Abstract
Populations of dioecious plants commonly exhibit dissimilarities to the equilibrium expectation of a 1:1 sex ratio. Differential expenditure for reproduction between genders is cited as the primary mechanism responsible for a male-biased sex ratio, with increased effects on long-living species, but these mechanisms are still poorly understood. We explore the sex ratio in the endemic gymnosperm Zamia boliviana (Zamiaceae) populations from the Brazilian savanna (the Cerrado). We aim to investigate what the Z. boliviana sex ratio is, and whether population density and ecological correlates lead to variation in the sex proportion among Cerrado habitats. The study was conducted on ten in situ populations of Z. boliviana at sexual maturity (tertiary sex ratio). We estimated the populations’ sex ratio and performed a redundancy analysis to assess the relationship between biotic traits, such as sex ratio, and associated environmental features. Soil texture classes were used to classify the cycad habitats and were expressed in a ternary phase diagram. The results show a significant male-biased sex ratio in seven of the ten populations surveyed. Environmental factors did not explain the redundancy in the reproductive characteristics. However, the cycad occurs in different habitats in their endemic zone. Our study provides new biological data for Z. boliviana, suggesting that the differential reproductive expenditure of sexes in reproduction is governing the mechanisms of sex ratio variation, compared to local environmental factors in this cycad. The pattern of effective sex ratio found here improves our understanding of mechanisms causing biased sex ratios in cycads and other dioecious species from tropical ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dioecy is characterized by the presence of male and female reproductive structures in different individuals (Bawa and Beach 1981). Although relatively uncommon, this reproductive system occurs commonly in gymnosperms and approximately 6% of angiosperms (Renner and Ricklefs 1995). Although almost half of all families and the majority of orders contain dioecious species, dioecy is rare within these higher taxa, as shown from its phylogenetic distribution, and mostly occurs in long-living species (Renner 2014; Käfer et al. 2016; Barrett et al. 2010). The obligate outcrossing mechanism imposed by dioecy means that the absence of potential mates of the opposite sex renders an effectively sterile individual (Käfer et al. 2016). Dioecy is commonly associated with a suite of life histories and reproductive traits (Barrett et al. 2010). The dioecious system is therefore complex, because it involves sexual specialization, incurring genetic, demographic, and ecological costs (Renner 2014).
The term “sex ratio” latu senso is defined as the number of males in relation to females in a given group of organisms (Calonje et al. 2011; Geodakyan 2012). However, the sex ratio of a population may change over time and can be formally split into three categories: primary sex ratio (ratio of males to females after fertilization); secondary sex ratio (ratio at birth); and tertiary sex ratio (ratio at sexual maturity) (Majerus 2003). The sex ratio or sexual rate equilibrium can indicate that there is no competitive advantage for males, despite a lower reproductive cost (Octavio-Aguilar et al. 2017). In Cycadaceae and Zamiaceae species it is recognized that if each year males and females produce strobili with equal frequency, then the larger the seedling group in a population, the smaller the chances become of it consisting exclusively of either males or females (Grobbelaar 2002). Therefore, chances increase that the recruitment of this population will contain both sexes (Grobbelaar 2002). These proportions are generally assumed for healthy populations (Ackleh and Zhang 2009; Barrett 2015). Thus, there can be different factors that cause sex ratio variations among populations in the natural habitat.
Populations of dioecious species show a nearly continuous variation in sex ratios from a strong male to strong female bias, especially in habitats affected by abiotic stresses such as poor soils or low humidity (reviewed in Barrett et al. 2010). The variations are usually related to specialization and competitive ability in resource allocation between genders (Delph 2019). This is an expected result of trade-offs in allocation, associated with sex-differential reproductive costs (Freeman et al. 1997; Zhang et al. 2014). Investigation of environmental influences on the sex ratio commonly reports it as a result of variation in site quality or local resources (e.g. climate, soil moisture, and altitude), as well as male and female proximity (Freeman et al. 1997; Stehlik et al. 2007; Morellato 2004). According to Allen and Antos (1988), the costs of reproduction are often higher in females, causing strong biases in the sex ratio due to earlier initiation of flowering, more frequent flowering, and higher mortality of females than of males (Allen and Antos 1993). Barrett et al. (2010) list at least three factors related to the costs of reproduction that can contribute to male bias: (i) earlier onset of male flowering; (ii) more frequent flowering in males; and (iii) greater gender-specific mortality in females. Particularly for cycads, Krieg et al. (2017) found several patterns of physiological and morphological differences between sexes, but their relationship to ecological factors needs to be investigated further.
Cycads are dioecious plants, with 356 taxa found around the world (Calonje et al. 2021). These plants typically take a long time to reach sexual maturity, with plants in a population that do not produce strobili regularly, and therefore, the reproductive phenology is often asynchronous in a particular given season or between seasons (Grobbelaar 2002; Jones 2002). In cycad populations, deviations from the expected 1:1 sex ratio were reported for species of the families Cycadaceae and Zamiaceae, with populations commonly showing a male bias in most assessments of tertiary sex ratio (Grobbelaar 2002; Calonje et al. 2011). According to Calonje et al. (2011), the cumulative sex ratio may vary widely in wild populations, but little is known about local habitat conditions that can influence the balance of male and females (Grobbelaar 2002).
Here we investigate the tertiary (phenotypical) sex ratio of Zamia boliviana (Brongn.) A. DC. (Cycadales, Zamiaceae) (Fig. 1), a species occurring in woody Cerrado vegetation (Fig. 2). We were interested in determining the sex ratios of reproductive plants of these cycad populations in situ and how this ratio varies in different Cerrado habitats (Fig. 2). Thus, we tested the hypothesis that the tertiary sex ratio in Z. boliviana may be affected by local environmental conditions found in different populations (light incidence and soil properties). Our study addressed the following specific questions: (1) What is the sex ratio across different populations of Z. boliviana? Does it differ from the expected 1:1 ratio? (2) What are the predominant characteristics of Z. boliviana habitats regarding soil physical properties and light intensity, and are those variables related to the sex ratio of local Z. boliviana populations?
