic (granites and gneiss), and the climate is typically alpine, characterised by negative temperatures in winter and low records of temperature (around 10 °C) in meteorological summer (Köppen 1900).
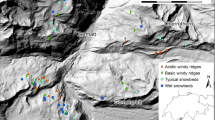
This study is part of the OPCC-POCTEFA EFA 235/11 project, which aims to create climate change indicators in Pyrenean alpine regions and study the long-term dynamics of Pyrenean snowbed species and vegetation (https://www.opcc-ctp.org/fr/florapyr). We selected eight sites where the snowbed survey had been conducted during a comparable time frame (Fig. 1; Table 1). On the northern Pyrenean slopes (France), four sites were analysed: Oô-Portillon (2507 m above sea level), Creussans (2600 m a.s.l.), Pas de la Case (2451 m a.s.l.) and Planès (2370 m a.s.l.). The four remaining sites are located on southern Pyrenean slopes, two in Andorra: Cataperdis (2535 m a.s.l.) and Arbella (2402 m a.s.l.), and two in Spain: Ratera (2560 m a.s.l.) and Ulldeter (2402 m a.s.l.).
Location of study sites in the Pyrenees. Pale grey represents areas below 750 m of altitude; grey areas correspond to 750–2000 m and dark grey areas above 2000 m. Names of the sites are Por: Oô-Portillon, Rat: Ratera, Cat: Cataperdis, Cre: Creussans, Arb: Arbella, Cas: Pas de la Case, Pla: Planès, Ull: Ulldeter
The alpine climate in Arbella and Ratera shows the lowest mean monthly temperatures, with a − 4 °C record for the coldest month. Regarding rainfall, only Planes, Ulldeter and Ratera show a contrasted water balance with the lowest records in winter and summer. Oppositely, the highest precipitations are registered in Portillon, Creussans and Arbella (Table 1).
Regarding geology, bedrock type is diverse among sites: shale forms the bedrock of Arbella, Cataperdis and Creussans. Ulldeter and Planes are on granite and orthogneiss substrates, while Pas de la Case, Portillon and Ratera are based on monzogranite and granodiorite bedrock.
Snowbed vegetations
Snowbeds support very specific plant communities. From a phytosociological point of view, these communities belong to the Salicetea herbaceae Braun-Blanquet 1948 class (Braun-Blanquet 1948; Corriol and Mikolajczak 2014; Mucina et al. 2016). According to Corriol and Mikolajczak (2014), this class can be divided into a neutro-basophilous order, Arabidetalia caeruleae Rübel ex Nordhagen 1937, and an acidophilous order, Salicetalia herbaceae Braun-Blanquet in Braun-Blanquet & Jenny 1926. Mucina et al. (2016) suggest a different syntaxonomic scheme for Europe with only one order divided into a relict glacial group of alliances (occurring in the mountains of the nemoral zone) and an arctic group of alliances (occurring in the boreo-arctic zone). Alliances can be considered the most relevant vegetation unit for broad-scale ecological and biogeographical processes (Willner 2020). For this reason, this study focuses on the Pyrenean snowbed vegetation on siliceous bedrock. This corresponds to the Salicion herbaceae Braun-Blanquet in Braun-Blanquet & Jenny 1926 in both mentioned above systems. The characteristic species of the alliance are Omalotheca supina, Cerastium cerastoides, Cardamine alpina, Sedum alpestre, Sibbaldia procumbens, Arenaria biflora, Luzula alpino-pilosa, to which should be added Salix herbacea and Veronica alpina as class characteristics (Corriol and Mikolajczak 2014).
Snowbed vegetations of the Salicion herbaceae are linked dynamically (when snowfall decreases) or spatially (in neighbouring areas where snow melts earlier) to chionophilous meadows that have been classified in the Caricetalia curvulae Braun-Blanquet in Braun-Blanquet & Jenny 1926, the most frequent association being the Trifolio alpine–Phleetum gerardii Braun-Blanquet 1948. Snowbeds nested in more rugged terrain may border scree communities such as the Saxifrago geranioidis–Rhododendretum ferruginei Braun-Blanquet 1948. Those neighbouring plant communities will act as a source of competitors for snowbeds species (Gruber 1978). Alpine grasslands grazed by livestock surround each site except Planès.
Field survey
Permanent survey
To select snowbed vegetation plots in 2012, we considered ranges above 2300 m of elevation, where we investigated northern slopes and looked for depressions where snow persists consistently until July. On each site, we set up three permanent plots (P1, P2 and P3) of 3 × 1 m along the snowmelt gradient (Appendix 1), where snowbed species dominated the vegetation. Plots were either contiguous or disjoint. Each plot was segmented into twelve 0.5 × 0.5 m quadrats.
Floristic data
On each study site, from 2013 until 2019 (except years 2014 and 2015 in Ulldeter, Creussans and Ratera sites), individual experts sampled the vegetation composition four times during summer: the first and third weeks of July, mid-August and early September. First, we summarised the information in a binary presence-absence species list for one complete year per quadrat. We then calculated the relative frequency of each species in each 3 m2 plot per year.
Plant nomenclature follows the Plants of the World Online portal (POWO 2019; complete list in Appendix 2).
