Abstract
Tropical monodominant forests are rare communities with low tree species diversity. Species monodominance is not the product of a single mechanism, but the result of a set of not yet fully understood integrated ecological factors acting together. We compared populations of Brosimum rubescens in monodominant and mixed forests in Southern Amazonia to test whether leaf functional traits are ecological factors related to monodominance. Individuals of B. rubescens in the mixed forest invest in conservative strategies, while those in the monodominant forest invest in acquisitive strategies. Leaf functional traits, such as petiole length and adaxial cuticle thickness, could be associated with the monodominance of B. rubescens. Our study highlights for the first time the power of integrating leaf functional traits as a component of the set of ecological conditions to explain species monodominance. B. rubescens showed different functional strategies to establish and maintain its population in different forests, which makes it a strong competitor for resources, such as water and light, through variation in its leaf functional traits. We also suggest that such high plasticity can be an important condition for the persistence of the species over time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The tropics hold by far the largest biodiversity worldwide, especially in tropical forests. For instance, the Amazonia harbor more than 16,000 tree species (ter Steege et al. 2013), reaching in a single hectare 200 species or even more (Gentry 1992). However, it is also possible to find in the tropics monodominant forests, rare phytophysiognomies with more than 60% of the canopy dominated by a single species (Hart et al. 1989; Peh et al. 2011). Few occurrences of this unusual situation have been recorded on a pan-tropical scale (Peh et al. 2011; Brookshire and Thomas 2013; Nik Norafida et al. 2018), with just two cases described for Amazonia: Peltogyne gracilipes Ducke (Fabaceae) in north (Nascimento et al. 1997; Nascimento and Proctor 1997), and Brosimum rubescens Taub. (Moraceae) in the southern region (Marimon et al. 2001a, b).
The causes of the tropical monodominance are not yet fully understood, but surely these are not related to just a special feature, but to a set of ecological and evolutionary mechanisms (Peh et al. 2011; Brookshire and Thomas 2013; Nascimento et al. 2017; Marimon-Junior et al. 2019). Some ecological mechanisms proposed are related to the lack of disturbance (Connell and Lowman 1989; Hart et al. 1989), shade tolerance and survival of seedlings under closed cover (Hart 1995; Torti et al. 2001), and soil-nutrient factors, as Mg/Ca ratio and N dynamics along the ecological succession (Brookshire and Thomas 2013; Nascimento et al. 2017; Elias et al. 2018). The proposed evolutionary mechanisms involve functional and structural traits of monodominant species as large seeds to overcome deep litter (Torti et al. 2001; Peh et al. 2011), ectomycorrhizal association (Connell and Lowman 1989; McGuire et al. 2008) and low efficiency in seed dispersal (Hart 1985).
Current studies have integrated the knowledge of interspecific variation of functional traits to explain the coexistence of tropical tree species and the relationship between functional diversity and dominance in plant assemblies (Cornwell and Ackerly 2010; Aiba et al. 2020), including trade-offs between functional traits, growth, and mortality (Wright et al. 2010). Functional traits such as wood density, seed volume, and total height have already been identified as good predictors of tree species competition and survival rates in Neotropical forests (Poorter et al. 2008), all of them possibly important for species dominance. It has also been shown that dominance can be positively correlated with leaf mass by area, and negatively with leaf size (Aiba et al. 2020). Recent studies have looking for more consistent explanations about monodominance at the functional level, for example, in Africa Gilbertiodendron dewevrei monodominance is associated with low local functional diversity (Kearsley et al. 2017), as well as functional acquisition strategies (Hall et al. 2020).
Many species have different survival strategies that allow them to thrive in various ecological conditions (Araújo et al. 2021a, b). These strategies can manifest in the population structural parameters of a species. Parameters such as growth and recruitment rates vary between populations due to different environmental conditions as a survival strategy (Marimon et al. 2020). Also, these population structural parameters may indicate underlying conditions, e.g., functional traits, which keep an ecosystem in its climax state or alternative successional state such as monodominance (Kearsley et al. 2017). Associating population structural parameters and leaf functional traits seems a logical step in elucidating many of the dynamics in forest ecosystems.
Understanding how functional traits shape the species monodominance is a major challenge in ecology. For example, the combination of leaf functional traits can represent distinct ecological strategies (Araújo et al. 2021b), such as drought tolerance and competition ability, influencing the survival, growth, and reproduction of the organisms (Ackerly 2003; Violle et al. 2007), and consequently regulating its abundance (Aiba et al. 2020). Some species can become such efficient competitors that they end up causing local competitive exclusion (Gause 1932), increasing their abundance (Grubb 1982; Miller and Werner 1987), which can favor monodominance.
As for the monodominant forests of B. rubescens, early studies suggested that the monodominance of this species is episodic and depends on small disturbances, such as tree fall gaps (Marimon et al. 2001a, b). The most recent study evaluated whether lower soil water retention could explain the monodominance (Marimon-Junior et al. 2019). The authors rejected this hypothesis and argue that only the integration of several studies involving different mechanisms will be able to reveal the causes and consequences of tropical monodominance.
This study with B. rubescens in Southern Amazonia is the first evidence of integration of leaf functional traits explaining tropical monodominance. This monodominant forest occurs adjacent to a mixed forest, with much greater species diversity, but both under the same climatic condition (Marimon and Felfili 2006; Marimon et al. 2014; Morandi et al. 2016). We evaluated and compared the leaf functional traits of B. rubescens in both monodominant and mixed forests to answer two questions: (i) Do the leaf functional traits differ between the populations of B. rubescens occurring in the monodominant and mixed forests? (ii) Can the relationship between leaf functional traits and structural parameters explain the monodominance of this species?
Materials and methods
Study area and species description
We carried out the study in a B. rubescens monodominant forest and a mixed forest in southern Amazonia (Marques et al. 2020) (Fig. 1) 800 m apart, located at the Legal Reserve of Vera Cruz Farm (14° 50′ 47″ S e 52° 08′ 37″ W), in Nova Xavantina municipality, Mato Grosso state. The climate is Aw type according to Köppen's classification, characterized by two well-defined seasons, the rainy, from March to October, and the dry, from April to September (Alvares et al. 2013). The annual averages of precipitation and temperature in the study area are 1600 mm and 25 °C, respectively (Marimon et al. 2002, 2010). The forests grew on dystrophic Ferrasols (FAO/UNESCO 1992), well-drained, acidic and with high Mg/Ca ratio (Marimon et al. 2001b, 2014).
Tropical forests (Monodominant-B. rubescens and mixed) in Southern Amazonia, Brazil, South America. Here we use the official IBGE map; however, for ecological purposes, we are considering the study area as Southern Amazonia, based on Marques et al. (2020)
B. rubescens has a wide distribution in South America, having been recorded in the Amazonia and Atlantic Forest (Lima et al. 2017; ter Steege et al. 2019), but being monodominant only in stretches of forest in southern Amazonia, transition with the Cerrado biome (Marimon et al. 2014; Marques et al. 2020). The trees can reach 45 m in height and 90 cm in diameter, presenting a long-life cycle that can reach up to 700 years (Laurance et al. 2004). Wood is widely exploited both for structural and supporting buildings purposes and used by indigenous communities for making utensils (Marimon and Felfili 2001). Its fruits are also used as food by those communities and by wild fauna (Marimon et al. 2008).