Geographic distribution and locality of occurrence according to ecoregion types and sampling of Zamia boliviana in South America, state of Mato Grosso (Brazil), and Brazil–Bolivia border region. Areas of the studied populations with schematic view of the sampling method and locations marked with green points indicate the studied populations. Note: vegetation types, according to the classification of Olson et al. (2001), and the manipulation of geospatial data was done using QGIS
Habitats and morphology of Z. boliviana: a, b Male plant: a. In Red-Yellow Latosols and b. In rocky calcareous soil. c, d, g In sandy Haplic Cambisols in cerrado, with occurrence of fire, clonal xylopodium and single xylopodium (g1-2) clonal adult plant with brevideciduous bud, (g3) adult plant. e In Oxisols (ironstone outcrops). f Female plant with single xylopodium in Red-Yellow Latosols. g Underground organ (xylopodium): (1) buds in development, (2) apical bud growth region, (3) ground level. h Underground organ (xylopodium): (1) buds in development, (2) apical bud growth region, (3) ground level–Credits: Rosane Segalla, personal collection
Methods
Study species
Zamia boliviana is a small perennial plant (0.80 m average height above ground), with a subterranean stem (xylopodium) and between one and three leaves in each crown in adult plants and individuals of both sexes are randomly distributed at distances varying from 1 m or more (Fig. 2d, e, g). We have adopted the name “xylopodium” in this study to denote the perennial thickened woody axis of the underground system of Z. boliviana (Fig. 2f, g, h). This xylopodium has a complex structure and includes the base of the hypocotyl, the root–stem transition region, and the proximal portion of the main root, as described for different species from the Cerrado (Hayashi and Appezzato-da-Glória 2007). Zamia boliviana can have a single xylopodium or multiple xylopodia. The multiple system comprises branched or clonal xylopodia, but with very limited spread and joined by portions of stem or roots at the base (Fig. 2h). This morphology, although there are exceptions, have distinguishing clumps of plants of the same sex above the ground. According to phenology records (Soares 2020), the vegetative and reproductive structures are formed in the soil during the dry season. The plants can become deciduous or brevideciduous for undetermined periods of time. Male plants can develop from one to five microstrobili per stem or branch (apical meristem) of xylopodium (Fig. 2a, b), whereas female plants produce only one megastrobilus per stem or branch (Fig. 2c–f). Starting from soil emergence, the lifespan of the microstrobili lasts more or less two months, while megastrobili can last up to ten months (Soares 2020).
Study region
Populations of Z. boliviana are distributed within the intertropical zone in the central portion of South America (Fig. 1) (Segalla et al. 2019). The habitats are characterized by high total solar radiation incidence throughout the year (SEPLAN 2000) and equatorial and tropical hot climates, with little seasonal or annual temperature variation (Köppen 1918; Geiger 1954; Kottek et al. 2006). The region has two distinct seasons: a dry season (May to September), with an average rainfall of 42.3 mm, and a wet season (October to April), with an average rainfall of 186 mm. Mean annual temperatures range from 22 ºC to 26 °C, low temperatures from 18 ºC to 23 ºC, and high temperatures from 25 ºC to 30 ºC (Karger et al. 2017).
Zamia boliviana distribution is restricted to parts of Bolivia (Beni, Cochabamba, La Paz, and Santa Cruz) and Brazil, in the state of Mato Grosso (MT), within an elevation range of 130 m to 450 m above sea level, and different vegetation types within the Cerrado biome (Oliveira-Filho and Ratter 2002; Ribeiro and Walter 2008), mainly the Cerrado sensu stricto (Brazilian woodland savanna), hereafter just Cerrado (Table 1, Fig. 1) (Segalla et al. 2019). Cycad occurs in relatively flat landscapes or terrain exhibiting minor changes in topography (Segalla et al. 2019; SEPLAN 2000). Slopes predominate in the region of Chapada dos Guimarães (MT), with the formation of deep valleys (SEPLAN 2000). Zamia boliviana frequently form dense clumps of individuals of both sexes in different successional stages or at different reproductive ages (Soares 2020). Most of the populations have been decimated or severely fragmented by the clearing of land for agriculture in both countries (Table 1, Fig. 1) (Segalla and Calonje 2019; Segalla et al. 2019). During this study, a fire event occurred in the region of the DES site population one year before sex ratio evaluation (Table 1). This happened within the reproductive period of the Z. boliviana and evidence showed that seeds and emerged (megastrobili of previous season) and emerging or newly emerged strobili were probably burned. This prevented us from identifying the sex of many individuals during our sampling in that area in 2017.
Sampling, sex determination, sex ratio variation, and habitat characterization
To investigate sex ratio variation in different habitats of Z. boliviana, we sampled populations in ten sites from four distinct areas of Cerrado in the Brazil–Bolivia border region and other localities of the state of Mato Grosso (MT), as described in Table 1. For the purpose of this study, we determined the sex ratio at sexual maturity, the tertiary sex ratio according to Majerus (2003). We define an individual as the set of leaves (1–3) belonging to a single ramet, regardless of the number of xylopodium ramets (Fig. 2b, c, g, h). This criterion was chosen due to the subterranean xylopodium and, in some cases, the proximity of potential clonal individuals, which is not always distinguishable from above ground (Fig. 2). The surveyed area covered the municipalities of Cáceres (JB 30.720 ha; TM 65.155 ha; VC 15.376 ha), Glória do Oeste (VB 39.605 ha), Mirassol do Oeste (IMP 10.581 ha), and Chapada dos Guimarães (BV 3.270 ha; BVB 33.884 ha; SB 6.977 ha; NP 3.843 ha and DES 15.440 ha) (Table 1). Groups or clusters of at least 1,000 male and female individuals within a radius of approximately 10,000 square meters were considered as populations. Initially, transects of 5 × 100 m were established, containing a minimum of 100 individuals of both sexes for each sampling unit. The transect area was extended as necessary to meet the established inclusion criteria. The visual census of Z. boliviana was performed by means of active searching for, and marking of, adult plants within the transects. The sex ratio was determined based on the identification and counting of plants with mega- and microstrobili between August and October 2017, which is the reproductive peak Z. boliviana (Soares 2020). Plants were classified as male, female, or unidentified plants (adult plants, but without strobili). This determination was made according to plant size (≥ to 0.80 m tall) and the number of leaflets per leaf (≥ 12). In this study we considered each observable above-ground branch as an independent plant due to the impossibility of observing the nature of xylopodia (clonal or separate individuals) below ground. This criterion was defined after a pilot project carried out from within the VB area to better understand the biological system of this species. The sampled material was deposited in the Central Herbarium of the Federal University of Mato Grosso. In each transect we recorded the population density, calculated as the number of males, females, and indeterminate plants per sampling unit (transect). Other ecological information collected for the Z. boliviana populations is shown in Table 1 and was obtained as follows: predominant soil type; habitat types and light condition––on-site sampling carried out by this study or reviews in SEPLAN publications; and history of anthropogenic disturbances––information provided by landowners and our observations of evidence of disturbances in sampled sites. The specific geographical coordinates of populations are omitted to minimize the risk of illegal extraction of this species from the wild.