Climatic data
At the site scale, we extracted climatic data from the topoclimate map of the Pyrenees (Batalla et al. 2018), which is composed of 35 layers synthesising mean climatic values for spring, summer, autumn, winter and annual for seven variables, namely potential evapotranspiration (PET), potential solar radiation (PSR), precipitation (PREC), maximum temperature (TMAX), mean temperature (TMEAN), minimum temperature (TMIN) and water availability (WAT; complete list in Appendix 3). The resolution of the dataset is 30 m per pixel. After the first analysis of collinearity based on a Principal Component Analysis (PCA) and an analysis of the Variance Inflation Factor (package vegan; Oksanen et al. 2007; see Appendix 4 for details), we retained only four variables: annual precipitation, average summer minimum temperature, average summer maximum temperature, and summer potential radiation.
At the plot scale, we used permanent loggers to register temperature every three hours. Each iButton was laid directly on the ground and covered by one small flat stone to avoid theft. To characterise the period when the ground was covered by snow, we computed the daily variance of the temperature, which tend to stabilise when snow buffers temperature variations. A threshold of 1 characterises snow cover (< 1 signifies that the temperature does not vary, so the logger is covered by snow). We ensure to use a window of 7 consecutive days based on the “mowing-maximum” variance to avoid punctual snow cover to bias our estimate. Then we deduced the “melting” and the “freezing” days for each year, which allowed us to calculate four indexes: the number of days from January the first to the melting day, the number of days from January the first to the freezing day, the duration of the vegetative (summer) period, the total snowpack duration from the melting day back to the previous freezing day. We then calculated the 95% quantile of the snow-free temperature per year (to approximate the maximum yearly temperature). Additionally, we computed the mean daily temperature as the maximum plus the minimum daily temperature divided by two. We calculated an equivalent Growing degree day adapted for snowpack, computed as the sum of daily mean temperature during the vegetative period. We assessed the collinearity among these variables and retained the duration of the vegetative period, the number of days to the freezing date and the yearly maximum temperature. Details are presented in Appendix 5. We joined the two climatic datasets to form one climate table.
Statistical analysis
Composition and structure of snowbed plant communities
Following preliminary multivariate analysis, we excluded the plot CRE1 based on its floristic composition as it corresponds to a particular vegetation type, i.e. windy crests, not considered in this study (details in Appendix 6).
Analysis of vegetation patterns
To compare the floristic composition of each plot, we ran a Principal Coordinates Analysis (PCoA) based on the Bray–Curtis distance matrix between all plots sampled over seven growing seasons (or five seasons for Ratera, Creussans and Ulldeter sites). Before analysis, frequencies were transformed following the Hellinger method to avoid similarities based on null values (Legendre and Gallagher 2001). To analyse the spatial structure of the samples, we ran a permutational MANOVA based on the adonis() function (Oksanen et al. 2007), with site and plot nested into site as explanatory variables. We report the proportion of the total variance explained by each variable based on the adjusted R2 of the model (Anderson 2001).
We then conducted a multivariate visualisation method with a heatmap based on Bray–Curtis distance. We used Ward's methods to carry out species composition and site relationship clustering to grasp the structure of plant communities. In order to investigate which species contribute the most to site clustering, we fitted generalised linear models based on a negative binomial distribution, with site and plot nested into site as explanatory variables, and tested at α < 0.05 thanks to ANOVA test (nboot = 500) with the anova.manyglm() function from package mvabund (Wang et al. 2012).
Species indicator scores were investigated and related to the heatmap clustering of sites. The function multipatt() from the package indicspecies (Cáceres and Legendre 2009) highlighted which species attested to the singularity of a sample (Legendre and Gallagher 2001).
Species–environment relationship
We then analysed how environmental variables shape between-site turnover. We ran one redundancy analysis with the climate dataset to explore the relationship between species composition and climatic conditions among sites. We selected the species-plots matrix for 2019, constituting the most complete dataset for a given year. We had to exclude the Portillon site as no data were available for that particular year. Species frequency was transformed based on the Hellinger method. To test the statistical relationship between the two matrices, we ran an ANOVA test (function anova.cca() from package vegan) and checked variable significance within the constrained space. To display the most important species of the model, we drew a triplot and represented species whose arrow distance was superior to 0.3, which limits the number of species presented to 15.
Temporal dynamic of snowbed communities
The dataset contains plant surveys from eight sites for 7 or 5 years (i.e., CRE, RAT and ULL sites).
Global trend
We assessed the temporal species turnover thanks to a beta-temporality index computed with the function TBI() from the package adespatial (Legendre 2019). That function computes the temporal beta diversity between two dates and decomposes this TBI-value into species loss and gains relative importance. We ran this comparison between the years 2013 and 2019, and we excluded Arbella 2 and 3 and Oô-Portillon sites because they lack those years of surveys. We draw B–C plots presenting TBI based on presence-absence data to identify which samples differ in B (gain) and C (loss) components between the 2 years. We performed a permutation procedure to test if there was a significant difference between the two matrices (number of permutations = 999), which allowed us to detect site showing “exceptional difference between t1 and t2, compared to other sites that have been observed at the same two times” (Legendre, 2019). We readjusted the significance threshold following the Holms–Bonferroni correction to avoid false-positive rate inflation.
Specific temporal changes, loss and gain over time
We specified the temporal community trajectories within sites based on the complete presence-absence data over the survey period. First, we analysed the temporal divergence of each site by comparing each year with the first year of the survey (2013), thanks to the TBI index, we decomposed between species gains and loss. Second, to investigate convergence or divergence in floristic assemblage between plots, we computed independently for each year the beta-diversity among each pair of plots (P1–P2, P1–P3 and P2–P3) based on Bray–Curtis distance. Finally, we discuss those trends with snow melt duration based on in situ surveys.