Structural parameters and leaf morphological and anatomical traits estimates for B. rubescens
Structural parameters
Each forest (monodominant and mixed) is represented by an area of 0.6 ha, subdivided into 10 × 10 m (100 m2) sub-plots. In each forest, we randomly selected 15 subplots with adult individuals of B. rubescens (> 10 cm DBH). In each subplot, we collected a set of leaves from only one representative of the species, being the leaves mature, open, and exposed to the sun following a standardized protocol. Within the same subplots, we used the density, height, diameter, and above-ground biomass of the B. rubescens trees, provided by Marimon et al. (2014).
Leaf morphological and anatomical traits estimates
We selected eight leaves for each individual in each forest, five for morphological characterization and three leaves for anatomical determinations. Complete list of traits measured and their description can be found in Table S1. As a standardization criterion, we collected fully expanded leaves, exposed to full sunlight, and free of pathogens (i.e., leaf standardization protocol). We kept the plastic bags with the samples inside coolers during transport to the laboratory, which is very close to the studied areas, and on the same day, we processed the samples morphological. Morphologically, we measured leaf thickness (mm) using an electronic digital micrometer (± 0.001 mm) and using the caliper (± 0.001 mm) the petiole length (mm). With the precision balance (± 0.001 g), fresh and dry mass and we calculate the water mass content in the leaves (mg g−1); with LI-COR model LI-3100C we measure the leaf area, and calculate the specific leaf area (cm2 g−1) by dividing the leaf area by the dry mass. To obtain the dry mass, we packed the leaves in paper bags, and we put them in an oven with forced air circulation at 65 °C until constant weight (Fidalgo and Bononi 1984; Pérez-Harguindeguy et al. 2016).
For the anatomical traits, we selected three leaves for each individual that followed the same collection and storage protocol, performed the procedure for reversing herborization (Smith and Smith 1942) and store the samples in 70% alcohol (Johansen 1940). We made freehand cross cuts with a steel blade's aid, we used the method of clarifying the cuts with 2% sodium hypochlorite and stained the material with Astra blue and basic fuchsin. (Roeser 1962; Kraus et al. 1998). For the epidermal analysis, we performed the Franklin method (1945), the leaf portions were submitted to an aqueous solution (hydrogen peroxide 30 volumes and glacial acetic acid in a 1:1 ratio) and kept in an oven at 65 °C for 24 h. After this period, we wash the samples in distilled water and separate the epidermal surfaces with a brush and stain them with basic fuchsin (Roeser 1962). With the colored sections, we set up semi-permanent slides and recorded photomicrographs with the LAZ EZ 1.7.0 software from a microscope. (Leica® ICC50) attached to a computer.
We calculated stomatal density, for each individual, being the average of the number of stomata counted in the same fields of view registered previously. Then we estimated the average stomatal density, length, and width, measuring 25 stomatal complexes per individual. We measure the length of the guard cells (L, in µm), the width of the guard cell pair (W, in µm), the size of the stomata (S, estimated as S = L*W, according Franks et al. 2009, 2012), and the maximum area of the stomatal pores (amax, in µm2). The maximum area of the stomatal pore was calculated as amax = α*S, being α = 0,12 (Franks et al. 2009). We measure leaf traits with the aid of the program Anati Quant 2® UFV (Aguiar et al. 2007) and software ImageJ (Schneider et al. 2012; http://rsb.info.nih.gov/ij) (Table S1).
We applied an index based on the equation of the maximum and minimum medians to determine the phenotypic plasticity of the leaf traits (Valladares et al. 2006).
Statistical analysis
We compared the structural parameters and leaf functional traits between forests (monodominant vs. mixed) using the permutation t-test with RVAideMemoire package (Hervé 2021). Also, we generated a Pearson’s correlation matrix to evaluate the relationship between the leaf functional traits. We produced linear models to assess the relationship between leaf functional traits and variation between forests, and developed a principal component analysis (correlation PCA), to investigate how leaf functional traits are distributed among forests, using vegan (Oksanen et al. 2020) and psych (Revelle and Revelle 2020).
We also created a variance partition to understand which factors best explain the variation of each functional traits using different groupings of generalized linear mixed models (Zuur et al. 2009) and adjusting the separate models for each functional trait (Rosas et al. 2019). Based on the methodology used by these authors, we introduced the vegetation type and individuals as nested random factors and leaf functional traits as response variables for each model. We emphasize that the term individuals is independent of the forest type.
To understand whether leaf functional traits can predict the monodominance of B. rubescens, we used generalized linear models (Zuur et al. 2009). We used as variable responses in the models the density of individuals, height, diameter, and above-ground biomass. To choose the predictor variables, we based on other studies about functional traits in plants (Rossatto and Kolb 2010, 2012; Kearsley et al. 2017; Hall et al. 2020), but we also used the statistical methods. Based on a correlation matrix and in the literature, we selected the predictors and ran the first models. However, due to the autocorrelation between the variables, we chose to apply an automatic method the vifcor function of usdm package (Naimi and Araújo 2016). Therefore, to select the predictive traits and at the same time eliminate those that were multicollinear, we used the variance inflation factor (VIF) method, considering a VIF < 2 (Quinn and Keough 2002). Thus, of a total of 13 variables, only seven were maintained in the models. After running GLM, we selected the best models based on the value of AICc (Borcard et al. 2011) and calculated the average model with the R model.avg function using the dredge function, both from the MuMIn package (Barton and Barton 2015). We considered the best models those with ΔAICc < 2 (Burnham and Anderson 2002). All analyzes were performed using the R program, version 3.6.0 (R Development Core Team 2019), considering 5% of significance level.
Results
For all leaf functional traits here considered, only adaxial epidermis thickness (AET) and leaf dry matter content (LDM) did not present different values between the two forests (monodominant and mixed). The specific leaf area (SLA), maximum opening of the stomatal pore (MOS), petiole length (PEL), and stomatal size (STS) of B. rubescens were higher in the monodominant forest (Fig. 2). The stomatal density (STD) and thickness of the cuticle (ACT), palisade (PPT), spongy parenchyma (SPT), and thickness leaf (LET) were higher in the mixed forest. In addition, we note that all structural parameters (i.e., density, biomass, height, and DBH of trees) differ between forests and are greater in the monodominant forest (Fig. S4).
Leaf functional traits of B. rubescens in monodominant and mixed forests in Southern Amazonia. Boxplots represent medians and confidence intervals, and different lowercase letters indicate significant differences (Permutation t test, P < 0.05). SLA specific leaf area, LET leaf thickness, MOS maximum opening of the stomatal pore, PEL petiole length, ACT adaxial cuticle thickness, AET adaxial epidermis thickness, SPT spongy parenchyma thickness, PPT palisade parenchyma thickness, STD stomata density, STS stomata size, LDM leaf dry matter content
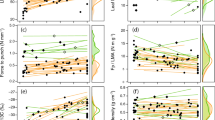
Our linear models showed strong relationship between several leaf functional traits (Table S2), with some trade-offs, as the increase in specific leaf area (SLA) predicting reduction in leaf thickness (LET), (Fig. 3A) and adaxial epidermis (AET), (Fig. 3B), as well as in the palisade and spongy parenchyma (PPT and SPT), (Fig. 3C and D) and leaf dry matter content (LDM), (Fig. 3E). On the other hand, the larger the specific leaf area (SLA), the larger the stomata size (STS), (Fig. 3F). On the other hand, thicker leaves (LET) or thicker adaxial epidermis (AET) presented thicker palisade parenchyma (PPT), (Fig. 3G and J). Furthermore, the greater the leaves thickness, the smaller the stomata size (STS) and the maximum opening of the stomatal pore (MOS), (Fig. 3H and I). Also, the greater the adaxial epidermis thickness (AET), the greater the spongy parenchyma thickness (SPT), (Fig. 3K). Also, the increase in stomata density (STD) predicts decrease in stomata size (STS) and in maximum opening of the stomatal pore (MOS), (Fig. 3L and M).