Environmental factors
Physical and chemical variables
To investigate whether physical and/or chemical soil variables in the habitat of Z. boliviana can affect the sex ratio, we sampled soils along the transect for each surveyed area at equidistant points (Fig. 2). Surface soil samples (0–30 cm) were obtained along each transect using a Dutch soil auger and then combined into composite samples. The soil samples obtained in all surveyed areas were air-dried and sieved (0.5 mm mesh). The samples were analyzed for physical and chemical properties according to the procedures adopted in Vourlitis et al. (2011). The physical properties of the soil tested include stoniness, moisture and organic matter (SOM) content, and particle size composition (percent sand, silt, and clay). The physical parameters tested were classified by particle size diameter in mm (Dane and Hopmans 2002; FAO 2006) as follows: (A) Five sand fractions––very coarse sand (VC); coarse sand (C); medium sand (M); fine sand (F); and very fine sand (VF)––total sand (TS); total clay; and water dispersible clay. The chemical properties measured were (as assessed colorimetrically) boron (B), copper (Cu), zinc (Zn), manganese (Mn), iron (Fe), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), and aluminum (Al) content, as well as pH. The analyses were carried out in the Soil Laboratory of the “Luiz de Queiroz” School of Agriculture, University of São Paulo (USP-ESALQ), São Paulo, Brazil.
Light variables
To investigate whether the incident light in the habitat of Z. boliviana interacts with the sex ratio of this cycad, we estimated the percentage of canopy openness light for each site, using a hemispherical image taken with a NIKON F-501 camera, coupled with a NIKON 8 mm fisheye lens with a 180° angle of view. Images were taken every 25 m along the transect, with the camera positioned at a height of 0.30 cm above the ground, in all areas and according to the recommendations of Galvani and Lima (2014). Solar radiation at each site was estimated according to the percentage of canopy openness (canopy ground cover) in each transect and the images were processed using Gap Light Analyzer (GLA) software version 2.0 (Lang et al. 2010).
Statistical analysis
Sex ratio variation
To test whether the male to female plant tertiary ratio intra and inter the ten populations’ sex ratio patterns deviate from the expected isoplethic equilibrium (1:1), we used the G-test for goodness of fit (Sokal and Rohlf 2011).
Habitat characterization
To detect possible environmental differences among the ten sites of Z. boliviana in terms of soil composition and structure, the sites were ordered according to soil texture classes for percentages of clay, silt, and sand, and illustrated in a ternary phase diagram, according to the FAO (2006).
Sex ratio and its correlation with environmental factors
Physical, chemical, and light variables
We used a redundancy analysis (RDA) to assess whether there were redundancies between summaries of cycad populations according to the biotic terms (considering the total area, total plant abundance, and abundance according to sex), sex proportion, and the environmental conditions (soil parameters and incident light). Out of 25 physical and chemical variables, the following were chosen: light, coarse sand, boron (B), copper (Cu), manganese (Mn), aluminum (Al), and V% (base saturation). These variables were selected based on the lowest Pearson correlation coefficients and variance inflation factor (VIF < 2) between variables using the vif function from the usdm package (Wei and Simko 2017) in R (TeamCore-R 2017). To perform the RDA analysis, the parameters for soil and incident light were standardized with a mean of zero using the “standardize” method of the decostand function in the Vegan package. Environmental factors were standardized using the Hellinger method (Borcard et al., 2018). The RDA analysis was performed using the rda function in Vegan (Oksanen et al. 2019), and the significance of environmental variables was tested using the function anova.cca (Legendre et al. 2011) with argument by = “terms.”
Results
Sex ratio pattern
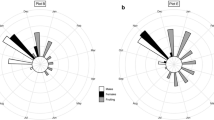
Estimates of the sex ratio were obtained from 1,572 individuals sampled from ten populations. Table 2 summarizes the results of the sex ratio for each population studied. Seven out of ten populations were male biased (VB 1.69♂:1♀, X2(61.277), p = 0.013; VC 1.58♂:1♀, X2(4.05), p = 0.044; IMP 3.1♂:1♀, X2(39.486), p = 3.304; JB 4.75♂:1♀, X2(19.565), p = 9.722; BV 2.91♂:1♀, X2(10.256), p = 0.001; BVB 2.04♂:1♀, X2(82.286), p = 0.004; DES 07♂:01♀, X2(76.5), p < 2.2) and three were close to the expected sex ratio of 1:1 (TM 1.61♂:1♀, X2(3.266), p = 0.070; NP 1.15♂:1♀, X 2(0.494), p = 0.481 and SB 1.32♂:1♀, X2(1.531), p = 0.215) (Table 2, Fig. 3).
Distribution of sampled individuals (male, female, and unidentified individuals) in ten populations of Zamia boliviana. The black and dark gray bars show the tertiary sex ratio (male:female), suggesting significant deviation from the expected 1:1 ratio as determined by the Chi-square test. Asterisks above bars denote significant sexual bias (P < 0.5)
Habitat characterization
Populations of Z. boliviana were categorized into three distinct habitat types according to the soil texture classes (Table 1; Fig. 4). Four out of ten populations (BV, BVB, NP, and DES) are from Chapada dos Guimarães, a region of wavy to very wavy reliefs and soils primarily composed of sandy Cambisols (Fig. 4–Habitat 1). Three sites (TM, JB, and VB) occur on a mainly flat to smooth-wavy relief, flat areas, or in the lower third of slopes, with potential movement or a shallow water table. These three sites are formed, predominantly, by Latosols, Plinthosols, and Neosols with a Loamy Sand texture (Fig. 4–Habitat 2). In the other three sites, the populations are distributed between Mirassol do Oeste and Cáceres, among the elevations of the rugged relief of the Província Serrana (IMP and VC), and in the valleys and sloped relief of Chapada dos Guimarães (SB). The main soils are Plinthosols and Neosols, with Sandy Clay Loam (SCL) transitioning to Sandy Loam (SL) textures (Fig. 4–Habitat 3). To summarize, the soils of the three habitats have distinct morphological, physical, and chemical characteristics, but all have a sandy to rocky texture and medium to high drainage. The distribution of Cerrado phytophysiognomies is related to the presence of medium to high levels of calcium (Ca), copper (Cu), iron (Fe), manganese (Mn), and aluminum (Al), whether associated with the lithology of soils or not.