Overall species changes in frequency
To detect species that exhibit significant changes throughout the survey, we compared the frequency of each taxon on all sampled plots between the first and the last survey dates. For each taxon, we selected samples in which species were noted at least during 1 year and were present in more than five plots during the first or the second survey. Next, we subtracted the 2013 matrix from the 2019 matrix to illustrate changes in frequency per taxon per plot. We then computed the mean change in frequency and 95% confidence intervals based on a bootstrap procedure with 10,000 runs with the boot function from the boot package. Finally, we represented those values on a graphic where we compare the value of each confidence interval to the absence of change in frequency (frequency change equals zero).
All analyses were coded in the R language (R development Core Team 2021). Codes are available on GP's Github account (github.com/Guillaume-Papuga/analyse_snowbed).
Results
Composition and structure of snowbed plant communities
Analysis of vegetation patterns
The first two axes of the Principal Coordinate analysis represent 39% of the variability of the dataset. Plots belonging to the same site are grouped inside an ellipse encompassing 90% of the samples; plots and sites appeared to be well separated with limited overlap among them (Fig. 2). However, plots 2 and 3 of Ratera and Ulldeter sites are close by, which indicates a high similarity in their composition. A similar observation can be noticed regarding ARB_1 with POR_1 and POR_3, while CRE_2 shows high similarity with ULL_2. Most sites exhibit little overlap between their own three plots, except for Planes and Portillon, which pinpoints the similarity among plots within those sites. The permutational MANOVA highlights the importance of the variable site in the partitioning of variance (R2 = 0.6), while plot in site displays a lower R2 of 0.24 (details in Table 2).
Principal-coordinate analysis based on Bray–Curtis distance metric of vegetation plots. Each dot represents a plot sampled on a specific year. Ellipses gather all the surveys of a same plots across years (plot name 1, 2, 3) with an inertia of 90%. Colours symbolise each study site. Data were transformed following Hellinger distance
Heatmap and species indicators
The species selection by the nbGLM model picked 70 of 99 significant species to build the global heatmap (Fig. 3). Regarding site clustering, the situation is similar to that observed in the previous PCoA. Three sites are well individualised (Planes, Pas de la Case and Cataperdis), and Portillon's plots are all grouped but belong to a cluster that includes other plots. Then, the remaining four sites show some overlap regarding the clustering of their plots. Creussans, Ratera and Ulldeter, are grouped under the same node of the dendrogram, but their plots remain individualised. This highlights a greater similarity among two plots of two different sites than within each site. ARB_1, ARB_2 and CRE_3 are included in the same dendrogram branch as Oô-Portillon, with ARB_1 somewhat isolated from the other plots. One sample of POR_3 (representing one annual survey) was included in Cataperdis' branch.
Heatmap analysis of all plots sampled throughout the six years of the survey, based on Bray–Curtis dissimilarity matrix and Ward’s clustering method. Species are noted in rows and plots in columns. The colour of each cell represents the species frequency based on the Hellinger distance (from blue: low to red: high). Selection of significant species is based on a negative binomial GLM analysis
Rows were split into four clusters of species. The lower cluster represents an association of a few species over-represented in the Planes site (e.g. Bistorta vivipara, Salix retusa or Trifolium thalii). The second cluster gathers a species pool that distinguishes Pas de la case's plant community (CASE), similarly to the PCoA analysis. The third cluster from the bottom gathers distinctive species of snowbeds and grasslands, widely distributed among sites. This group includes characteristic snowbed species of the Salicion herbaceae Braun-Blanquet in Braun-Blanquet & Jenny 1926 alliance (Cardamine alpina, Sibbaldia procumbens, Sedum alpestre) and grassland species (Nardus stricta, Carex pyrenaica, Agrostis rupestris, Cardamine resedifolia, Gentiana alpina, Phyteuma hemisphaericum, Poa alpina and Trifolium alpinum). Most grassland species are under-represented in Cataperdis (CAT) and Arbella (ARB) sites. Finally, the top cluster includes scarce species which individually enhance site differentiation (Fig. 3). Detailed results from the mulitpatt analysis are presented in Appendix 7.
Environmental gradients
The proportion of the constrained part of the variance in our model (~ 66%) is higher than the unconstrained part (~ 34%), which suggests that an important component of the community composition can be explained by the environmental variables tested (Table 3). Axis 1 and axis 2 of the RDA represent 36.5% of the total variance and are both significant (ANOVA test p value (axis 1) and p value(axis 2) < 0.05). The first axis (RDA1) is driven by two peculiar variables, i.e. the local maximum annual temperature (loc.t_max), which displays a coefficient correlation of 0.69 and precipitation (prec), with a coefficient correlation of −0.53. The maximum summer temperature (t_max) shows a weaker positive correlation with the first axis (0.30). Concerning axis 2, the summer potential solar radiation (rad_sum) presents a positive relation (0.56), together with the duration of the vegetative period (0.53). The minimum summer temperature (t_min) is negatively correlated (−0.26; Fig. 4).
