Abstract
This study intended to clarify whether low irradiance, high temperature or both acted as a trigger inducing summer dormancy in Daphne pseudomezereum (Dpm), a wintergreen shrub native to Japanese mixed forests. A 2-year growth chamber experiment was conducted in Otsu, Japan where, in the first year, 3 provenances of Dpm were raised in two irradiance levels by two temperature regimes to observe their effects on leaf phenology. The results showed a clear effect of temperature, but not irradiance, on leaf phenology. In the second year, the experiment was repeated using different Dpm plants and applying only the two temperature regimes. The results confirmed those obtained in the first year, where the temperature regime that tracked normal field condition induced summer dormancy similar to field populations. In both years, when plants were kept over the summer under a cool temperature regime mimicking field conditions in April, most plants did not undergo summer dormancy (i.e., becoming “evergreen”). In contrast to phenology, leaf morphology (i.e., LMA), and photosynthetic capacity (e.g., Amax) did respond to irradiance levels consistent with shade adaptive changes, but were not affected by temperature. Simulated carbon gain using previously determined parameters for Dpm and the chamber microclimate data found that only plants in the warm treatment experienced carbon deficit in mid-summer. These findings suggest that summer temperature alone and the attendant rise in respiration can alter internal carbon balance and trigger the onset of summer dormancy in Dpm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leaf functions have an overwhelming influence on plant growth and survival. It is thought that leaf functions are optimized through phenological control of the timing and duration of leaf presence on a plant (Jurik and Chabot 1986; Kikuzawa and Lechowicz 2006). Most perennial plants undergo cycles of leaf change during their life time, the main reasons for this appear to be (1) to avoid maintaining energetically unprofitable leaves during periods of environmental stress, and (2) to replace older photosynthetically inefficient leaves by reallocating resources to new active ones (Chabot and Hicks 1982; Kikuzawa and Lechowicz 2011). The signal for leaf exchange would therefore be the onset of energetically unprofitable period for maintaining existing leaves and the decline in carbon gain in older leaves. Although leaf phenology is clearly regulated by a number of environmental and endogenous cues, such as aridity and winter freeze, we still do not know the precise trigger and fitness outcome of leaf phenology (e.g., Kikuzawa and Lechowicz 2006; Woo et al. 2013). The recent reports of accelerated and extended timing of leaf and flower phenology highlight the sensitivity of plants to warming temperatures (Korner and Basler 2010; Diez et al. 2012; Fridley 2012). Matching leaf phenology with the best period for carbon gain is clearly a critical factor in a plant’s fitness and competitive strategy. For example, spring ephemerals take advantage of the brighter early spring and avoid the onset of canopy shade (Augspurger et al. 2017). A longer growing season afforded by a longer leafing period has been attributed to the success of invasive species (Fridley 2012, but see Smith and Palmer 2013) or improve whole plant carbon gain. However, phenological responses of species must weigh the risks of longer leaf lifespan (such as risking damage from early spring freeze with the benefit of greater carbon gain). Thus, responding to and managing risks during periods of unfavorable period is of primary concern for a plant.
For temperate-deciduous species, falling temperatures or a shortened photoperiod are known triggers of leaf senescence to avoid the winter freeze. In the case of the curious Daphne psudomezereum A. Gray. (Dpm), however, the onset of winter is met with a fresh sprout of autumn leaves. These leaves are maintained through winter and shed in mid-summer (Fig. 1) and the plant enters a period of summer dormancy. Our research has suggested that this unusual leaf habit is an adaptation to surviving in the temperate forest despite its ecophysiological intolerance of the warm summer shade (Lei et al. 2020). In that study, we showed that such adaptation involved a complex interaction between light and temperature in the environment which makes whole plant replacement of leaves in late summer energetically beneficial and sustainable. Given our assumption that the trigger for avoiding the unfavorable summer period through dormancy is low irradiance and high temperatures, we wondered if changing temperature or light regimes would alter Dpm’s normal leaf phenological response. Leaf phenology has been found to respond to both temperature (Tanino et al. 2010) and irradiance where tropical plants showed longer leaf longevity under experimentally reduced irradiance (Vincent 2006). Just as some temperate-deciduous plants can keep their leaves over winter if the trigger for the onset of winter is removed, could Dpm be induced to keep its leaves over summer by providing them with a brighter or cooler environment over the summer? This study will test the hypothesis that by removing factors linked to unprofitable carbon gain, Dpm would extend its leaf longevity, and render summer dormancy unnecessary.