The relationship between, or percentages of, clay, silt, and sand for soil texture classes was indicated by the ternary phase diagram, and the coarse sand gradient is indicated by the biplot (Figs. 4, 5). Generally, the cycad was found in sandy soils of low-fertility, intermediate levels of incident light, compared to the average values obtained in the sampling of the sites, and marked climatic seasonality in the Cerrado. The individuals form clumps mainly in the Cerrado (Figs. 2, 3, 4, 5) and the percentage of light reaching the habitat may exceed 70% during the dry period (Table 1). Cambisols are more permeable and, in this study, were associated with the development of plants with an extensive system of branched or clonal xylopodia (Table 1; Fig. 2g–h). In contrast to this, cycad populations associated with Red-Yellow Latosol have, predominantly, single (non-branched) and shallower xylopodia (Table 1; Fig. 2f).
Biplot of the relationship between physicochemical parameters and tertiary sex ratio of 10 populations of Zamia boliviana. The numbers represent the populations on the ordination axes 1–2 extracted from redundancy analysis. The arrows indicate the orientation of populations based on the physicochemical parameters of soil and incident light. According to the significance of the variable coarse sand, we include its surface (in green) that shows how the populations are distributed according to this characteristic. The numbers (1–10) represent the 10 populations studied (1 = VB; 2 = TM; 3 = VC; 4 = IMP; 5 = JB; 6 = NP; 7 = SB; 8 = BV; 9 = BVB; 10 = DES)
Sex ratio correlation with environmental factors
The correlation of sex ratio with physical and chemical parameters of soil and incident light in each of the ten populations of Z. boliviana revealed that the environmental variables do not explain redundancy in the reproductive characteristics of the populations (RDA model, F7.2 = 2.366; p = 0.256), even with an accumulation of 85% of the variation of these reproductive characteristics (see supplementary material) in the first two axes of the ordination. However, the coarse sand exhibited significance in the composition of the model, showing a gradient in the studied populations (F1.2 = 8.900; p = 0.037; Fig. 5). The sandy texture was present in all populations, but decreasing, with the populations (number = population in RDA, Fig. 5, Table 3) NP’6, BV’8, BVB’9, and DES’10 presenting the highest percentage of sand in their soils, followed by the TM’2, JB’5, and VB’1 populations with intermediate values, and IMP’4, SB’7, and VC’3 with lower values.
Discussion
Our investigations on the tertiary sex ratio of Z. boliviana populations in the Cerrado (Brazilian savanna) and local environmental factors revealed the following findings: (1) most populations present a male bias (70%); (2) Z. boliviana occupies different habitats within its area of distribution, with good adaptability to sandy and well-drained soils; and (3) we did not find any evidence that physical or chemical soil variables or light levels affect the sex ratio of Z. boliviana. However, we found an environmental gradient related to the soil properties. From these results, we discussed presumed causes, as well as similar studies.
Sex ratio in Zamia boliviana
The male bias in the tertiary sex ratio of Z. boliviana appears to be related to a differential energy expenditure to produce ovulate strobili compared to pollen strobili, as reported for other species of the families Cycadaceae and Zamiaceae and as argued by Tang (1990) and Calonje et al. (2011). At the biological level, the male bias in Zamiaceae may possibly be caused by the following factors or a combination thereof, according to Calonje et al. (2011) and the Z. boliviana phenology study (Soares 2020): (1) male individuals produce reproductive structures at a younger age than females; (2) male individuals can produce strobili more regularly than females; (3) male individuals of a population have a longer variation in time of the emergence and development of strobili within the same reproductive season than females; and (4) microstrobili have a shorter lifespan (~ two months) than megastrobili (~ ten months or more), preventing, oftentimes, the identification of sex in phenotypic evaluations. All these factors increase the chances of male sex identification in tertiary assessments.
Presumably the production of pollen strobili requires less energy expenditure than that of ovulate strobili and they are therefore produced more often during consecutive episodes of reproduction (Tang 1990; Grobbelaar 2002). The deviations toward the male sex occur in particular due to asynchronous reproductive phenological activity and short-term sampling (Tang 1990; Grobbelaar 2002; Calonje et al. 2011; Soares 2020). Thus, we assumed that the differential energy expenditure to produce sexual structures is preponderant in tertiary sex ratio variation in Z. boliviana, explaining the deviation from a genotypic sex ratio of 1:1, as expected in dioecious populations (Ornduff 1987; Barrett et al. 2010). A few other studies have also demonstrated greater female reproductive effort in dioecious species (see Allen and Antos 1988, 1993; Antos and Allen 1990, 1994; Delph 2019). We believe that there is no biological evidence that differential mortality in female plants of Z. boliviana causes male deviations in the sex ratio, although this needs to be further investigated. We attribute the morphology and ecological adaptations of this perennial cycad from the Cerrado, such as the presence of a xylopodium, to a large nutrient reserve and brevideciduous events, as the main reason to refute, at least partially, the mortality in female plants. Oliveira (1996) suggested that of the ca. 30% of dioecious species of Cerrado, 12% are in open areas, with a predominantly brevideciduous habit. Additionally, according to the findings of Octavio-Aguilar et al. (2017), mortality is not a crucial factor involved in the differential performance of the sexes in the genus Zamia.
Sex ratios of cycad populations have been investigated for different genera, such as Ceratozamia Brongniart, Dioon Lindley, Encephalartos Lehmann, Lepidozamia, Macrozamia Miquel, Microcycas (Miq.) A. DC., and Zamia L. (reviewed by Calonje et al. 2011). Deviation from the 1:1 sex ratio in cycads appears to be the norm in relatively small populations or when sampling adult-sized individuals only to determine the tertiary sex ratio (Clark and Clark 1987). In these cases, the bias toward males is usually higher (Calonje et al. 2011). However, most cumulative studies indicate no significant differences in genotypic or primary sex ratio, either for living collections (Ornduff 1987; Calonje et al. 2011) or in situ populations (Grobbelaar 2002; Octavio-Aguilar et al. 2017), tending toward equality in the sex ratio.