Linear models between leaf functional traits of B. rubescens in monodominant and mixed forests in Southern Amazonia. MOS maximum opening of the stomatal pore, STS stomata size, STD stomata density, SPT spongy parenchyma thickness, PPT palisade parenchyma thickness, AET adaxial epidermis thickness, ACT adaxial cuticle thickness, PEL petiole length, LET leaf thickness, LDM leaf dry matter content, SLA specific leaf area
The differences in leaf functional traits were strong enough to segregate individuals from both forests (Table S3). Such a condition can be seen in PC1 (Fig. S3), mainly determined by higher values of specific leaf area (SLA), petiole length (PEL), stomatal size (STS), and maximum opening of the stomatal pore (MOS) registered for individuals in the monodominant forest (Fig. S3). The PCA explained 64% of the total data variation in the first two axes.
The highest values of phenotypic plasticity were recorded for leaf functional traits linked to water saving, such as adaxial cuticle thickness (ACT) and stomatal pore, density (STD), and size (STS). The same was observed for leaf functional traits related to the acquisition of resources, such as specific leaf area (SLA) and petiole length (PEL). On the other hand, the lowest values were observed for leaf dry matter content (LDM) and leaf thickness (LET) (Fig. S2).
For most of the leaf functional traits here evaluated, the most significant variations were traits to differences between individuals (Fig. 4). The forest type explained 47–84% of the change in stomatal dimensions (MOS, STD, and STS) and petiole length (PEL), and the individual level explained 53–89% of the variation in the thickness of the spongy and palisade parenchymas (PPT and SPT), as well as in the cuticle and epidermis thickness (AET and ACT) (Fig. 4). The percentage of explanation at the individual level draws attention to the leaf dry matter content (LDM), which was higher than 80% (Fig. 4).
Partitioning of variance of the nested linear models of the leaf functional traits of B. rubescens in monodominant and mixed forests in Southern Amazonia. MOS maximum opening of the stomatal pore, STS stomata size, STD stomata density, SPT spongy parenchyma thickness, PPT palisade parenchyma thickness, AET adaxial epidermis thickness, ACT adaxial cuticle thickness, LDM leaf dry matter content, SLA specific leaf area, PEL petiole length, LET leaf thickness. Within means the residual error, all data were transformed (log-10) before analysis
We observed that the petiole length (PEL) is an important functional trait because it can positively predict the density of individuals and the height of B. rubescens trees (Fig. 5 and Table S4, S5). On the other hand, adaxial cuticle thickness (ACT) influences negatively both the height and diameter of B. rubescens trees (Fig. 5 and Table S6). No functional leaf traits were effective on the variation in biomass (Fig. 5 and Table S7). The complete models explained between 41 and 53% of the change in monodominance and population structure of B. rubescens (adjusted R2 values).
Correlates of structural parameters of B. rubescens populations in monodominant and mixed forests in Southern Amazonia. Variables that were not captured as good predictors in the models were adjusted toward the zero-point line. DBH diameter at breast height, LET leaf thickness, PEL petiole length, SPT spongy parenchyma thickness, STD stomata density, ACT adaxial cuticle thickness, LDM leaf dry matter content
Discussion
In this first study evaluating the relationship between leaf functional traits and the monodominance of B. rubescens, we found strong differences between the populations of a monodominant and a mixed forest. The set of functional traits of individuals of B. rubescens in the mixed forest reveals that such population invests in conservative strategies, in contrast with acquisitive strategies of the monodominant population. Such a condition indicates that individuals from both forests may have suffered different selective pressures resulting in bidirectional segregation in the leaf economics spectrum. Differences between individuals and forests types were the factors that best explained the variation of leaf functional traits. Our results also reveal that the petiole length and the adaxial cuticle thickness could be associated with B. rubescens monodominance. Explanations for these results are detailed below.
Trade-offs in leaf functional traits and implications for B. rubescens monodominance
The variation in leaf traits showed a functional divergence in the ecological strategies of B. rubescens. Individuals in the monodominant forest presented strategies linked to “acquisition and use of resources”. In contrast, individuals in the mixed forest showed the opposite strategies linked to “conservation of resources”, constituting extreme patterns of the leaf economics spectrum (Wright et al. 2004).
B. rubescens individuals in the monodominant forest showed higher values of specific leaf area, stomata size, maximum opening of the stomatal pore and petiole length, which are linked to a higher photosynthetic rate, primary productivity and, consequently, greater growth of individuals (Poorter and Bongers 2006; Ogburn and Edwards 2010). These functional traits allow maximize light capture (Takenaka 1994), being advantageous during a canopy opening (gaps) or an acquisition strategy (e.g., higher values of specific leaf area and length of the petiole). This can increase the efficiency in capturing the resource and, consequently, allow B. rubescens to overlap its density above other species, occupying a large part of the space (~ 90%) and maintaining its monodominant pattern (Marimon et al. 2014).
In contrast, individuals of B. rubescens in the mixed forest showed higher values of the adaxial cuticle thickness, palisade and spongy parenchymas thickness and also leaf thickness, normally linked to protection and support mechanisms (conservative strategy), and can also facilitate the uptake of water and their maintenance in the tissues (Fahn and Cutler 1992; Gratani et al. 2006). This condition increases the efficiency of these individuals in the conservation of nutrients and water use (Pallardy 1981; De Micco and Aronne 2012), indicating higher resistance to drought (Franco 2002; Goldstein et al. 2008) and better competition for water. Also, it reduces leaf damage caused by herbivores, excessive sunlight and high temperatures (Turner 1994; Rozendaal et al. 2006; Rossatto and Kolb 2010; Araújo et al. 2021b), ensuring higher integrity of the leaf mesophyll. On the other hand, these traits represent lower productivity rate and, in turn, slower growth (Reich 2014), which may be associated with the lower population density and lower dominance of B. rubescens in the mixed forest.
This network of trade-offs between leaf functional traits can be useful to understand the differences in the population density of B. rubescens between monodominant and mixed forests. We argue that the highest values of specific leaf area, petiole length, stomata size, and maximum opening of the stomatal pore are important to ensure competitive advantage in the search for light, water, and space (Aiba et al. 2020; Araújo et al. 2021b). Resource acquisition strategies have also been suggested as the main factor contributing to Gilbertiodendron dewevrei monodominance in Africa (Hall et al. 2020). Therefore, leaf functional traits linked to acquisitive strategies (Donovan et al. 2011) can be a proxy to help explain the monodominance of B. rubescens in Southern Amazonia. Thus, we believe that new studies should evaluate whether monodominant species present convergent ecological strategies, as well as the efficiency and safety in physiological mechanisms to predict the vulnerability and resistance of monodominant species in a hotter and drier climate.