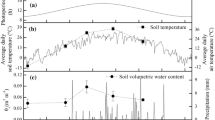
Leaf phenology of a representative Daphne pseudomezereum plant grown in the warm treatment (2012) showing leaf numbers on a terminal and a lateral shoot. Field leaf phenology was based on a census of individual shoots of the Ibu population (Lei et al. 2020) over several seasons. The curve fitting the field phenology data is a 4th-order polynomial
To ascertain that the phenological responses to conditions imposed in the experiments are general in nature, individuals from several geographical locations were used in this 2-year study. In addition to D. pseudomezereum occurring in south and central Japan, D. jezoensis (Dj), with a northern distribution was also included as both species exhibit the unusual summer-dormant leaf phenology (Lei and Koike 1998; Lei et al. 2020) and both are low shrubs found under deciduous or mixed forest canopy. The recent molecular evidence suggests that these two species do not show sufficient taxonomic differences (Kawahara, unpublished data), and; therefore, can be treated as southern (Dpm) and northern (Dj) provenances of a single species. Species names follow those accepted by the Plant List (2010) (theplantlist.org).
In addition to monitoring changes in leaf phenology, I also measured leaf-level traits including gas exchange and leaf construction (i.e., LMA) as these traits reflect the overall carbon economy of the leaf and can affect leaf phenology and longevity (Lei et al. 2020). Floral development was also recorded since flowering is a well-known temperature-dependent process. Lastly, I simulated the effect of experimental conditions on monthly carbon gain and assessed the connection between leaf carbon budget and leaf phenology.
Materials and methods
Two separate growth chamber experiments were conducted in 2011 and 2012. The 2011 experiment used 28 D. jezoensis (Dj) plants raised from seeds collected from one population in Sapporo (43° N) and 30 D. pseudomezereum (Dpm) plants, also raised from seeds collected from two populations (Dpm-Ibu and Dpm-Ryo) in Shiga Prefecture, western Japan (both 35° N; Lei et al. 2020). Plants were raised in a common garden (canopy opening at 6.2% and received 7 min of direct sunlight on July 22) for 2–3 years prior to the experiment conducted at Ryukoku University (34° 58′ N; 135° 56′ E). Individually potted plants were moved into two growth chambers on February 10, 2011. The growth chambers (Conviron A1000, Conviron, Winnipeg) each had two illuminated shelves; the plants were randomly allocated to four treatment groups and plants in each group were placed in one of the 2-chamber × 2-shelf setup. Initially, both chambers received the same daily irradiance and temperature settings specified for each month (February to April, Table S1) which track microclimate conditions of a natural D. pseudomezereum population in Shiga (Ibu site, Lei et al. 2020). The monthly regime was reset at the beginning of each month. At the beginning of March, an irradiance treatment was imposed where shade cloth was drawn under the fluorescent lamps of one shelf in each chamber, reducing the irradiance to ca. 30% of the unshaded shelf (e.g., from a daily mean of 90–110 μmol m−2 s−1 to 20–40 μmol m−2 s−1 PAR, photosynthetically active radiation). The two irradiance treatments are referred to henceforth as “ambient” (no shade cloth) and “shade” (with shade cloth). Plants in the two irradiance treatments continue to share a common temperature setting continued until the end of April before temperature settings diverged between chambers. On May 2, 2011, one chamber continued to track the increasing ambient temperatures of Ibu (warm treatment) and the other chamber was kept at the April setting for the remainder of the year (cool treatment, Table S1). As a result, the warm-ambient treatment tracked closest to actual field conditions while warm-shade simulated a dimmer environment at the lower range of irradiance for a Dpm habitat. Plants raised in the cool treatments never experienced daytime temperatures greater than 14 °C (versus 25 °C in August for warm) and were kept at 8 °C at night for the entire summer period (versus 22 °C in August for warm). To monitor leaf phenology, at the start of the experiment, one shoot (terminal or lateral) of each plant was randomly selected, and all leaves on the shoot were numbered and recorded. Subsequently, at 2 to 3-week intervals, the tagged shoots were repeated censused for presence (surviving) or absence (leaf senesced) of existing leaves and newly emerged (recruited) leaves. New leaves are recorded when they reach 1 cm in length. Observations continued until Nov. 15 when emergence of the autumn leaf flush has ceased. The presence of flower buds was also monitored and recorded for each plant throughout the study period. The sample sizes of each temperature × PAR treatment by seed provenance were warm-ambient (Dj = 7, Dpm-Ryo = 5, Dpm-Ibu = 3), warm-shade (Dj = 7, Dpm-Ryo = 5, Dpm-Ibu = 2), cool-ambient (Dj = 7, Dpm-Ryo = 5, Dpm-Ibu = 3), and cool-shade (Dj = 7, Dpm-Ryo = 5, Dpm-Ibu = 2).