Redundancy analysis of each plot sampled in 2019 with Pyrenean climatic parameters extracted from Batalla et al. (2018) and microclimatic data recorded by loggers. Variables were previously selected to limit multicollinearity, acronyms are rad_sum: Summer potential solar radiation, t_max: average maximum summer temperatures, t_min: average minimum summer temperature, prec: annual precipitation, veg_day: length of the vegetative period, f_day: starting day of winter snow cover, loc_t.max: local maximum temperature. All variables are significant (p value < 0.05). Axes explained 32% of variance. Grey numbers represent species: 1. Armeria alpina, 2. Luzula spicata, 3. Agrostis rupestris, 4. Euphrasia minima, 5. Trifolium alpinum, 6. Poa alpina, 7. Phyteuma hemisphaericum, 8. Salix herbacea, 9. Scorzoneroides pyrenaica, 10. Gentiana alpina, 11. Veronica alpina, 12. Phleum alpinum, 13. Festuca rubra, 14. Festuca glacialis, 15. Linaria alpina
On the multivariate graph (hereafter ‘triplot’), higher precipitation along axis one matches the plots of Cataperdis and Arbella. Planes and Pas de la Case show the lowest values on axis two, with lower solar radiation, shorter vegetative period due to earlier freezing date, and higher minimum summer temperature. Finally, Ulldeter and Creussans display the highest values along axis 2, supporting higher summer solar radiation and a longer vegetative period.
To ease interpreting, we selected species to display an arrow distance > 0.3; thus, only 15 species with the strongest contribution are represented on the RDA triplot (Fig. 4). Linaria alpina, Armeria alpina, Festuca glacialis and Luzula spicata show affinity with higher annual precipitation and correlates negatively with axis 1. The rest of the species display a null to positive correlation on the first axis. Scorzoneroides pyrenaica, Phyteuma hemisphaericum and Poa alpina seem to be associated with higher maximum summer temperatures. Festuca rubra, Phleum alpinum and Veronica alpina show a strong negative correlation with the second axis and are associated with Planes and Pas de la Case sites. Conversely, Agrostis rupestris and Euphrasia minima are positively correlated to axis 2, showing affinities to Ratera and Ulldeter sites.
Temporal dynamic of snowbed communities
The comparison of the Temporal Beta-diversity Index allows us to investigate which plots exhibit changes in species composition over two dates. Most sites have gained species between 2013 and 2019, ARB_1 being the only one to show the opposite trend (Fig. 5). Thus, the barycentre of the cloud of points exhibits a deviation from the equality line toward species gain. Plots of Planès and Pas de la Case shared the highest gains with CAT_3. Four plots exhibit a significant change in their temporal beta diversity index based on permutation tests (PLA 1–2 and CAT 2–3), but only one remains significant after correction for multiple testing (CAT_3, see Appendix 8). Therefore, most plots do not exhibit significant changes in their species composition between T1 and T2 (Fig. 5 & Appendix 8).
Analysis of vegetation changes between surveys completed in 2013 and 2019. B–C plot (Legendre et al. 2019) represents a decomposition of temporal beta-diversity between two dates into species gain (y-axis) and loss (x-axis). Dots size is proportional to the index values. The number of samples is 19. The green line is the equality line (when gains equal losses), and the red line represents its parallel that runs through the centroid of the points
Whereas between-plots dissimilarity remains globally constant throughout the monitoring period, there is a slight differentiation over time of most plots compared to the reference community sampled in 2013, mainly due to a gain of species. The importance of species loss in the evolution of the TBI is close to 0 (except for the Cataperdis site). Four sites between 2018 and 2019 shared a noticeable decrease in TBI, mostly explained by a sharp decrease in the species gain component (Fig. 6).
Intraplot dissimilarities, B–C and D (dissimilarity) plots of 6 study sites. At left: intraplot dissimilarity trends, pale orange: P1P2 dissimilarity, green: P1P3 dissimilarity, blue: P2P3 dissimilarity. At right: temporal beta diversity of each plot compared to the initial survey run in 2013, with a representation of dissimilarity (black line), loss (red) and gains (blue) at the site scale
We compared the changes in frequency for 26 species out of 99 between 2013 and 2019 (Fig. 7). Five showed increased frequency, including Festuca rubra, Lotus alpinus or Phyteuma hemisphaericum, typical alpine grassland species, and two snowbed species, Veronica alpina and Sibbaldia procumbens. Only Cerastium cerastoides and Sedum candollei showed a significant loss in frequency in this period.
Variation of species frequency between 2013 and 2019. Dots represent the mean change in frequency, while error bars represents a 95% confidence interval based on bootstrap computation. Non significant changes are represented in grey, while significant changes are represented in green (increased frequency) or red (decreased frequency)
However, several species show a quasi-significant trend, and it is interesting to note that most of the species that show an increase in frequency are grassland species (e.g. Nardus stricta, Carex curvula), while those that tend to decrease are rather typical snowbed species (e.g. Omalotheca supina, Cardamine alpina, Salix herbacea).
Additional analyses between other years support the same trend and are presented in Appendix 9.
Discussion
Understanding vegetation shifts in the face of global change is a key challenge worldwide to anticipate forthcoming conservation actions. However, some ecosystems are more prone to exhibit species turnover regarding the magnitude of the expected environmental changes. It is the case of snow-associated vegetation at their rear distribution limit, as global warming is supposed to decrease snow cover and alter the micro-ecological conditions, which could, in turn, shuffle the competition among taxa. In the Pyrenees, snowbed vegetation exhibits marked taxonomic differences ruled by complex ecological gradients, presumably driven by the interplay of climate, micro-climate, and soil characteristics. Here we discuss such changes observed over a 7-year survey and highlight potential drivers of vegetation structure. Finally, we discuss the implication of this work for predicting the future evolution of those vegetation types.