Our findings confirm that populations of B. rubescens in adjacent monodominant and forests, present ample phenotypic plasticity in leaves functional traits. This ecological condition can provide higher adaptive capacity to different environmental and climatic patterns (Silveira et al. 2013; Lima et al. 2017), conditioning the species persistence (Chevin and Lande 2010; Franks et al. 2014; Araújo et al. 2021b) and possible increases in their populations and geographic distribution over time and space.
Extreme climatic events, such as increased temperature and drought in Southern Amazonia (Meehl and Tebaldi 2004; Collins et al. 2013; Rifai et al. 2018), can cause severe changes in species abundance, composition, and distribution (Walther et al. 2002; Menzel et al. 2006), consequently increasing the tree mortality (Phillips et al. 2010). This is particularly important for B. rubescens in the monodominant forest, as the species have leaf functional traits that are less tolerant to increases in intensity and frequency of drought events and increase in temperature, and therefore may be negatively affected if future climate changes (Marimon et al. 2020). However, theoretically, phenotypic plasticity can ensure its persistence as a monodominant species (Vitasse et al. 2010), through potential changes in the leaf economics spectrum. In this way, revealing the individual responses of this species will make it possible to launch a perspective of future risk management for B. rubescens populations in both forests, especially if we consider the economic importance of the wood of this species and also its use by local indigenous communities (Marimon and Felfili 2001).
Possibly the difference in community structure of both forests and subtle variations in soil water availability (Marimon et al. 2014; Elias et al. 2018; Marimon-Junior et al. 2019) may have generated alterations in leaf tissues, where individuals of B. rubescens have a wide range variation at the individual level. This reinforces the species' ability to adapt to different ecosystems, such as the Amazonia and the Atlantic Forest (Silveira et al. 2013; Lima et al. 2017), as well as being a strong competitor in Southern Amazonia, to the point of becoming monodominant in several patches.
Leaf functional traits can predict B. rubescens monodominance
We found that the petiole length is an essential predictor of the increase in the density of individuals and the height of B. rubescens trees, these results corroborate our findings in Fig. S4. This trait maximizes the absorption of photosynthetically active radiation (Poorter and Bongers 2006), reducing leaf clumping and overlap (Takenaka 1994), which allows the plant to be more efficient in capturing light in shaded environments (Weijschedé et al. 2008). Longer petiole length improves leaf distribution and orientation (King and Maindonald 1999) and can promote higher growth rates and density of individuals, as in the case of B. rubescens. However, we cannot rule out the importance of the specific leaf area and the size of the stomata. The literature suggests that longer petioles support larger leaves (Reich 2014) that enable an increase in photosynthetic rate and primary productivity, reflecting rapid growth (Westoby 1998; Wright et al. 2004). In this way, these morphological traits acting together can further increase the efficiency in the absorption of light (King and Maindonald 1999). Also, a larger specific leaf area with larger stomatal sizes can facilitate CO2 absorption (Beaulieu et al. 2008; Rossatto et al. 2009) and, consequently, assist in higher investments in the height and density of individuals, as we can see in Fig. S4.
The negative relationship between the thickness of adaxial cuticle with the height and diameter of the individuals was not expected in this study. This trait increases the capacity of plants to retain water (Rossatto and Kolb 2010; Araújo et al. 2021b), preventing the rapid dehydration of tissues and the loss of excessive water to the atmosphere, which can allow B. rubescens to increase the water use efficiency. It is also commonly observed that plants under conditions of water deficit and intense solar radiation presented higher cuticle thickness (Shepherd and Griffiths 2006; Araújo et al. 2021b), which may be the case of B. rubescens individuals in the mixed forest, which has a more open canopy (Marimon et al. 2008). In contrast, the adaxial cuticle is thin in the forest where the species is monodominant, which allows higher photosynthetic efficiency and tree growth (Westoby 1998; Koch et al. 2009).
The cuticle has a fundamental role in maintaining the water status of the plant (Larcher 1995), especially for B. rubescens, which can reach up to 45 m in height. As the height of the trees increases the hydraulic vulnerability also increases and can lead trees to death (Klein et al. 2018). In this case, conserving more water in the leaves may be essential for B. rubescens to maintain the growth and survival (Ambrose et al. 2009). Thus, the higher cuticle thickness of individuals in the mixed forest may be a strategy of the species to better compete with the others for water. Perhaps, this contributed to the species becoming monodominant in the past; however, reducing the thickness of the cuticle according to the increase in dominance. If climate change does not occur B. rubescens can increase the number of individuals and become denser in forests where it is still present with few individuals, mainly driven by its phenotypic plasticity in leaf functional traits, which can be an essential component for the persistence of the species over time.
Our findings demonstrate that individuals of B. rubescens have different functional strategies to establish and maintain their population in monodominant and mixed forests, proving to be a strong competitor for resources, such as water and light, through the variation in their leaf functional traits. Also, we revealed that the integration of some leaf functional traits, such as the length of the petiole and the thickness of the adaxial cuticle, could be an important part of the set of conditions driving the monodominance of B. rubescens in Southern Amazonia.
References
Ackerly DD (2003) Community assembly, niche conservatism, and adaptive evolution in changing environments. Int J Plant Sci 164:165–184
Aguiar TV, Sant’anna-Santos BF, Azevedo AA, Ferreira RS (2007) Anati quanti: software de análises quantitativas para estudos em anatomia vegetal. Planta Daninha 25:649–659. https://doi.org/10.1590/S0100-83582007000400001
Aiba M, Kurokawa H, Onoda Y, Nakashizuka T (2020) Trait–abundance relationships in tree communities along temperature and successional gradients. J Veg Sci 31:551–560
Alvares CA, Stape JL, Sentelhas PC, De Moraes Gonçalves JL, Sparovek G (2013) Köppen’s climate classification map for Brazil. Meteorol Zeitschrift 22:711–728