In 2012, a follow-up experiment with a larger sample size of 32 Daphne pseudomezereum plants was carried out. To avoid using plants already subjected to the experimental conditions in 2011, plants were collected from a population in Shiga Prefecture in November 2011. These plants were about 30 cm in height and estimated to be 3–4 years old and mature. The plants were excavated with substrate still intact around the roots, transported back to the university and transplanted into 10 × 10 × 15 cm pots on the same day. The pots were filled with commercial potting soil and placed in a common garden (see 2011 experiment) until February 2012. At the beginning of February, the plants were randomly assigned to one of two groups (2 temperature treatments: warm 17 individuals and cool 15 individuals) with plants in each group placed in separate Conviron A1000 chambers. From February to the end of April 2012, the two chambers were set to the same monthly irradiance and temperature regimes specified in Table S1. Similar to 2011 described above, temperature settings of the two chambers diverged on May 1 where one maintained the April conditions throughout summer (cool) and the other followed field (warm) conditions. Irradiance in both chambers were set to track natural field levels (i.e., as specified in Table S1). Leaf phenology was monitored weekly beginning in February 2012 and continued to December 2012. Each plant was assigned a number and all existing leaves on the plants were numbered and recorded beginning from the base of the shoot. Some plants had only a single main stem while others had one or more additional lateral stems, for the latter, leaf sequence on the lateral shoots were similarly marked and censused. Potted plants of both years were watered regularly, but were not fertilized during the experiment and there were no signs of nutrient deficiency.
Gas exchange and leaf morphological measurements
Using plants in the growth chambers, gas exchange measurements were made in November 2011 on one fully developed autumn-flushed leaf (ca. 5th–7th position from the shoot tip) per plant. Photosynthetic rates at saturating PAR (1000 μmol m−2 s−1, Amax), and at 100 μmol m−2 s−1 PAR (diffuse shade light, Adim) were determined with a LiCor 6400 Photosynthesis System (LI-COR, Lincoln, Nebraska) with a LED-fitted leaf chamber (Li6400-40). Measurements were taken at two temperatures that represent a cooler spring condition at 10.0 °C (chamber block temperature) and a higher summer condition at 20.0 °C; CO2 concentration was set at 380 ppm and RH kept at 50%. Following gas exchange measurements, the leaf was detached and several discs removed (using a cork borer with a diameter of 8.5 mm) from the central portion of the leaf, these were oven-dried at 70 °C for 48 h and used for leaf mass to area ratio (LMA) determination.
Simulation of carbon gain during the year
To further reveal the possible underlying cause for the expected phenological response, a simulation of daily carbon gain across the season was made using predetermined temperature- and PAR-dependent photosynthetic rates where a response surface of photosynthetic rates (Pn, z) generated from a series of temperatures (Temp, ×) × PAR (y) conditions were fitted to quadratic equations (see methods in Lei et al. 2020). These data were derived from five 2–3 year-old Dpm plants raised from seed in the same common garden as the 2011 individuals, but were not used in the experiment. To generate estimated daily carbon gain (per leaf area), the equations were applied to the chamber microclimate data (Table S1). To mimic age-related changes in leaf carbon gain across time, the simulation used age-corrected photosynthetic rates where fully expanded autumn leaves decrease in rates by 5% monthly beginning in October through to the following summer. The age corrections follow the methodology described in Lei et al. (2020).