Composition of snowbed vegetations and spatial turnover
Plant community composition results from the interplay between large-scale processes such as species distribution over climatic gradients and local scale processes that filter the initial species pool to allow a limited fraction of species to grow and reproduce (Vellend 2010). Our analysis detected a strong spatial turnover among the sites sampled. Nevertheless, characteristic species did not present any biogeographical patterns that could have supported this structure, as most species are widespread along with the Pyrenean range and Palearctic mountains, and very few small range endemics have been selected as characteristics of a site, as Armeria muelleri in Planes, for example (Appendix 7). Additionally, only a few taxa are geographically limited in the Pyrenees (or reaching their distribution limit within our sampling scheme): Arenaria biflora in Ratera, Carex ericetorum in Ulldeter or Pedicularis kerneri, a western-Pyrenean species reaching its distribution limit in Ratera, and absent from other sites. Thus, species that discriminate sites are distributed evenly across the mountain range, restraining the role of dispersal limitation and restricted distribution from explaining such turnover (Ozinga et al. 2005; Kudo and Hirao 2006).
Similar observations have been made when analysing species composition with climatic variables (Fig. 4). We found a marked climatic gradient of decreased precipitation from west to east, associated with a north-to-south increase of solar radiation. Those two gradients broadly represent the climactic structure of the Pyrenees, where oceanic influences are more marked towards the western part of the range (Rica and Recoder 1990; Ninot et al. 2007), and a strong increase of radiation characterises the southern slopes. Nevertheless, the association of taxa to such gradients is uninformative and not supported by autecological evidence. For example, there is no association between Linaria alpina and higher precipitation among sites, as this taxon is largely distributed over the whole chain. Similarly, there is no rationale behind the association of Euphrasia minima or Agrostis rupestris with higher summer radiation and less precipitation.
Large-scale processes and dispersal limitations might not drive this strong inter-site differentiation but rather local processes that sort species to shape those communities (Domènech et al. 2016). Two main ecological factors are important but complex to measure. First, local microclimate can differ from global climate due to the topography, the vegetation, and local features (Lawson et al. 2014; Maclean et al. 2015). The importance of microclimatic variation is enhanced in mountain environments due to sharp altitudinal gradient and land heterogeneity, including slope orientation, peak and depression (Lembrechts et al. 2019). Such variations can be significant and impact plant survival and reproduction to a scale of a few meters (Löffler 2007; Pickering and Green 2009). In our study, we did not detect a clear microclimate effect, as the computed variables only explained a limited part of the variance of the redundancy analysis compared to large-scale climate variables.
Second, soil characteristics in terms of structure, water potential, and chemical properties (such as pH and organic matter content) might impact the micro-ecological niche where individuals can grow (Yapp 1922; Schöb et al. 2009; Papuga et al. 2018). The discrete nature of the spatial distribution of soil features is key to generating fine-scale vegetation spatial turnover, and such heterogeneity has been highlighted by previous pedological analysis (Rigou 2019). Yet, we could not explicitly quantify these factors, which prevented us from including them within our statistical approach.
Therefore, the influence of local ecological factors is likely to blur large-scale biogeographical gradients, which is paramount to account for when analysing large-scale vegetation structure (Vellend 2010; Pironon et al. 2017). Then, increasing the sampling effort and characterising fine-scale ecological factors appears to be key to a better understanding of assembly processes of snowbed communities (Schöb et al. 2009).
Temporal dissimilarity within sites
The dynamic aspect of vegetation is conditioned by intrinsic processes such as biological type and associated lifetime and extrinsic factors that affect the survival and reproduction of individuals (Leibold et al. 2004). The frequency, intensity and predictability of environmental changes strongly structure the temporal dynamic of plant communities. Snowbed vegetation is dominated by long-lived species, including dwarf shrubs with low growth rates such as Salix herbacea or S. retusa, often observed through our study sites. Such vegetation highly depends on the long-lasting snowpack that reduces the vegetative period to 2–4 months. However, this short growing period is supposed to change drastically in response to climate warming. Through our study, we observed different dynamics over 7 years (Fig. 5); although some plots exhibit significant (CAT3) or nearly significant (CAT2, PLA1-2) vegetation turnover, most plots exhibit slow turnover indicated by low TBI values. While climatic scenarios predict a stronger decrease in snow cover towards the eastern and southern Pyrenees (López-Moreno et al. 2009), we did not detect any spatial or ecological structure of TBI values. This reinforces the importance of local ecological variables in influencing snow cover. Additionally, such temporal changes are driven exclusively by species gain, which we cannot split between true gains (species colonisation) and the improvement of operator capabilities to detect species, a common bias in vegetation science (Archaux et al. 2006; Lisner and Lepš 2020).
Therefore, the investigation of year-to-year dynamics is supposed to add information regarding the gradual/abrupt nature of changes in species composition. First, the computation of pairwise beta diversity over the 7-year study time shows no convergence between plots, which would have been detected through a decrease in the distance index (Fig. 6). This supports the global stability of vegetation and goes against the hypothesis of vegetation homogenisation (Sandvik and Odland 2014; Winkler et al. 2018; Liberati et al. 2019), at least in the short term. Then, for each site, the temporal trend shows a very slow trend of increased differentiation (i.e. higher beta-diversity values) compared to the initial inventory, quasi-exclusively driven by species gain, while species loss remains anecdotal, in line with previous analysis (Carbognani et al. 2014). This raises an issue regarding the graduality of species change in such communities. The impact of climate warming on snow cover duration has not been strictly gradual over the last 30 years: despite a trend toward shorter snow cover, some exceptional years have seen longer snow cover (Chueca et al. 2007). This chaotic dynamic could structure vegetation dynamics following phases of colonisation by generalist species punctuated by purges when extreme climatic events kill colonisers. Thus the duration between extreme climatic events would condition the probability for generalist species to outcompete snowbed specialists. Additionally, the local persistence of long-live adult plants might induce a lag in the community turnover, despite changing environmental conditions and global species distribution (Dullinger et al. 2012; Alexander et al. 2018). Therefore, it is clear that longer-term surveys are requested to investigate such questions and better understand the pace of community changes to avoid important extinction debt.