Ambrose AR, Sillett SC, Dawson TE (2009) Effects of tree height on branch hydraulics, leaf structure and gas exchange in California redwoods. Plant Cell Environ 32:743–757
Araújo I, Marimon BS, Scalon MC, Fauset S, Junior BHM, Tiwari R, Galbraith DR, Gloor MU (2021a) Trees at the Amazonia-Cerrado transition are approaching high temperature thresholds. Environ Res Lett 16:034047
Araújo I, Marimon BS, Scalon MC, Cruz WJ, Fauset S, Vieira TC, Galbraith DR, Gloor MU (2021b) Intraspecific variation in leaf traits facilitates the occurrence of trees at the Amazonia-Cerrado transition. Flora 279:151829
Beaulieu JM, Leitch IJ, Patel S, Pendharkar A, Knight CA (2008) Genome size is a strong predictor of cell size and stomatal density in angiosperms. New Phytol 179:975–986
Borcard D, Gillet F, Legendre P (2011) Numerical ecology with R, 1st edn. Springer, New York
Brookshire ENJ, Thomas SA (2013) Ecosystem consequences of tree monodominance for nitrogen cycling in lowland tropical forest. PLoS ONE 8:1
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Chevin LM, Lande R (2010) When do adaptive plasticity and genetic evolution prevent extinction of a density-regulated population? Evolution (NY) 64:1143–1150
Collins M, Knutti R, Arblaster J, Dufresne JL, Fichefet T, Friedlingstein P, Gao X, Gutowski JW, Johns T, Krinner G, Shongwe M, Tebaldi C, Weaver AJ, Wehner MF, Allen MR, Andrews T, Beyerle U, Bitz CM, Bony S, Booth BBB (2013) Long-term climate change: projections, commitments and irreversibility. In: Stocker TF, Qin D, Plattner GK, Tignor MBM, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Climate change 2013: the physical science basis: Working Group I contribution to the fifth assessment report of the Intergovernmental Panel on Climate Change, 1st edn. Cambridge University Press, New York, pp 1029–1136
Connell JH, Lowman MD (1989) Low-diversity tropical rain forests: some possible mechanisms for their existence. Am Nat 134:88–119
Cornwell WK, Ackerly DD (2010) A link between plant traits and abundance: evidence from coastal California woody plants. J Ecol 98:814–821
De Micco V, Aronne G (2012) Morpho-anatomical traits for plant adaptation to drought. In: Aroca R (ed) Plant responses to drought stress, 1st edn. Springer, Berlin, pp 37–61
Donovan LA, Maherali H, Caruso CM, Huber H, de Kroon H (2011) The evolution of the worldwide leaf economics spectrum. Trends Ecol Evol 26:88–95
Elias F, Marimon BS, Marimon-Junior BH, Budke JC, Esquivel-Muelbert A, Morandi PS, Reis SM, Phillips OL (2018) Idiosyncratic soil-tree species associations and their relationships with drought in a monodominant Amazon forest. Acta Oecologica 91:127–136
Fahn A, Cutler DF (1992) Xerophytes, 1st edn. Gebrueder Brontraeger, Berlin
FAO/UNESCO (1992) Soil map of the world - South America. FAO SOILS PORTAL. http://www.fao.org/fileadmin/user_upload/soils/docs/Soil_map_FAOUNESCO/acrobat/South_America_IV.pdf. Accessed 26 Jun 2020
Fidalgo O, Bononi, VLR (1984) Técnicas de coleta, preservação e herborização de material botânico. Manual Instituto de Botânica, São Paulo, Brasil
Franco AC (2002) Ecophysiology of woody trees. In: Oliveira PS, Marquis RJ (eds) The cerrados of Brazil: ecology and natural history of a neotropical savanna, 1st edn. Columbia University Press, New York, pp 178–197
Franklin GL (1945) Preparation of thin sections of synthetic resins and wood-resin composites, and a new macerating method for wood. Nature 155:51–51
Franks PJ, Drake PL, Beerling DJ (2009) Plasticity in maximum stomatal conductance constrained by negative correlation between stomatal size and density: an analysis using Eucalyptus globulus. Plant Cell Environ 32:1737–1748. https://doi.org/10.1111/j.1365-3040.2009.002031.x
Franks PJ, Leitch IJ, Ruszala EM, Hetherington AM, Beerling DJ (2012) Physiological framework for adaptation of stomata to CO2 from glacial to future concentrations. Philos Trans R Soc Lond B 367:537–546. https://doi.org/10.1098/rstb.2011.0270
Franks SJ, Weber JJ, Aitken SN (2014) Evolutionary and plastic responses to climate change in terrestrial plant populations. Evol Appl 7:123–139
Gause GF (1932) Experimental studies on the struggle for existence: I. mixed population of two species of yeast. J Exp Biol 9:389–402
Gentry AH (1992) Tropical forest biodiversity: distributional patterns and their conservational significance. Oikos 63:19
Goldstein G, Meinzer FC, Bucci SJ, Scholz FG, Franco AC, Hoffmann WA (2008) Water economy of Neotropical savanna trees: six paradigms revisited. Tree Physiol 28:395–404
Gratani L, Covone F, Larcher W (2006) Leaf plasticity in response to light of three evergreen species of the Mediterranean maquis. Trees 20:549–558
Grubb PJ (1982) Control of relative abundance in Roadside Arrhenatheretum: results of a long-term garden experiment. J Ecol 70:845
Hall JS, Harris DJ, Saltonstall K, Medjibe VP, Ashton MS, Turner BL (2020) Resource acquisition strategies facilitate Gilbertiodendron dewevrei monodominance in African lowland forests. J Ecol 108:433–448
Hart TB (1985) The ecology of a single-species-dominant forest and of a mixed forest in Zaire, Africa. Dissertation, Michigan State University
Hart TB (1995) Seed, seedling and sub-canopy survival in monodominant and mixed forests of the ituri forest, Africa. J Trop Ecol 11:443–459
Hart TB, Hart JA, Murphy PG (1989) Monodominant and species-rich forests of the humid tropics: causes for their co-occurrence. Am Nat 133:613–633
Hervé M (2021) Package ‘RVAideMemoire’ (V. 0.9-79). Testing and Plotting Procedures for Biostatistics. https://CRAN.Rproject.org/package=RVAideMemoire
Johansen DA (1940) Plant microtechnique. McGraw-Hill Book Company, New York
Kearsley E, Verbeeck H, Hufkens K, Van de Perre F, Doetterl S, Baert G, Beeckman H, Boeckx P, Huygens D (2017) Functional community structure of African monodominant Gilbertiodendron dewevrei forest influenced by local environmental filtering. Ecol Evol 7:295–304
King DA, Maindonald JH (1999) Tree architecture in relation to leaf dimensions and tree stature in temperate and tropical rain forests. J Ecol 87:1012–1024
Klein T, Zeppel MJB, Anderegg WRL, Bloemen J, De Kauwe MG, Hudson P, Ruehr NK, Powell TL, von Arx G, Nardini A (2018) Xylem embolism refilling and resilience against drought-induced mortality in woody plants: processes and trade-offs. Ecol Res 33:839–855
Koch K, Bhushan B, Barthlott W (2009) Multifunctional surface structures of plants: an inspiration for biomimetics. Prog Mater Sci 54:137–178
Kraus JE, De Sousa HC, Rezende MH, Castro NM, Vecchi C, Luque R (1998) Astra blue and basic fuchsin double staining of plant materials. Biotech Histochem 73(5):235–243. https://doi.org/10.3109/10520299809141117
Larcher W (1995) Ecophysiology and stress: physiology of functional groups, 3rd edn. Springer, New York
Laurance WF, Nascimento HEMM, Laurance SG, Condit R, D’Angelo S, Andrade A (2004) Inferred longevity of Amazonian rainforest trees based on a long-term demographic study. For Ecol Manag 190:131–143