Statistical procedures
To assess whether temperature or irradiance played a role in affecting dates of leaf senescence, autumn resprout, and the length of summer dormancy, data were analyzed using the non-parametric Kruskal–Wallis test. The frequencies of deciduous and evergreen leaf habits due to treatment effects were assessed using Pearson's chi-squared test with Yates' continuity correction given that the counts were integers with small cell numbers (R Core Team 2013). The effect of temperature and irradiance on leaf morphology (leaf area, leaf dry mass and LMA, 2011 experiment) were assessed using data from all provenances in a two-way ANOVA. When no treatment effects were detected, data were combined for analysis of remaining factors. Gas exchange measurements were analyzed in a similar manner. Orthogonal contrast was used to compare specific pairs among multiple treatment combinations.
Results
When Daphne pseudomezereum were grown under experimental conditions that mimicked its natural habitat (i.e., warm treatment 2012), they exhibited a leaf phenology that resembled those observed in the field (Fig. 1). The field data were based on a multiple-season census of leaf phenology made on individuals in a natural Dpm population (cf. Ibu site in Lei et al. 2020). Both experimental (shown by a representative 2012 warm-grown individual in Fig. 1) and field plants senesced at about the same time, but experimental plants tended to have a shorter dormancy and resprouted autumn leaves more rapidly than those in the field (Fig. 1). Although normally leaf senescence and leaf abscission constitute different developmental stages prior to dormancy (e.g., Munné-Bosch and Alegre 2004; Tanino et al. 2010), the distinction was not made for Daphne in this study given the short (a few days) duration between the two stages.
In 2011, phenological results, such as timing of summer dormancy and autumn leaf resprout did not differ statistically between Dpm plants from the two populations in western Japan. As a result, further analyses were made with combined data for the two populations. Regarding the effect of temperature or irradiance on the timing of summer dormancy, a clear difference was found between cool and warm treatments but not between irradiance levels. Plants grown in the cool regime tended to delay their leaf senescence, often to a time after the new autumn leaves have flushed, thus becoming evergreen. The higher prevalence of “evergreenness” was particularly evident in Dpm (Table 1). In the warm treatment, the tendency for the deciduous habit (defined as having a distinct period of total leaflessness) was particularly obvious in Dj. The frequency distribution of leaf habit between temperature treatments was clearer in Dj (χ2 = 5.211, df = 1, P = 0.022) than in Dpm (χ2 = 2.503, df = 1, P = 0.114). Irradiance regime; however, had no effect on the timing of summer dormancy (i.e., P = 0.71 for warm-shade vs warm-ambient and P = 0.60 for cool-shade vs cool-ambient).
In addition to differences in the frequencies of leaf habit, Dpm, and Dj were different in their timing of summer dormancy despite having been raised under the same temperature and light regimes. For Dj, dormancy began much earlier than Dpm 2011 (median date: JD 179 vs 235, Kruskal–Wallis test P = 0.026 in warm and JD 269 vs 297, P < 0.001 in cool treatments). Timing of resprout was mixed with Dj and Dpm occurring, respectively, at median date: JD 235 vs 252, Kruskal–Wallis test P < 0.001 in warm and JD 285 vs 252, P < 0.030 in cool treatments, Fig. 2a). There was much closer correspondence in the phenological scheduling between Dj and Dpm 2012.
Box and whisker plots of timing of complete leaf fall (LF) and autumn resprout (RS) pairs in Dj and Dpm (2011 and 2012 as indicated) grown under cool (blue bars) and warm (orange bars) conditions (a); and the length of dormancy in the two provenances where 0 day defines complete leaf fall coinciding with the beginning of new autumn resprout. Minus values indicate resprouting occurring before complete leaf fall, or “evergreenness” (b). Sample sizes were between 8 and 15 for each group except for LF and dormancy of cool-grown Dpm 2012 where only 3 individuals achieved complete leaf fall (see Fig. 3)
In terms of length of dormancy, plants in the warm regime had longer periods of leaflessness than those in the cool regime for both Dj and Dpm 2011 (Fig. 2b). For Dj, we see a longer and more distinct dormancy, i.e., from 0 (cool) to 32 days (warm), while Dpm showed a stronger absence of dormancy, i.e., from − 39 (cool) to 0 days (warm). Temperature effect on both provenances was significant (Kruskal–Wallis test P = 0.01 for Dj and P = 0.02 for Dpm). There was no irradiance effect on the length of dormancy. Dpm 2012 was more similar to Dj in dormancy but most Dpm 2012 in the cool treatment had no dormancy (Fig. 3).