Evolution of species composition
Species coexistence within communities is a multifactorial process, among which competitive exclusion is central (Bengtsson et al. 1994). This statement has led to the niche differentiation theory, whose corollary is that changing ecological conditions might modify competition output among two species, altering the probability for those species to persist in the community. Our study compared the change in species frequency between 2013 and 2019; among the 26 species analysed, 5 presented a significant increase in frequency: two typical of snowbed communities (Veronica alpina, Sibbaldia procumbens) and three generalist species, often found in subalpine to alpine grasslands (Phyteuma hemisphaericum, Festuca rubra, Lotus alpinus). Other generalist species counted among the strongest (but not significant) increases in frequency, such as Nardus stricta. On the other hand, the only two taxa that showed a significant decrease are snowbed specialists (Sedum candollei, Cerastium cerastoides), and most typical snowbed taxa have seen little changes in their frequency, such as Salix herbacea or Cardamine alpina. We repeated the same procedure between 2014 and 2019 to avoid bias in species detection during the implementation of the field protocol, and the results were globally coherent (see Appendix 9).
This result tends to support the colonisation of generalist grassland taxa within snowbed communities. Therefore, we can postulate that a reduced snowbed longevity could allow widespread alpine generalist species to colonise such vegetation, which is coherent with the previous analysis based on overall temporal beta-diversity. This scenario follows a core–edge spatial structure where the snowbed represents islands of unfavourable habitat scattered in a matrix of alpine grasslands, whose species tend to colonise through propagule and/or vegetative dispersal (Ninot et al. 2013). Nevertheless, no sign of important competitive exclusion was detected in our dataset, as most characteristic chionophilous species showed no decrease in their frequency. This can be considered an artefact due to our coarse cell size (50 × 50 cm) that cannot depict plant-to-plant interactions and minimise declines. Precisely measuring plant cover would shed light on such a process. Alternatively, vegetation extirpation might lag behind environmental change due to the local persistence of individuals (Dullinger et al. 2012; Alexander et al. 2018). Additionally, we cannot rule out the improvement of observers in detecting small species, although the increase of easy-to-determine species (such as Lotus alpinus) somehow brings robust information.
Therefore this observational study raises important questions to understanding mechanisms that rule community dynamics, particularly regarding the colonisation of snowbed vegetation by adjacent grassland taxa. We would support a joint analysis based on a fine-scale field survey of plant-to-plant interaction, coupled with experimental approaches following traditional common-garden competition experiments under different ecological scenarios to delve into this issue.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Codes are freely available on the GP Github account (github.com/Guillaume-Papuga/analyse_snowbed).
References
Ackerly DD et al (2010) The geography of climate change: implications for conservation biogeography. Divers Distrib 16:476–487
Alexander JM et al (2018) Lags in the response of mountain plant communities to climate change. Glob Change Biol 24:563–579
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Archaux F et al (2006) Effects of sampling time, species richness and observer on the exhaustiveness of plant censuses. J Veg Sci 17:299–306
Batalla M et al (2018) Digital long-term topoclimate surfaces of the Pyrenees mountain range for the period 1950–2012. Geosci Data J 5:50–62
Bengtsson J et al (1994) Competition and coexistence in plant communities. Trends Ecol Evol 9:246–250
Björk RG, Molau U (2007) Ecology of alpine snowbeds and the impact of global change. Arct Antarct Alp Res 39:34–43
Braun-Blanquet J (1948) Übersicht der pflanzengesellschaften rätiens. Vegetatio 1:29–41
Buckley HL et al (2021) Changes in the analysis of temporal community dynamics data: a 29-year literature review. PeerJ in press
Cáceres MD, Legendre P (2009) Associations between species and groups of sites: indices and statistical inference. Ecology 90:3566–3574
Callaway RM, Walker LR (1997) Competition and facilitation: a synthetic approach to interactions in plant communities. Ecology 78:1958–1965
Carbognani M et al (2014) Current vegetation changes in an alpine late snowbed community in the south-eastern Alps (N-Italy). Alp Bot 124:105–113
Chueca J et al (2007) Recent evolution (1981–2005) of the Maladeta glaciers, Pyrenees, Spain: extent and volume losses and their relation with climatic and topographic factors. J Glaciol 53:547–557
Corriol G, Mikolajczak A (2014) Contribution au prodrome des végétations de France: les Salicetea herbaceae Braun-Blanq. 1948. J Bot Soc Bot Fr 68:15–49
Domènech M et al (2016) Site-specific factors influence the richness and phenology of snowbed plants in the Pyrenees. Plant Biosyst Int J Deal Asp Plant Biol 150:741–749
Dullinger S et al (2012) Extinction debt of high-mountain plants under twenty-first-century climate change. Nat Clim Change 2:619–622
Engler R et al (2011) 21st century climate change threatens mountain flora unequally across Europe. Glob Change Biol 17:2330–2341