Still with regard to cycads, studies showed that, whereas the gender ratio appears to change as cycad populations decrease in size, large and vigorous populations of Encephalartos spp. have a sex ratio of approximately 1:1 (as reviewed by Cousins and Witkowski 2017). Although the potential causes of deviations in the tertiary sex ratio include a higher mortality of older females relative to males as suggested by Grobbelaar et al. (1989), and strong male-biased ratios in small cycad populations, attributed to selective harvesting of females by cycad collectors, such as in South Africa (as reviewed by Cousins and Witkowski 2017), these factors do not seem applicable to Z. boliviana. However, we found that up until ~ 50 years ago, the DES and NP site areas were used for crops, with frequent weeding and cutting of vegetation at ground level. These activities probably stimulated individuals to branch and form a clonal xylopods tendency intensified by Cambisol characteristics. These conditions, together with the fire that occurred in those populations, may have combined to increase the frequency and reproductive asynchronicity of the sexes, facts that also concur with the sampling method, and consequently may have contributed to the male bias in these populations.
Finally, the tertiary sex ratio results of this study may have been influenced by a set of deterministic biological and ecological factors, but also by stochastic events, such as precious cropping and fires (Table 1), affecting the cycad populations. Although in some cycads strobili may be regulated, or at least influenced, by summer fires (Jones 2002), no study has been conducted to specifically assess the effects of fire on plants of Z. boliviana. However, Z. boliviana seems well adapted to some habitat conditions because the energy reserves in the underground organs allow it to develop new shoots when the aerial parts are lost to fire, brevideciduous events (Fig. 2g-h), or other related causes. These effects may interact with other aspects deterministic of life history, such as longevity, or stochastic to cause amplification of the sex ratio bias in recurrent episodes of reproduction (Field et al. 2012). This is supported since the biased sex ratio may impact the dioecious species’ viability by reducing the effective reproductive population size, influencing female fecundity and/or decreasing pollen availability through an increase in the distance between reproductive individuals (Riba-Hernández et al. 2014; Octavio-Aguilar et al. 2017; Lazcano-Lara and Ackerman 2018).
Sex ratio correlation with environmental factors
With regard to cycad environmental conditions, our results have shown that Z. boliviana can occur in a considerable diversity of soil types and their physical and chemical compositions. The Cerrado’s soils among our studied sites are characterized, in general, as alkaline (Reatto et al. 2008), with low natural fertility but good structure, and are predominantly sandy and well drained (SEPLAN 2000). Zamia boliviana also seems well adapted to intermediate levels of incident light, inherent to the climatic seasonality of the Cerrado. The pedological zone where this cycad occurs also includes Oxisols, mostly dystrophic and acidic but with good water permeability; Cambisols, chemically dystrophic with low chemical fertility and a predominantly sandy texture; Plinthosols, generally compact but hydromorphic with great variability in their chemical properties; and Neosols, shallow soils associated with rock outcrops (e.g. in this study, limestone rocks), high levels of primary minerals, morphologically quite heterogeneous, little evolved, and with an arbitrary depth to less than 50 cm (Reatto et al. 2008).
The presence of Z. boliviana in different environmental contexts suggests occupation and growth in a wide variety of habitats throughout its distribution, revealing fitness for, and ecological plasticity to adapt to, different micro-conditions, with no interference in sex ratio. For instance, the absence of an association between incident light and the tertiary sex ratio probably reflects the adaptability of Z. boliviana to their habitat of occurrence. This was also found in dioic Mercurialis perennis EU., where no correlation was found between light and the sex ratio (Vandepitte et al. 2009). The study of Nicotra (1998), with Siparuna grandiflora (Kunthin Hub. & Bonpl., A. DC.,) Siparunaceae, showed that the light availability affected reproductive activity in both sexes.
The coarse sand was the only soil attribute that separated Z. boliviana populations and seems to reflect the soil typology of the cycad habitats. In particular, the populations BV, BVB, NP, and DES occur in habitats composed predominantly of sandy soils, typical of Cambisols, which probably influences the fitness of Z. boliviana, a matter for future research. Given the assumed differences in the energy expenditure of the sexes and the occurrence of these populations in characteristically sandy habitats, sandy soils may drive differences in the allocation of resources between the sexes. Male plants in these sites would meet their energy requirements for vegetative and reproductive functions and maintain a more frequent reproduction without interruption. In contrast, female plants have additional requirements, which can lead to irregular reproduction, accentuating the male bias in those populations.
Therefore, physiological differences between males and females can influence the sex ratio within a population when environmental conditions are heterogeneous (as reviewed in Krieg et al. 2017). The classic study of Ornduff (1987) with populations of Zamia pumila L. was the first to record the idea that soil quality affects the primary sex ratio. This researcher found that, in shallow, poor soils, populations were male biased, and females reproduced less frequently. In sites with more favorable soils, the proportion of males to females was equal, and females reproduced more frequently. Together with these factors, we presume that on an ecological scale, stochastic forces and the founder effect (e.g. small populations consisting predominantly of either males or females) can also act on natural habitats and cause a biased sex ratio. Variation in population density, the spatial distribution of sexes, resource availability, the structure of the founder population, pollinator visits, anthropic actions, spontaneous colonization, and genetic and demographic characteristics are examples of such forces we want to highlight. These factors were also pointed out as relevant in other species (Cunha et al. 2014; Zhang et al. 2015) and cycad studies (Pérez-Farrera et al. 2017; Mankga and Yessoufou 2017; Octavio-Aguilar et al. 2017).
It should be noted that the small number of populations sampled in our study (N = 10) is common for the rare, restricted Z. boliviana and other cycads. According to Yu and Lu (2011) and Vandepitte et al. (2009), the male-biased sex ratio may be quite pronounced in small populations and corroborate our results, since Z. boliviana populations are mostly small and fragmented. Small endemic populations, such as in Z. boliviana, are especially vulnerable to changes in land use and habitat loss (Lopez-Gallego 2015; Segalla et al. 2019). This fact further reduces the probability of finding a superior N and is an obstacle for future study. Additionally, this fact limits opportunities to detect robust patterns, assess the health of populations, and to distinguish which forces act most strongly to cause sex ratio deviations in long-living tropical plants. Despite the challenges, long-term studies that consider each species separately as a unique case study, combining biological, ecological, and functional approaches, are promising and will help improve our knowledge, as emphasized by Juvany and Munné-Bosch (2015).