Phenological responses of Daphne pseudomezereum to the two temperature growth regimes in the 2012 experiment. Plants in cool summer were grown under April temperature regime throughout the summer, warm summer plants were exposed to rising ambient temperatures during the summer tracking the meteorological conditions of a field population. For each plant, timing of complete leaf fall (open symbols) and date of first autumn resprout (green symbols) are shown. Plants without a gap between the two symbols did not undergo dormancy, plants without either symbols exhibited continuous leaf emergence through the summer
The single population 2012 experiment using transplanted Dpm confirmed that cool temperature during the summer months delayed summer leaf senescence significantly (df = 1, 27; F = 102.3; P < 0.001, Fig. 3). In addition, the delayed senescence led to 12 out of 15 cool-treatment plants sprouting before the last spring leaves were dropped rendering them effectively evergreen (Fig. 3). The three dormant plants in that treatment had a mean leafless period of 17 days. For the warm treatment, 14 of 17 plants exhibited dormancy with a mean length of 20 days and in two plants, leaf emergence continued throughout the summer.
Floral development response
Together with leaf phenology, all plants were censused for the presence of flower and flower buds during the experiment. Unexpectedly, floral development showed a strong response to temperature treatment. In spring 2011, plants assigned to each temperature treatment had similar numbers of flowering individuals with 11:18 (flowers present: absent) in the cool regime and 12:17 in the warm regime (χ2 = 0, df = 1, P = 1.0). By October 2011, the ratio of flowering to non-flowering individuals in the cool regime was 0:29 and in the warm regime it was 25:4 (χ2 = 40.5, df = 1, P < 0.001) indicating that flower bud production is strongly associated with having a distinct summer dormancy (warm treatment) while the lack of complete leaflessness appears to have inhibited floral initiation.
Leaf morphology and gas exchange
Individual leaf area and leaf dry mass were not affected by temperature or growth irradiance but there was a difference in leaf size among provenances where Dpm leaves from Ibu were larger (14.1 ± 2.7 cm2) and heavier (61.6 ± 6.7 mg) than D. jezoensis (8.9 ± 3.0 cm2 and 37.0 ± 12.5 mg), P = 0.005 for area and P = 0.001 for dry mass. For LMA, there was a significant effect of irradiance (4.92 ± 0.63 mg m−2 in ambient, 3.65 ± 0.51 mg m−2 in shade; P < 0.001) but not by temperature (P = 0.232), LMA did not differ among provenances (df = 2, 21, F = 0.218, P = 0.806).
Photosynthetic rates measured at 100 (Adim) and 1000 μmol m−2 s−1 (Amax) PAR were determined to answer the question: Is assimilation capacity of new leaves sprouted in the fall different between those that emerged after complete summer dormancy and those that sprouted before complete shedding of old leaves? Photosynthesis was measured at 10 °C and 20 °C, but no statistical difference was found for either Adim (P = 0.12) or Amax (P = 0.51); hence, the data of the two temperatures were combined for subsequent analyses. There was also no among provenance differences (F = 1.08, P = 0.35). Using combined data from all provenances, it was revealed that the growth irradiance had an effect on both Amax (7.53 ± 2.47 [ambient] > 5.39 ± 1.61 μmol m−2 s−1 [shade] and Adim (4.35 ± 1.29 [ambient] > 3.31 ± 1.29 μmol m−2 s−1 [shade]). ANOVA results were for Adim: df = 1,44; F = 7.47, P = 0.009; and for Amax: df = 1,45; F = 10.3, P = 0.002).
Regarding how plant growth rate might have been affected by different treatment regimes, measurements taken between March and November 2011 found relative growth rate (RGR) of stem basal diameter ranged between 0.37 and 0.44 mm year−1 among the four treatment combinations. There was no effect of either temperature (cool vs. warm, P = 0.38) or growth irradiance (ambient vs. shade, P = 0.76) on RGR.
Carbon gain simulation
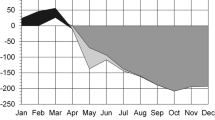
The simulation of daily carbon balance for Dpm grown in warm and cool chamber conditions showed a distinct between-treatment difference (Fig. 4) after the temperatures diverged in May. Although the age-corrected carbon gain declined in both treatments, it was much steeper in the warm regime descending into carbon deficit territory by mid-summer. Simulating the effect of low irradiance showed a similar trend between temperature treatments where the same separation in carbon balance was maintained.