Fernandes M et al (2018) Spatial distribution and morphometry of permafrost-related landforms in the Central Pyrenees and associated paleoclimatic implications. Quat Int 470:96–108
Fischer J, Lindenmayer DB (2007) Landscape modification and habitat fragmentation: a synthesis. Glob Ecol Biogeogr 16:265–280
Galen C, Stanton ML (1995) Responses of snowbed plant species to changes in growing-season length. Ecology 76:1546–1557
Gottfried M et al (2012) Continent-wide response of mountain vegetation to climate change. Nat Clim Change 2:111–115
Götzenberger L et al (2012) Ecological assembly rules in plant communities—approaches, patterns and prospects. Biol Rev 87:111–127
Gruber M (1978) La végétation des Pyrénées ariégeoises et catalanes occidentales. PhD thesis. Faculté des Sciences Techniques de St. Jerome, Université Aix - Marseille III, 305 pages
Guisan A et al (2006) Making better biogeographical predictions of species’ distributions. J Appl Ecol 43:386–392
Hampe A, Petit RJ (2005) Conserving biodiversity under climate change: the rear edge matters. Ecol Lett 8:461–467
Heegaard E, Vandvik V (2004) Climate change affects the outcome of competitive interactions—an application of principal response curves. Oecologia 139:459–466
Hülber K et al (2011) Effects of snowmelt timing and competition on the performance of alpine snowbed plants. Perspect Plant Ecol Evol Syst 13:15–26
Izard M (1985) Le Climat. In: Végétation des Pyrénées. Notice détaillée de la partie pyrénéenne des feuilles 69 Bayonne—70 Tarbes—71 Toulouse—72 Carcassonne—76 Luz—77 Foix—78 Perpignan. Edition du CNRS, pp 17:36
Köppen W (1900) Versuch einer Klassifikation der Klimate, vorzugsweise nach ihren Beziehungen zur Pflanzenwelt. Geogr Zeitschr 6:593–611, 657–679
Körner C (2003) Alpine plant life: functional plant ecology of high mountain ecosystems. Springer, Berlin
Körner C, Riedl S, Keplinger T, Richter A, Wiesenbauer J, Schweingruber F, Hiltbrunner E (2019) Life at 0° C: the biology of the alpine snowbed plant Soldanella pusilla. Alp Bot 129:63–80
Kudo G (1999) A review of ecological studies on leaf-trait variations along environmental gradients; in the case of tundra plants. Jpn J Ecol Jpn 49:21–35
Kudo G (2020) Dynamics of flowering phenology of alpine plant communities in response to temperature and snowmelt time: analysis of a nine-year phenological record collected by citizen volunteers. Environ Exp Bot 170:103843
Kudo G, Hirao AS (2006) Habitat-specific responses in the flowering phenology and seed set of alpine plants to climate variation: implications for global-change impacts. Popul Ecol 48:49–58
Lavergne S et al (2010) Biodiversity and climate change: integrating evolutionary and ecological responses of species and communities. Annu Rev Ecol Evol Syst 41:321–350
Lawson CR et al (2014) Topographic microclimates drive microhabitat associations at the range margin of a butterfly. Ecography 37:732–740
Legendre P (2019) A temporal beta-diversity index to identify sites that have changed in exceptional ways in space–time surveys. Ecol Evol 9:3500–3514
Legendre P, Gallagher ED (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280
Leibold MA et al (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7:601–613
Lembrechts J et al (2019) Incorporating microclimate into species distribution models. Ecography 42:1267–1279
Lenoir J et al (2008) A significant upward shift in plant species optimum elevation during the 20th century. Science 320:1768–1771
Lenormand M et al (2019) Biogeographical network analysis of plant species distribution in the Mediterranean region. Ecol Evol 9:237–250
Liberati L et al (2019) Contrasting impacts of climate change on the vegetation of windy ridges and snowbeds in the Swiss Alps. Alp Bot 129:95–105
Lisner A, Lepš J (2020) Everyone makes mistakes: sampling errors in vegetation analysis—the effect of different sampling methods, abundance estimates, experimental manipulations, and data transformation. Acta Oecologica 109:103667
Lluent A (2007) Estudi de l’estructura i funcionament de les comunitats quionofiles als pirineus en relacio del factors PhD thesis. Universitat de Barcelona, 196 pages
Löffler J (2007) The influence of micro-climate, snow cover, and soil moisture on ecosystem functioning in high mountains. J Geogr Sci 17:3–19
López-Moreno JI et al (2009) Impact of climate change on snowpack in the Pyrenees: horizontal spatial variability and vertical gradients. J Hydrol 374:384–396
López-Moreno JI, García-Ruiz JM (2004) Influence of snow accumulation and snowmelt on streamflow in the central Spanish Pyrenees. Hydrol Sci J in press
Maclean IMD et al (2015) Microclimates buffer the responses of plant communities to climate change. Glob Ecol Biogeogr 24:1340–1350
Matteodo M et al (2016) Snowbeds are more affected than other subalpine–alpine plant communities by climate change in the Swiss Alps. Ecol Evol 6:6969–6982
Morán-Tejeda E et al (2017) Changes in climate, snow and water resources in the Spanish Pyrenees: observations and projections in a warming climate. In: Catalan J, Ninot JM, Mercè Aniz M (eds) High mountain conservation in a changing world. Springer, Cham, pp 305–323
Mucina L et al (2016) Vegetation of Europe: hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Appl Veg Sci 19:3–264
Ninot JM et al (2007) Altitude zonation in the Pyrenees. A geobotanic interpretation. Phytocoenologia 37:371–398
Ninot JM et al (2013) Functional plant traits and species assemblage in Pyrenean snowbeds. Folia Geobot 48:23–38