Lima RBA, Marangon LC, Freire FJ, Feliciano ALP, Silva RKS (2017) Potencial regenerativo de espécies arbóreas em fragmento de Mata Atlântica, Pernambuco, Brasil. Rev Verde Agroecol e Desenvolv Sustentável 12:666
Marimon BS, Felfili JM (2001) Ethnobotanical comparison of “Pau Brasil” (Brosimum rubescens Taub.) forests in a Xavante Indian and a non-Xavante community in eastern Mato Grosso state. Brazil Econ Bot 55:555–569
Marimon BS, Felfili JM (2006) Chuva de sementes em uma floresta monodominante de Brosimum rubescens Taub. e em uma floresta mista adjacente no Vale do Araguaia, MT. Brasil Acta Bot Bras 20:423–432
Marimon BS, Felfili JM, Haridasan M (2001a) Studies in monodominant forests in eastern Mato Grosso, Brazil: i. a forest of Brosimum rubescens Taub. Edinburgh J Bot 58:123–137
Marimon BS, Felfili JM, Haridasan M (2001b) Studies in monodominant forests in eastern Mato Grosso, Brazil: ii. a forest in the Areões Xavante Indian reserve. Edinburgh J Bot 58:483–497
Marimon BS, Felfili JM, Lima ES (2002) Floristics and phytosociology of the gallery forest of the Bacaba Stream, Nova Xavantina, Mato Grosso, Brazil, Edinburgh. J Bot 59:303–318
Marimon BS, Felfili JM, Marimon Júnior BH, Franco AC, Fagg CW (2008) Desenvolvimento inicial e partição de biomassa de Brosimum rubescens Taub. (Moraceae) sob diferentes níveis de sombreamento. Acta Bot Bras 22:941–953
Marimon BS, Felfili JM, Lima ES, Duarte WMG, Marimon-Júnior BH (2010) Environmental determinants for natural regeneration of gallery forest at the Cerrado/Amazonia boundaries in Brazil. Acta Amaz 40:107–118
Marimon BS, Marimon-Junior BH, Feldpausch TR, Oliveira-Santos C, Mews HA, Lopez-Gonzalez G, Lloyd J, Franczak DD, de Oliveira EA, Maracahipes L, Miguel A, Lenza E, Phillips OL, Oliveira EA, Maracahipes L, Miguel A, Lenza E, Phillips OL (2014) Disequilibrium and hyperdynamic tree turnover at the forest-cerrado transition zone in southern Amazonia. Plant Ecol Divers 7:281–292
Marimon BS, Oliveira-Santos C, Marimon-Junior BH, Elias F, de Oliveira EA, Morandi PS, Nayane NCC, Mariano LH, Pereira OR, Feldpausch TR, Phillips OL (2020) Drought generates large, long-term changes in tree and liana regeneration in a monodominant Amazon forest. Plant Ecol 221:733–747
Marimon-Junior BH, Hay JDV, Oliveras I, Jancoski H, Umetsu RK, Feldpausch TR, Galbraith DR, Gloor EU, Phillips OL, Marimon BS (2019) Soil water-holding capacity and monodominance in Southern Amazon tropical forests. Plant Soil 450:65–79
Marques EQ, Marimon-Junior BH, Marimon BS, Matricardi EA, Mews HA, Colli GR (2020) Redefining the Cerrado-Amazonia transition: implications for conservation. Biodivers Conserv 29:1501–1517
McGuire KL, Henkel TW, Granzow De La Cerda I, Villa G, Edmund F, Andrew C (2008) Dual mycorrhizal colonization of forest-dominating tropical trees and the mycorrhizal status of non-dominant tree and liana species. Mycorrhiza 18:217–222
Meehl GA, Tebaldi C (2004) More intense, more frequent, and longer lasting heat waves in the 21st century. Science 305:994–997
Menzel A, von Vopelius J, Estrella N, Schleip C, Dose V (2006) Farmers’ annual activities are not tracking the speed of climate change. Clim Res 32:201–207
Miller TE, Werner PA (1987) Competitive effects and responses between plant species in a first-year old-field community. Ecology 68:1201–1210
Morandi PS, Marimon BS, Eisenlohr PV, Marimon-Junior BH, Oliveira-Santos C, Feldpausch TR, Oliveira EA, Reis SM, Lloyd J, Phillips OL (2016) Patterns of tree species composition at watershed-scale in the Amazon ‘arc of deforestation’: implications for conservation. Environ Conserv 43:317–326
Naimi B, Araújo MB (2016) sdm: a reproducible and extensible R platform for species distribution modelling. Ecography 39:368–375
Nascimento MT, Proctor J (1997) Population dynamics of five tree species in a monodominant peltogyne forest and two other forest types on Maraca Island, Roraima, Brazil. For Ecol Manag 94:115–128
Nascimento MT, Proctor J, Villela DM (1997) Forest structure, floristic composition and soils of an amazonian monodominant forest on Maraca Island, Roraima, Brazil, Edinburgh. J Bot 54:1–38
Nascimento MT, Barbosa RI, Dexter KG, de Castilho CV, da Silva Carvalho LC, Villela DM (2017) Is the Peltogyne gracilipes monodominant forest characterised by distinct soils? Acta Oecologica 85:104–107
Nik Norafida N, Nizam M, Wan Juliana W, Faezah P (2018) Edaphic relationships among tree species in the Kapur (Dryobalanops aromatica Gaertn.f.) forests of peninsular Malaysia. Adv Environ Biol 12:11–16
Ogburn RM, Edwards EJ (2010) The ecological water-use strategies of succulent plants. Adv Bot Res 55:179–225
Oksanen J, Blanchet FG, R K (2020) vegan: Community Ecology Package (V. 2.5–7). The Comprehensive R Archive Network. https://cran.r-project.org/web/packages/vegan/index.html. Accessed 20 Jun 2020
Pallardy SG (1981) Chapter 8—closely related woody plants. In: Kozlowski TT (ed) Water deficits and plant growth. Woody plant communities, 1st edn. Academic Press, New York, pp 511–548
Peh KSHH, Lewis SL, Lloyd J (2011) Mechanisms of monodominance in diverse tropical tree-dominated systems. J Ecol 99:891–898
Pérez-Harguindeguy N, Díaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P, Bret-Harte MS, Cornwell WK, Craine JM, Gurvich DE, Urcelay C, Veneklaas EJ, Reich PB, Poorter L, Wright IJ, Ray P, Enrico L, Pausas JG, Vos ACD, Buchmann N, Funes G, Quétier F, Hodgson JG, Thompson K, Morgan HD, Steege HT, Sack L, Blonder B, Poschlod P, Vaieretti MV, Conti G, Staver AC, Aquino S, Cornelissen JHC (2016) Corrigendum to: New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 64:715–716. https://doi.org/10.1071/BT12225_CO
Phillips OL, van der Heijden G, Lewis SL, López-González G, Aragão LEOCOC, Lloyd J, Malhi Y, Monteagudo A, Almeida S, Dávila EA, Amaral I, Andelman S, Andrade A, Arroyo L, Aymard G, Baker TR, Blanc L, Bonal D, de Oliveira ÁCA, Chao K-JJ, Cardozo ND, da Costa L, Feldpausch TR, Fisher JB, Fyllas NM, Freitas MA, Galbraith D, Gloor E, Higuchi N, Honorio E, Jiménez E, Keeling H, Killeen TJ, Lovett JC, Meir P, Mendoza C, Morel A, Vargas PN, Patiño S, Peh KS-HH, Cruz AP, Prieto A, Quesada CA, Ramírez F, Ramírez H, Rudas A, Salamão R, Schwarz M, Silva J, Silveira M, Ferry Slik JW, Sonké B, Thomas AS, Stropp J, Taplin JRDD, Vásquez R, Vilanova E (2010) Drought-mortality relationships for tropical forests. New Phytol 187:631–646
Poorter L, Bongers F (2006) Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 87:1733–1743
Poorter L, Wright SJ, Paz H, Ackerly DD, Condit R, Ibarra-Manríquez G, Harms KE, Licona JC, Martínez-Ramos M, Mazer SJ, Muller-Landau HC, Peña-Claros M, Webb CO, Wright IJ (2008) Are functional traits good predictors of demographic rates? Evidence from five neotropical forests. Ecology 89:1908–1920
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists, 1st edn. Cambridge University Press, Cambridge
R Development Core Team (2019) R: a language and environment for statistical computing. R Found. Stat. Comput.