Conclusions
The male-biased sex ratio dominates in most in situ populations of Z. boliviana. However, environmental variables did not explain the biased sex ratios in this cycad. The biased sex ratios are likely explained by the differential energy expenditure of sexes in reproduction. Our study is the first systematic assessment of the tertiary sex ratio for Zamia and other dioecious species from central South America. It showed the current tertiary sex ratio pattern of a Zamia species, in a scenario of continuing decline in the extent and/or quality of habitats of cycad populations (see Calonje et al. 2021). Our finding may help us improve the understanding of the mechanisms causing biased sex ratios in dioecious species (e.g. impact of costs of reproductions). Based on the findings and also the limitations of this study, we highlight three general considerations to guide others’ research in dioecious species from tropical ecosystems: (i) a precise determination of the stages at which the sexual bias occurs and the factors influencing the survival, growth, and flowering of females and males of in situ populations (e.g. Barrett 2002; Stehlik and Barrett 2005; Calonje et al. 2011; Field et al. 2012; Khorsand Rosa et al. 2014); (ii) genomic approaches should provide accurate methods for the rapid identification of large numbers of sex-specific markers (e.g. Prakash and Van Staden 2006; Field et al. 2012). The different genomic techniques currently available may help identify several ecological processes within sex ratio determination, including cycad populations (on primary, secondary, and tertiary levels), patterns of gene exchange by seeds and pollen, population structure, and species cohesion (e.g. Barrett 2002; Sharma et al. 1998, 2004; Leal et al. 2016; Pinheiro et al. 2018); and (iii) finally, the mapping and understanding of habitats of cycads and other dioecious species in South America must be improved (e.g. Lopez-Gallego 2015; Segalla et al. 2019).
References
Ackleh AS, Zhang P (2009) Competitive exclusion in a discrete stage-structured two species model. MMNP 4:156–175. https://doi.org/10.1051/mmnp/20094606
Allen GA, Antos JA (1988) Relative reproductive effort in males and females of the dioecious shrub Oemleria cerasiformis. Oecol 76:111–118
Allen GA, Antos JA (1993) Sex ratio variation in the dioecious shrub Oemleria cerasiformis. Am Nat 141:537–553
Antos JA, Allen GA (1990) A comparison of reproductive effort in the dioecious shrub Oemleria cerasiformis using nitrogen, energy and biomass as currencies. Am Midl Nat 124:254–262. https://doi.org/10.2307/2426174
Antos JA, Allen GA (1994) Biomass allocation among reproductive structures in the dioecious shrub Oemleria cerasiformis—A functional interpretation. J Ecol 82:21–29. https://doi.org/10.2307/2261382
SEPLAN (Secretaria de Estado de Planejamento) (2000) Aspectos geomorfológicos da folha vila Guarita –MIR-300 (SC.21-Z-B) - Memória Técnica. Portal de Dados e Metadados Geográficos do Estado de Mato Grosso. http://metadados.seplan.mt.gov.br/metadados/srv/por/catalog.search#/home. Accessed 5 February 2020.
Barrett SCH (2015) Influences of clonality on plant sexual reproduction. PNAS 112:8859–8866. https://doi.org/10.1073/pnas.1501712112
Barrett SCH (2002) The evolution of plant sexual diversity. Nat Rev Genet 3:274–284. https://doi.org/10.1038/nrg776
Barrett SCH, Yakimowski SB, Field DL, Pickup M (2010) Ecological genetics of sex ratios in plant populations. Philos Trans R. Soc Lond, B, Biol Sci 365:2549–2557. https://doi.org/10.1098/rstb.2010.0002
Bawa KS, Beach JH (1981) Evolution of sexual systems in flowering plants. Ann Mo Bot Gard 68:254–274
Borcard D, Gillet F, Legendre P (2018) Numerical Ecology with R! Springer International Publishing, Cham, Use R
Calonje C, Calonje AM, Husby CE (2011) Cycad sex ratios at Montgomery Botanical Center. In: Cycad Biology and Conservation: Proceedings of 9th International Conference on Cycad Biology, The New York Botanical Garden, NYBG Press, Southern Boulevard, Bronx, NY, pp 360–370.
Calonje M, Stevenson DW, Osborne R (2013–2021) The World List of Cycads. http://www.cycadlist.org. Accessed 26 January 2021.
Clark DA, Clark DB (1987) Temporal and Environmental Patterns of Reproduction in Zamia Skinneri. A Tropical Rain Forest Cycad J Ecol 75:135–149. https://doi.org/10.2307/2260540
Cousins SR, Witkowski ETF (2017) African cycad ecology, ethnobotany and conservation: a synthesis. Bot Rev 83:152–194. https://doi.org/10.1007/s12229-017-9183-4
da Cunha NL, Fischer E, Lorenz-Lemke AP, Barrett SCH (2014) Floral variation and environmental heterogeneity in a tristylous clonal aquatic of the Pantanal wetlands of Brazil. Ann Bot 114:1637–1649. https://doi.org/10.1093/aob/mcu181
Dane JH, Hopmans JW (2002) Pressure plate extractor. In: Dane JH, Topp EC (eds) Methods of soil analysis part 4: physical methods. SSSA Book Ser. 5, SSSA, Madison, WI, pp 688–690
Delph LF (2019) Water availability drives population divergence and sex-specific responses in a dioecious plant. Am J Bot 106:1–10. https://doi.org/10.1002/ajb2.1359
FAO (Food and Agriculture Organization) (2006) Guidelines for soil description, 4th edn. Food and Agriculture Organization of the United Nations, Rome
Field DL, Pickup M, Barrett SCH (2012) Comparative analyses of sex-ratio variation in dioecious flowering plants. Evolution 67:661–672. https://doi.org/10.5061/dryad.28kd1
Freeman DC, Doust JLA, El-Keblawy A, Miglia KJ, McArthur ED (1997) Sexual specialization and inbreeding avoidance in the evolution of dioecy. Bot Rev 63:65–92