Oksanen J et al (2007) The vegan package. Commun Ecol Packag 10:719
Ooi MK (2012) Seed bank persistence and climate change. Seed Sci Res 22:S53–S60
OPCC-CTP (2018) Climate change in the Pyrenees: impacts, vulnerability and adaptation. In: Bases of knowledge for the future adaptation strategy of the Pyrenees, p 34
Ozinga WA et al (2005) Predictability of plant species composition from environmental conditions is constrained by dispersal limitation. Oikos 108:555–561
Papuga G et al (2018) Ecological niche differentiation in peripheral populations: a comparative analysis of eleven Mediterranean plant species. Ecography 41:1650–1664
Pauli H et al (2012) Recent plant diversity changes on Europe’s mountain summits. Science 336:353–355
Petraglia A et al (2014) Responses of flowering phenology of snowbed plants to an experimentally imposed extreme advanced snowmelt. Plant Ecol 215:759–768
Pickering CM, Green K (2009) Vascular plant distribution in relation to topography, soils and micro-climate at five GLORIA sites in the Snowy Mountains, Australia. Aust J Bot 57:189–199
Pironon S et al (2017) Geographic variation in genetic and demographic performance: new insights from an old biogeographical paradigm. Biol Rev 92:1877–1909
POWO (2019) Plants of the world online. Plants World Online Facil. R. Bot. Gard. Kew
R development Core Team (2021) R: a language and environment for statistical computing
Rica JM, Recoder PM (1990) Biogeographic features of the Pyrenean range. Mt Res Dev 10:235–240
Rigou L (2019) Typologie des sols des combes à neige.: 103
Rivas-Martínez S (1996) Bioclimatic map of Europe. Cartographic service of the University of Leon, Leon
Rogora M et al (2018) Assessment of climate change effects on mountain ecosystems through a cross-site analysis in the Alps and Apennines. Sci Total Environ 624:1429–1442
Root TL et al (2003) Fingerprints of global warming on wild animals and plants. Nature 421:57–60
Sandvik SM, Odland A (2014) Changes in alpine snowbed-wetland vegetation over three decades in northern Norway. Nord J Bot 32:377–384
Schöb C et al (2009) Small-scale plant species distribution in snowbeds and its sensitivity to climate change. Plant Ecol 200:91–104
Schweiger O et al (2010) Multiple stressors on biotic interactions: how climate change and alien species interact to affect pollination. Biol Rev 85:777–795
Spasojevic MJ et al (2013) Changes in alpine vegetation over 21 years: are patterns across a heterogeneous landscape consistent with predictions? Ecosphere 4:1–18
Thuiller W et al (2008) Predicting global change impacts on plant species’ distributions: future challenges. Perspect Plant Ecol Evol Syst 9:137–152
Tylianakis JM et al (2008) Global change and species interactions in terrestrial ecosystems. Ecol Lett 11:1351–1363
Vellend M (2010) Conceptual synthesis in community ecology. Q Rev Biol 85:183–206
Walther GR (2003) Plants in a warmer world. Perspect Plant Ecol 6:169–185
Walther G-R (2010) Community and ecosystem responses to recent climate change. Philos Trans r Soc B Biol Sci 365:2019–2024
Walther GR et al (2005) Trends in the upward shift of alpine plants. J Veg Sci 16:541–548
Wang YI et al (2012) mvabund—an R package for model-based analysis of multivariate abundance data. Methods Ecol Evol 3:471–474
Willner W (2020) What is an alliance? Veg Classif Surv 1:139
Winkler DE et al (2018) Snowmelt timing regulates community composition, phenology, and physiological performance of Alpine plants. Front Plant Sci. in press
Woodward FI, Diament AD (1991) Functional approaches to predicting the ecological effects of global change. Funct Ecol 5:202–212
Yapp RH (1922) The concept of habitat. J Ecol 10:1–17
Acknowledgements
The authors deeply thank all botanists (bachelor's and master's students, colleagues from the different structures) for their help in conducting field surveys.
Funding
Part of this work has been conducted as a development of the POCTEFA project “Florapyr” coordinated by the Conservatoire Botanique National des Pyrénées et de Midi-Pyrénées CBNPMP. All fieldwork and climatic surveys have benefited from this European funding. In addition, TM acknowledges support from the University of Montpellier under a grant for Master's students. GP thanks the University of Montpellier for financial support.
Author information
Authors and Affiliations
Contributions
T.M. analysed the data, wrote the paper, and conducted additional surveys for the project. G.P., E.I. and O.A. conceived the study, wrote the paper and analysed the data. CBNMP & CENMA & UB created the original protocol. G.L. designed and coordinated the European program. All authors participated in the fieldwork.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Consent to participate
No consent to participate was required for this study.
Consent for publication
No consent for publication was required for this study.
Ethics approval
Ethics approval was not required for this study according to French, Spanish and Andorran national legislation.
Additional information
Communicated by Simon Pierce.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Illa Estela, Argagnon Olivier and Papuga Guillaume are co-senior authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Masclaux, T., Largier, G., Cambecèdes, J. et al. Large-scale diachronic surveys of the composition and dynamics of plant communities in Pyrenean snowbeds. Plant Ecol 223, 1103–1119 (2022). https://doi.org/10.1007/s11258-022-01261-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-022-01261-6