Reich PB (2014) The world-wide “fast-slow” plant economics spectrum: a traits manifesto. J Ecol 102:275–301
Revelle W, Revelle MW (2020) psych: procedures for psychological, psychometric, and personality Research (V. 2.0.12). The Comprehensive R Archive Network. https://cran.r-project.org/web/packages/psych/index.html. Accessed 20 Jun 2020
Rifai SW, Girardin CAJ, Berenguer E, del Aguila-Pasquel J, Dahlsjö CAL, Doughty CE, Jeffery KJ, Moore S, Oliveras I, Riutta T, Rowland LM, Murakami AA, Addo-Danso SD, Brando P, Burton C, Ondo FE, Duah-Gyamfi A, Amézquita FF, Freitag R, Pacha FH, Huasco WH, Ibrahim F, Mbou AT, Mihindou VM, Peixoto KS, Rocha W, Rossi LC, Seixas M, Silva-Espejo JE, Abernethy KA, Adu-Bredu S, Barlow J, da Costa ACL, Marimon BS, Marimon-Junior BH, Meir P, Metcalfe DB, Phillips OL, White LJT, Malhi Y (2018) ENSO Drives interannual variation of forest woody growth across the tropics. Philos Trans R Soc B 373:20170410
Roeser KR (1962) Die nadel der Schwarzkiefer-masenprodukt und Keinstwert der Natur. Microkosmos 61:33–36
Rosas T, Mencuccini M, Barba J, Cochard H, Saura-Mas S, Martínez-Vilalta J (2019) Adjustments and coordination of hydraulic, leaf and stem traits along a water availability gradient. New Phytol 223:632–646
Rossatto DR, Kolb RM (2010) Gochnatia polymorpha (Less.) Cabrera (Asteraceae) changes in leaf structure due to differences in light and edaphic conditions. Acta Bot Bras 24:605–612
Rossatto DR, Kolb RM (2012) Structural and functional leaf traits of two Gochnatia species from distinct growth forms in a sclerophyll forest site in Southeastern Brazil. Acta Bot Bras 26:849–856
Rossatto DR, Hoffmann WA, Franco AC (2009) Características estomáticas de pares congenéricos de cerrado e mata de galeria crescendo numa região transicional no Brasil central. Acta Bot Bras 23:499–508
Rozendaal DMA, Hurtado VH, Poorter L (2006) Plasticity in leaf traits of 38 tropical tree species in response to light; relationships with light demand and adult stature. Funct Ecol 20:207–216
Schneider C, Rasband W, Eliceiri K (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Shepherd T, Griffiths DW (2006) The effects of stress on plant cuticular waxes. New Phytol 171:469–499
Silveira LHC, Rezende AV, Vale AT (2013) Teor de umidade e densidade básica da madeira de nove espécies comerciais Amazônicas. Acta Amaz 43:179–184
Smith FH, Smith EC (1942) Anatomy of the inferior ovary of Darbya. Am J Bot 29:464–471. https://doi.org/10.2307/2437312
Takenaka A (1994) Effects of leaf blade narrowness and petiole length on the light capture efficiency of a shoot. Ecol Res 9:109–114
ter Steege H, Pitman NCA, Sabatier D, Baraloto C, Salomao RP, Guevara JE, Phillips OL, Castilho C V., Magnusson WE, Molino J-F, Monteagudo A, Nunez Vargas P, Montero JC, Feldpausch TR, Coronado ENH, Killeen TJ, Mostacedo B, Vasquez R, Assis RL, Terborgh J, Wittmann F, Andrade A, Laurance WF, Laurance SGW, Marimon BS, Marimon B-H, Guimaraes Vieira IC, Amaral IL, Brienen R, Castellanos H, Cardenas Lopez D, Duivenvoorden JF, Mogollon HF, Matos FD d. A, Davila N, Garcia-Villacorta R, Stevenson Diaz PR, Costa F, Emilio T, Levis C, Schietti J, Souza P, Alonso A, Dallmeier F, Montoya AJD, Fernandez Piedade MT, Araujo-Murakami A, Arroyo L, Gribel R, Fine PVA, Peres CA, Toledo M, Aymard C. GA, Baker TR, Ceron C, Engel J, Henkel TW, Maas P, Petronelli P, Stropp J, Zartman CE, Daly D, Neill D, Silveira M, Paredes MR, Chave J, Lima Filho D d. A, Jorgensen PM, Fuentes A, Schongart J, Cornejo Valverde F, Di Fiore A, Jimenez EM, Penuela Mora MC, Phillips JF, Rivas G, van Andel TR, von Hildebrand P, Hoffman B, Zent EL, Malhi Y, Prieto A, Rudas A, Ruschell AR, Silva N, Vos V, Zent S, Oliveira AA, Schutz AC, Gonzales T, Trindade Nascimento M, Ramirez-Angulo H, Sierra R, Tirado M, Umana Medina MN, van der Heijden G, Vela CIA, Vilanova Torre E, Vriesendorp C, Wang O, Young KR, Baider C, Balslev H, Ferreira C, Mesones I, Torres-Lezama A, Urrego Giraldo LE, Zagt R, Alexiades MN, Hernandez L, Huamantupa-Chuquimaco I, Milliken W, Palacios Cuenca W, Pauletto D, Valderrama Sandoval E, Valenzuela Gamarra L, Dexter KG, Feeley K, Lopez-Gonzalez G, Silman MR (2013) Hyperdominance in the amazonian tree flora. Science (80- ) 342:1243092–1243092
ter Steege H, Henkel TW, Helal N, Marimon BS, Marimon-Junior BH, Huth A, Groeneveld J, Sabatier D, Coelho L de S, Filho D de AL, Salomão RP, Amaral IL, Matos FD de A, Castilho C V., Phillips OL, Guevara JE, Carim M de JV, Cárdenas López D, Magnusson WE, Wittmann F, Irume MV, Martins MP, Guimarães JR da S, Molino J-FF, Bánki OS, Piedade MTF, Pitman NCAA, Mendoza AM, Ramos JF, Luize BG, Moraes de Leão Novo EM, Núñez Vargas P, Silva TSF, Venticinque EM, Manzatto AG, Reis NFC, Terborgh J, Casula KR, Honorio Coronado EN, Montero JC, Feldpausch TR, Duque A, Costa FRCC, Arboleda NC, Schöngart J, Killeen TJ, Vasquez R, Mostacedo B, Demarchi LO, Assis RL, Baraloto C, Engel J, Petronelli P, Castellanos H, de Medeiros MB, Quaresma A, Simon MF, Andrade A, Camargo JL, Laurance SGWW, Laurance WF, Rincón LM, Schietti J, Sousa TR, de Sousa Farias E, Lopes MA, Magalhães JLL, Mendonça Nascimento HE, Lima de Queiroz H, Aymard C. GA, Brienen R, Revilla JDC, Vieira ICG, Cintra BBL, Stevenson PR, Feitosa YO, Duivenvoorden JF, Mogollón HF, Araujo-Murakami A, Ferreira LV, Lozada JR, Comiskey JA, de Toledo JJ, Damasco G, Dávila N, Draper F, García-Villacorta R, Lopes A, Vicentini A, Alonso A, Dallmeier F, Gomes VHFF, Lloyd J, Neill D, de Aguiar DPP, Arroyo L, Carvalho FA, de Souza FC, do Amaral DD, Feeley KJ, Gribel R, Pansonato MP, Barlow J, Berenguer E, Ferreira J, Fine PVAA, Guedes MC, Jimenez EM, Licona JC, Peñuela Mora MC, Villa