Galvani E, Lima NGB (2014) Fotografias hemisféricas em estudos microclimáticos: Referencial teórico-conceitual e aplicações [Hemispherical photographs in microclimatic studies: theoretical-conceptual and applications]. Ciência e Natura 36:215–221. https://doi.org/10.5902/2179460X13216
Geiger R (1954) Classification of climates after W. Köppen. In: Geiger R (ed) Landolt-Börnstein–Zahlenwerte und Funktionen aus Physik, Chemie, Astronomie, Geophysik und Technik, alte Serie. Springer, Berlin
Geodakyan SV (2012) Two Sexes. Why? The evolutionary theory of sex. Amazon, CreateSpace, Wilmington, DE
Grobbelaar N (2002) Cycads: with special reference to the southern African species. South Africa, Pretoria
Hayashi AH, Appezzato-da-Glória B (2007) Anatomy of the underground system in Vernonia grandiflora Less. and V. brevifolia Less. (Asteraceae). Braz Arch Biol Technol 50:979–988
Jones DL (2002) Cycads of the World. Smithsonian Institution Press, Washington
Juvany M, Munné-Bosch S (2015) Sex-related differences in stress tolerance in dioecious plants: a critical appraisal in a physiological context. J Exp Bot 66:6083–6092. https://doi.org/10.1093/jxb/erv343
Käfer J, Marais GAB, Pannell JR (2016) On the rarity of dioecy in flowering plants. Mol Ecol 26:1225–1241. https://doi.org/10.1111/mec.14020
Karger DN, Conrad O, Böhner J, Kawohl T, Kreft H, Soria-Auza RW, Zimmermann N, Peter Linder H, Kessler M (2017) Climatologies at high resolution for the earth’s land surface areas. Sci Data 4:170–122
Khorsand Rosa R, Barbosa RI, Koptur S (2014) Which factors explain reproductive output of Mauritia flexuosa (Arecaceae) in forest and savanna Habitats of Northern Amazonia? Int J Plant Sci 175:307–318
Köppen W (1918) Klassification der Klimate nach Temperatur, Niederschlag and Jahreslauf. Petermanns Geogr Mitt 64(193–203):243–248
Kottek M, Grieser J, Beck C, Rudolf B, Rubel F (2006) World Map of the Köppen-Geiger climate classification updated. Meteorol Z 15:259–263
Krieg C, Watkins JE, Chambers S, Husby CE (2017) Sex-specific differences in functional traits and resource acquisition in five cycad species. AoB Plants. https://doi.org/10.1093/aobpla/plx013
Lang M, Kuusk A, Mõttus M, Rautiainen M, Nilson T (2010) Canopy gap fraction estimation from digital hemispherical images using sky radiance models and a linear conversion method. Agr Forest Meteorol 150:20–29. https://doi.org/10.1016/j.agrformet.2009.08.001
Lazcano-Lara JC, Ackerman JD (2018) Best in the company of nearby males: female success in the threatened cycad Zamia portoricensis. PeerJ 6:e5252. https://doi.org/10.7717/peerj.5252
Leal BSS, Palma-Silva PF (2016) Phylogeographic studies depict the role of space and time scales of plant speciation in a highly diverse Neotropical region. Crit Rev Plant Sci 35:215–230. https://doi.org/10.1080/07352689.2016.1254494
Legendre P, Oksanen J, ter Braak CJF (2011) Testing the significance of canonical axes in redundancy analysis. Methods Ecol Evol 2:269–277. https://doi.org/10.1111/j.2041-210X.2010.00078.x
Lopez-Gallego C (2015) Plan de acción para la conservación de las Zamias de Colombia. Bogotá D.C.: Colombia. Ministerio de Ambiente y Desarrollo, Sostenible: Universidad de Antioquia, Colombia.
Majerus MEN (2003) Sex wars: genes, bacteria, and biased sex ratios. Princeton University Press, Princeton, NJ
Mankga LT, Yessoufou K (2017) Factors driving the global decline of cycad diversity. AoB Plants 9:1–10. https://doi.org/10.1093/aobpla/plx022
Morellato LPC (2004) Phenology, sex ratio, and spatial distribution among dioecious species of Trichilia (Meliaceae). Plant Biol 6:491–497. https://doi.org/10.1055/s-2004-817910
Nicotra AB (1998) Sex ratio variation and spatial distribution of Siparuna grandiflora, a tropical dioecious shrub. Oecologia 115:102–113. https://doi.org/10.1007/s004420050496
Octavio-Aguilar P, Iglesias-Andreu LG, de Cáceres-González FN, Galván-Hernández DM (2017) Fine-Scale Genetic Structure of Zamia furfuracea: Variation with Life-Cycle Stages. Int J Plant Sci 178:57–66. https://doi.org/10.1086/689200
Oksanen J, Blanchet FG, Friendly M et al (2019) Vegan: Community Ecology Package. R package version 2.5–6. https://CRAN.R-project.org/package=vegan
Oliveira PE (1996) Dioecy in the Cerrado vegetation of Central Brazil. Flora 191:235–243. https://doi.org/10.1016/S0367-2530(17)30718-1
Oliveira-Filho AT, Ratter JA (2002) Vegetation physiognomies and woody flora of the Cerrado biome. In: Oliveira PS, Marquis RJ (eds) The Cerrados of Brazil: ecology and natural history of a Neotropical savanna. Columbia University Press, New York, pp 121–140
Olson DM, Dinerstein E, Wikramanayake ED et al (2001) Terrestrial ecoregions of the world: a new map of life on Earth. Bioscience 51:933–938. https://doi.org/10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2
Ornduff R (1987) Sex ratios and coning frequency of the cycad Zamia pumila L (Zamiaceae) in the Dominican Republic. Biotropica 19:361–364
Pérez-Farrera MA, Vovides AP, González D, López S, Hernandez-Sandoval L, Martínez M (2017) Estimation of genetic variation in closely related cycad species in Ceratozamia (Zamiaceae: Cycadales) using rapds markers. Rev Biol Trop 65:303–319. https://doi.org/10.15517/rbt.v65i1.15266
Pinheiro F, Dantas-Queiroz MV, Palma-Silva C (2018) Plant species complexes as models to understand speciation and evolution: a review of South American studies. Crit Rev Plant Sci 37:54–80. https://doi.org/10.1080/07352689.2018.1471565
Prakash S, Van Staden J (2006) Sex identification in Encephalartos natalensis (Dyer and Verdoorn) using RAPD markers. Euphytica 152:197–200. https://doi.org/10.1007/s10681-006-9198-0
QGIS Development Team (2019) Geographic Information System 3.4. Open Source Geospatial Foundation Project. https://qgis.osgeo.org. (accessed 8 Oct 2019).