Simulated daily carbon balance (mean ± SD, n = 5) of Dpm using experimental conditions of the months shown. Results ambient (orange) treatment descending in to carbon deficit in July and August while cool (blue) regime plants remain positive. Dotted lines show the same two treatments under additional shading (30% of the ambient level), error bars were omitted for visual clarity. Simulations were made using protocols described in Lei et al. (2020)
Discussion
The outcome of the 2011 experiment made clear that temperature was more important than irradiance in affecting the leaf phenology of D. pseudomezereum and the effect of temperature was confirmed in the 2012 experiment. By imposing summer conditions mimicking those in the field (i.e., warm treatment), chamber-grown plants displayed leaf fall, summer dormancy and rates of resprout similar to field observations (Fig. 1) with the exception of a shorter dormancy period in experimental plants. Although there were large variations in leaf phenology for warm-grown Dpm individuals in 2011 (Fig. 2, Table 1), other temperature by provenance responses support the conclusion that summer dormancy is mainly triggered by higher temperature in the environment. When plants were grown in the cool regime, they delayed leaf senescence beyond the time of autumn resprout resulting in an evergreen habit.
Effect of temperature on phenology
Temperature has been implicated as an important cue for initiating leaf senescence and dormancy. Higher night time temperature can have a preponderant effect on leaf phenology in poplar hybrids where warm autumn, in combination with a short-day photoperiod, can hasten growth cessation and induce deeper winter dormancy (Tanino et al. 2010). In contrast, some plants display an extended spring and fall phenology under warmer temperatures attributed to global warming (Peñuelas and Filella 2001; Menzel et al. 2006). Having an extended leaf phenology can confer an advantage in carbon gain and competitiveness (Friedley 2012; Augspurger et al. 2017), and Dpm could garner similar benefits by retaining its leaves over the summer. However, what sets Dpm apart from other studies is that it is responding to the risks and benefits of retaining leaves under the stress of low light and high temperature rather than that of drought and winter freeze. Simulations of carbon gain (Fig. 4) show Dpm grown in the warm regime suffered significant decline in daily carbon budget similar to earlier results based on the field data (Lei et al. 2020). These findings point to a reduced carbon gain facilitated by high cost of respiration under warm summer temperatures and the dormancy response is energetically consistent with the avoidance of excessive carbon deficit (Lei et al. 2020). When high temperature stress is absent, plants in the cool treatment can maintain positive carbon gains without triggering summer dormancy (Fig. 4).
Although the timing of autumn resprout was generally later under the cool treatment, it was not simply a shifting of dormancy to a later period relative to the warm treatment. In both experiments, autumn resprout in most plants did not occur later than ca. JD280 (or beginning of October) which suggests a temporal threshold for recruiting new leaves perhaps in sync with the opening of forest canopy prior to winter. This timing may be critical for Dpm as it retains its autumn leaves through winter. As many broadleaf perennials with cold-tolerant leaves are capable of photosynthesizing even at below freezing temperatures (Wingler 2014), so, it is conceivable that the time needed to acquire cold tolerance may be critical for Dpm. Since cold tolerance itself is associated with sugar accumulation in the leaves (Wingler 2014), we may speculate that timely flushed autumn leaves will allow Dpm to accumulate sufficient sugars under an open canopy before winter sets in. These water-soluble carbohydrates can act as cryoprotectants (Guy 1990) and their accumulation in leaves and the meristems also serve as antioxidants (Peshev et al. 2013) protecting tissue from winter damage. Interestingly, simple sugars also play a role as signaling molecules where an elevated concentration can delay leaf senescence and promote early budbreak (Park et al. 2009). For Dpm, in addition to the role of temperature, whether soluble sugars are also involved in the timing of summer dormancy and in leaf retention overwinter will be the focus of subsequent investigations.