B, Cerón C, Maas P, Silveira M, Stropp J, Thomas R, Baker TR, Daly D, Dexter KG, Huamantupa-Chuquimaco I, Milliken W, Pennington T, Ríos Paredes M, Fuentes A, Klitgaard B, Pena JLM, Peres CA, Silman MR, Tello JS, Chave J, Cornejo Valverde F, Di Fiore A, Hilário RR, Phillips JF, Rivas-Torres G, van Andel TR, von Hildebrand P, Noronha JC, Barbosa EM, Barbosa FR, de Matos Bonates LC, Carpanedo R de S, Dávila Doza HP, Fonty É, GómeZárate z R, Gonzales T, Gallardo Gonzales GP, Hoffman B, Junqueira AB, Malhi Y, Miranda IP de A, Pinto LFM, Prieto A, Rodrigues D de J, Rudas A, Ruschel AR, Silva N, Vela CIAA, Vos VA, Zent EL, Zent S, Weiss Albuquerque B, Cano A, Carrero Márquez YA, Correa DF, Costa JBP, Flores BM, Galbraith D, Holmgren M, Kalamandeen M, Nascimento MT, Oliveira AA, Ramirez-Angulo H, Rocha M, Scudeller VV, Sierra R, Tirado M, Umaña Medina MN, van der Heijden G, Vilanova Torre E, Vriesendorp C, Wang O, Young KR, Ahuite Reategui MA, Baider C, Balslev H, Cárdenas S, Casas LF, Farfan-Rios W, Ferreira C, Linares-Palomino R, Mendoza C, Mesones I, Torres-Lezama A, Giraldo LEU, Villarroel D, Zagt R, Alexiades MN, de Oliveira EA, Garcia-Cabrera K, Hernandez L, Palacios Cuenca W, Pansini S, Pauletto D, Ramirez Arevalo F, Sampaio AF, Sandoval EHVV, Valenzuela Gamarra L, Levesley A, Pickavance G, Melgaço K, Aymard C GA, Brienen R, Revilla JDC, Vieira ICG, Cintra BBL, Stevenson PR, Feitosa YO, Duivenvoorden JF, Mogollón HF, Araujo-Murakami A, Ferreira LV, Lozada JR, Comiskey JA, de Toledo JJ, Damasco G, Dávila N, Draper F, García-Villacorta R, Lopes A, Vicentini A, Alonso A, Dallmeier F, Gomes VHFF, Lloyd J, Neill D, de Aguiar DPP, Arroyo L, Carvalho FA, de Souza FC, do Amaral DD, Feeley KJ, Gribel R, Pansonato MP, Barlow J, Berenguer E, Ferreira J, Fine PVAA, Guedes MC, Jimenez EM, Licona JC, Peñuela Mora MC, Villa B, Cerón C, Maas P, Silveira M, Stropp J, Thomas R, Baker TR, Daly D, Dexter KG, Huamantupa-Chuquimaco I, Milliken W, Pennington T, Ríos Paredes M, Fuentes A, Klitgaard B, Pena JLM, Peres CA, Silman MR, Tello JS, Chave J, Cornejo Valverde F, Di Fiore A, Hilário RR, Phillips JF, Rivas-Torres G, van Andel TR, von Hildebrand P, Noronha JC, Barbosa EM, Barbosa FR, de Matos Bonates LC, Carpanedo R de S, Dávila Doza HP, Fonty É, GómeZárate z R, Gonzales T, Gallardo Gonzales GP, Hoffman B, Junqueira AB, Malhi Y, Miranda IP de A, Pinto LFM, Prieto A, Rodrigues D de J, Rudas A, Ruschel AR, Silva N, Vela CIAA, Vos VA, Zent EL, Zent S, Weiss Albuquerque B, Cano A, Carrero Márquez YA, Correa DF, Costa JBP, Flores BM, Galbraith D, Holmgren M, Kalamandeen M, Nascimento MT, Oliveira AA, Ramirez-Angulo H, Rocha M, Scudeller VV, Sierra R, Tirado M, Umaña Medina MN, van der Heijden G, Vilanova Torre E, Vriesendorp C, Wang O, Young KR, Ahuite Reategui MA, Baider C, Balslev H, Cárdenas S, Casas LF, Farfan-Rios W, Ferreira C, Linares-Palomino R, Mendoza C, Mesones I, Torres-Lezama A, Giraldo LEU, Villarroel D, Zagt R, Alexiades MN, de Oliveira EA, Garcia-Cabrera K, Hernandez L, Palacios Cuenca W, Pansini S, Pauletto D, Ramirez Arevalo F, Sampaio AF, Sandoval EHVV, Valenzuela Gamarra L, Levesley A, Pickavance G, Melgaço K (2019) Rarity of monodominance in hyperdiverse Amazonian forests. Sci Rep 9:13822
Torti SD, Coley PD, Kursar TA (2001) Causes and consequences of monodominance in tropical lowland forests. Am Nat 157:141–153
Turner IM (1994) Sclerophylly: primarily protective? Funct Ecol 8:669
Valladares F, Sanchez-Gomez D, Zavala MA (2006) Quantitative estimation of phenotypic plasticity: bridging the gap between the evolutionary concept and its ecological applications. J Ecol 94:1103–1116
Violle C, Navas ML, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E (2007) Let the concept of trait be functional! Oikos 116:882–892
Vitasse Y, Bresson CC, Kremer A, Michalet R, Delzon S (2010) Quantifying phenological plasticity to temperature in two temperate tree species. Funct Ecol 24:1211–1218
Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin JM, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416:389–395
Weijschedé J, Berentsen R, De Kroon H, Huber H (2008) Variation in petiole and internode length affects plant performance in Trifolium repens under opposing selection regimes. Evol Ecol 22:383–397
Westoby M (1998) A leaf-height-seed (LHS) plant ecology strategy scheme. Plant Soil 199:213–227
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas M-L, Niinemets Ü, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Wright SJ, Kitajima K, Kraft NJB, Reich PB, Wright IJ, Bunker DE, Condit R, Dalling JW, Davies SJ, DíAz S, Engelbrecht BMJ, Harms KE, Hubbell SP, Marks CO, Ruiz-Jaen MC, Salvador CM, Zanne AE (2010) Functional traits and the growth-mortality trade-off in tropical trees. Ecology 91:3664–3674
Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R, 1st edn. Springer, New York
Author information
Authors and Affiliations
Contributions
Conceived and designed the investigation: IA, PSM, AOM, LHM, FA, BSM, BHMJ. Performed field and laboratory work: IA, AOM, LHM, IVS, FA. Analyzed the data: IA, PSM, AOM, LHM. Wrote the paper: IA, PSM, LHM, IVS, FA, BSM, BHMJ.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare to have no conflict of interest.
Informed consent
All authors agree with the publication of the work.
Additional information
Communicated by William E. Rogers.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Araújo, I., Morandi, P.S., Müller, A.O. et al. Leaf functional traits and monodominance in Southern Amazonia tropical forests. Plant Ecol 223, 185–200 (2022). https://doi.org/10.1007/s11258-021-01201-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-021-01201-w