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Reatto A, Correia JR, Spera ST (2008) Solos do Bioma Cerrado: aspectos pedológicos. In: Sano SM, Almeida SP (eds) Cerrado: ambiente e flora. Planaltina, EMBRAPA-CPAC, pp 107–149
Renner SS (2014) The relative and absolute frequencies of angiosperm sexual systems: Dioecy, monoecy, gynodioecy, and an updated online database. Am J Bot 101:1588–1596. https://doi.org/10.3732/ajb.1400196
Renner SS, Ricklefs RE (1995) Dioecy and its correlates in the flowering plants. Am J Bot 82:596–606. https://doi.org/10.1002/j.1537-2197.1995.tb11504.x
Riba-Hernández P, Segura JL, Fuchs EJ, Moreira J (2014) Population and genetic structure of two dioecious timber species Virola surinamensis and Virola koschnyi (Myristicaceae) in southwestern Costa Rica. For Ecol Manage 323:168–176. https://doi.org/10.1016/j.foreco.2014.03.018
Ribeiro JF, Walter BMT (2008) As principais fitofisionomias do Bioma Cerrado. In: Sano SM, Almeida SP (eds) Cerrado: ambiente e flora. Planaltina, Brazil, EMBRAPA-CPAC, pp 151–212
Segalla R, Calonje C (2019) Zamia brasiliensis, a new species of Zamia (Zamiaceae, Cycadales) from Mato Grosso and Rondônia, Brazil. Phytotaxa 404:001–011. https://doi.org/10.1007/s10531-018-1555-5
Segalla R, Telles FJ, Pinheiro F, Morellato P (2019) A Review of current knowledge of Zamiaceae, with emphasis on Zamia from South America. Trop Conserv Sci 12:1–21. https://doi.org/10.1177/1940082919877479
Sharma IK, Jones DL, Forster PI, Young AG (1998) The extent and structure of genetic variation in the Macrozamia pauli-guilielmi complex (Zamiaceae). Biochem Syst Ecol 26:45–54
Sharma IK, Jones DL, Forster PI (2004) Genetic differentiation and phenetic relatedness among seven species of the Macrozamia plurinervia complex (Zamiaceae). Biochem Syst Ecol 32:313–327. https://doi.org/10.1016/j.bse.2003.07.002
Soares, R (2020) Fenologia, ecologia reprodutiva e razão sexual de Zamia boliviana (Brongn.) A. DC. (Cycadales, Zamiaceae): uma cicada tropical rara e ameaçada. Ph.D. Dissertation, Universidade Estadual Paulista (UNESP), Instituto de Biociências, Rio Claro, São Paulo, Brazil.
Sokal RR, Rohlf FJ (2011) Biometry, 4th edn. Freeman, New York, W. H
Stehlik I, Barrett SCH (2005) Mechanisms governing sex-ratio variation in dioecious Rumex nivalis. Evolution 59:814–825. https://doi.org/10.1554/04-417
Stehlik I, Kron P, Barrett SCH, Husband BC (2007) Sexing pollen reveals female bias in a dioecious plant. New Phytol 175:185–194. https://doi.org/10.1111/j.1469-8137.2007.02093.x
Tang W (1990) Reproduction in the cycad Zamia pumila in a fire-climax habitat – an 8 year study. Bull Torrey Bot Club 117:368–374. https://doi.org/10.2307/2996834
Vandepitte K, Honnay O, De Meyer T, Jacquemyn H, Roldán-Ruiz I (2009) Patterns of sex ratio variation and genetic diversity in the dioecious forest perennial Mercurialis perennis. Plant Ecol 206:105–114. https://doi.org/10.1007/s11258-009-9627-y
Vourlitis GL, Lobo FA, Biudes MS, Rodríguez Ortíz CE, Nogueira JS (2011) Spatial variations in soil chemistry and organic matter content across a invasion front in the Brazilian Pantanal. Soil Sci Soc Am J 75:1554. https://doi.org/10.2136/5S5aj2010.0412
Wei T, Simko V (2017) R package “corrplot”: Visualization of a Correlation Matrix (Version 0.84). https://github.com/taiyun/corrplot.
Yu L, Lu J (2011) Does landscape fragmentation influence sex ratio of dioecious plants? A case study of Pistacia chinensis in the Thousand-Island Lake Region of China. PLoS ONE 6:e22903. https://doi.org/10.1371/journal.pone.0022903
Zhang X, Zhang C, Zhao X (2014) Effect of sex ratio, habitat factors and neighborhood competition on stem growth in the dioecious tree Fraxinus mandshurica. Ecol Res 29:309–317. https://doi.org/10.1007/s11284-013-1125-y
Zhang X, Pu Z, Li Y, Han X (2015) Stochastic processes play more important roles in driving the dynamics of rarer species. Plant Ecol 9:328–332. https://doi.org/10.1093/jpe/rtv058
Acknowledgements
RS thanks the Instituto Federal de Educação, Ciência e Tecnologia de Mato Grosso for allowing the development of the doctoral thesis in the “Programa de Pós-graduação em Biologia Vegetal, UNESP–Rio Claro.” This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brazil (CAPES)–Finance Code 001, and by the National Council for Scientific and Technological Development (CNPq). The authors thank Pró-Reitoria de Pesquisa–UNICAMP – for the language services provided. LPCM receives a research productivity fellowship from CNPq and the Phenology Lab facilities are supported by the São Paulo Research Foundation (FAPESP) grant to LPCM. GJB is grateful for a postdoctoral fellowship awarded by CAPES/PNPD/UFVJM (Process Number 88887.352134/2019-00). We also thank Juberto Babilônia de Souza (IFMT), Eduardo Guimarães Couto and Maria Hunter of Universidade Federal de Mato Grosso for help with soil identification and interpretation. The authors are grateful to the landowners for allowing access to cycad populations and to Wynand Van Eeden and anonymous reviewers for helpfully improving this manuscript.
Author information
Authors and Affiliations
Contributions
RS and LPCM conceived and designed the study; RS conducted all fieldwork; RS and GJB analyzed and interpreted data. RS wrote the first draft of the manuscript. RS, FP, and LPCM contributed to further versions of the manuscript. All the authors have read and agreed the final content of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Claus Holzapfel.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Segalla, R., Pinheiro, F., Barônio, G.J. et al. Male-biased effective sex ratio across populations of the threatened Zamia boliviana (Zamiaceae). Plant Ecol 222, 587–602 (2021). https://doi.org/10.1007/s11258-021-01127-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-021-01127-3