Irradiance on phenology, gas exchange and leaf morphology
Although growth irradiance had no effect on leaf phenology, the two light levels were sufficient to produce differences in leaf traits (LMA, Amax, and Adim) that are consistent with shade acclimation. Because these traits were taken on autumn flushed leaves, they only demonstrate that irradiance had an effect on the development and expansion of these newly emerged leaves. However, if irradiance can alter these autumn leaf properties, we may also expect that increased shading will also have an effect on spring leaves prompting a deeper carbon deficit and hasten leaf fall. The lack of such a response may be explained by a similar pattern of carbon balance between ambient and shade regimes based on the simulated data (Fig. 4). However, the expectation of earlier leaf senescence under increased shade in Dpm runs contrary to other species that show prolonged leaf lifespan to shade due to slower leaf aging. Vincent (2006) found that low irradiance-grown tropical woody plants that displayed shade-adapted leaf morphology and rates of CO2 assimilation also had longer leaf lifespan than those grown under higher irradiance. Further research is needed to uncover the role of irradiance and its interaction with temperature in leaf phenology.
Effect of dormancy on reproduction
During the leaf phenology monitoring, it was noted that cool treatment plants did not produce any flower buds in the autumn while most of the warm treatment plants did. What does this unexpected result mean, does it suggest that the evergreenness under cool treatment inhibited floral development or is the cool temperature itself a factor in the reproductive process? Because all Dpm plants were subjected to the same diurnal cycle in the chambers, photoperiod, a major determinant of floral initiation (Andrés and Coupland 2012), did not play a role. Temperature, particularly vernalization, is also critical to floral opening, but does not appear relevant to this study. The lack of complete leaf fall in the cool regime though could mean reduced recovery and re-allocatable resources for reproduction. Furthermore, as a resource demanding process, flower bud formation (beginning in September) would be competing directly for resources against the temporally synchronous autumn leaf production. While the link between the vegetative and floral processes remains unknown, suggestions that both flowering and leaf phenology are regulated by common hormones (i.e., florigen, Shalit et al. 2009) is an area of potential interest.
Life history significance
Traditionally, being deciduous or evergreen has been viewed as a fundamental dichotomy in leaf habit with associated functional trade-offs (Kikuzawa and Lechowicz 2011). However, in this study, evergreenness appears to be simply an artifact of the shifted timing of leaf fall and resprout. As such, perhaps the dichotomy is better viewed as a continuum determined by the processes that come before and after leaf dormancy (Fig. 3). If winter-deciduous plants can gain fitness by avoiding cold stress and injury through an optimally timed dormancy (Tanino et al. 2010), then there is clear benefit for Dpm in prolonging leaf longevity when the risk of carbon deficit (such as a cooler environment) is alleviated. We may also interpret the loss of dormancy in Dpm as a form of relaxed selection (Lahti et al. 2009) whereby a weakened stimulus for its current leaf phenology leads to a reversion of leaf phenology, to that expressed by an ancestral form growing in cooler uplands (similar to the extant D. koreana) where summer dormancy is absent. As a life history trait, plants show significant latitude in their leaf habit, expressing phenological plasticity under different climatic settings. This is amply demonstrated by ecotypes of high arctic plants freely switching from evergreen to wintergreen and from wintergreen to summergreen (Bell and Bliss 1977). Perhaps, observations made in this study contribute to an additional glimpse of how leaf longevity and leaf habits are interconnected.
There is still much to learn about the unique phenological responses of Dpm to environmental cues including the timing of summer leaf fall and autumn resprout. These events, results suggest, could be independently regulated and uncoordinated processes that can sometimes overlap resulting in a change in whole plant leaf habit. Given the continuing efforts to comprehend the complex genetic and biochemical pathways regulating plant phenology (Park et al. 2009; Shalit et al. 2009; Tanino et al. 2010; Woo et al. 2018), D. psudomezereum as a plant amenable to experimental manipulations, may serve as a useful model for unlocking the underlying mechanisms regulating bud burst, leaf senescence, and flowering phenology (Wingler 2014).
References
Andrés F, Coupland G (2012) The genetic basis of flowering responses to seasonal cues. Nat Rev Genet 13:627–639
Augspurger CK, Salk CF, Wilson S (2017) Constraints of cold and shade on the phenology of spring ephemeral herb species. J Ecol 105:246–254
Bell KL, Bliss LC (1977) Overwinter phenology of plants in a polar semi-desert. Arctic 30:118–121
Chabot BF, Hicks DJ (1982) The ecology of leaf life span. Ann Rev Ecol Syst 13:229–259
Diez JM, Ibanez I, Miller-Rushing AJ, Mazer SJ, Crimmins TM, Crimmins MA, Bertelsen CD, Inouye DW (2012) Forecasting phenology: from species variability to community patterns. Ecol Lett 15:545–553
Fridley JD (2012) Extended leaf phenology and the autumn niche in deciduous forest invasions. Nature 485:359–362
Guy CL (1990) Cold acclimation and freezing stress tolerance: role of protein metabolism. Annu Rev Plant Physiol Plant Mol Biol 41:187–223
Jurik TW, Chabot BF (1986) Leaf dynamics and profitability in wild strawberries. Oecologia 69:296–304
Kikuzawa K, Lechowicz M (2006) Toward synthesis of relationships among leaf longevity, instantaneous photosynthetic rate, lifetime leaf carbon gain, and the gross primary production of forests. Am Nat 168:373–383
Kikuzawa K, Lechowicz M (2011) Ecology of leaf longevity. Springer, Tokyo
Korner C, Basler D (2010) Plant science. Phenology under global warming. Science 327:1461–1462
Lahti DC, Johnson NA, Ajie BC, Otto SP, Hendry AP, Blumstein DT, Coss RG, Donohue K, Foster SA (2009) Relaxed selection in the wild. Trends Ecol Evol 24:487–496
Lei TT, Koike T (1998) Some observations of phenology and ecophysiology of Daphe kamtschatica maxim ver jezoensis maxim ohwi a shade deciduous shrub in the forest of north japan. J Plant Res 111:207–212
Lei T, Yamashita N, Watanabe T, Kawahara T, Miyaura T (2020) Why does Daphne pseudomezereum drop its leaves in the summer? An adaptive alternative to surviving forest shade. Physiol Plant 168:77–87
Menzel A, Sparks TH, Estrella N, Koch E, Aasa A, Ahas R, Alm-Kübler K, Bissolli P, Braslavská OG, Briede A, Chmielewski FM, Crepinsek Z, Curnel Y, Dahl Å, Defila C, Donnelly A, Filella Y, Jatczak K, Måge F, Mestre A, Nordli Ø, Peñuelas J, Pirinen P, Remišová V, Scheifinger H, Striz M, Susnik A, Van Vliet AJH, Wielgolaski F-E, Zach S, Zust ANA (2006) European phenological response to climate change matches the warming pattern. Glob Change Biol 12:1969–1976
Munné-Bosch S, Alegre L (2004) Die and let live: leaf senescence contributes to plant survival under drought stress. Funct Plant Biol 31:203–216
Park JY, Canam T, Kang KY, Unda F, Mansfield SD (2009) Sucrose phosphate synthase expression influences poplar phenology. Tree Physiol 29:937–946
Peñuelas J, Filella I (2001) Responses to a warming world. Science 294:793–795
Peshev D, Vergauwen R, Moglia A, Hideg E, Van den Ende W (2013) Towards understanding vacuolar antioxidant mechanisms: a role for fructans? J Exp Bot 64:1025–1038
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. https://www.R-project.org/. Accessed 17 Dec 2019
Shalit A, Rozman A, Goldshmidt A, Alvarez JP, Bowman JL, Eshed Y, Lifschitz E (2009) The flowering hormone florigen functions as a general systemic regulator of growth and termination. Proc Natl Acad Sci USA 106:8392–8397
Smith LM, Palmer M (2013) Extended leaf phenology in deciduous forest invaders: mechanisms of impact on native communities. J Veg Sci 24:979–987
Tanino KK, Kalcsits L, Silim S, Kendall E, Gray GR (2010) Temperature-driven plasticity in growth cessation and dormancy development in deciduous woody plants: a working hypothesis suggesting how molecular and cellular function is affected by temperature during dormancy induction. Plant Mol Biol 73:49–65
The Plant List (2010) Version 1. Published on the internet. https://www.theplantlist.org/. Accessed 14 Mar 2016
Vincent G (2006) Leaf life span plasticity in tropical seedlings grown under contrasting light regimes. Ann Bot 97:245–255
Wingler A (2014) Comparison of signaling interactions determining annual and perennial plant growth in response to low temperature. Front Plant Sci 5:794
Woo HR, Kim HJ, Nam HG, Lim PO (2013) Plant leaf senescence and death-regulation by multiple layers of control and implications for aging in general. J Cell Sci 126:4823–4833
Woo HR, Masclaux-Daubresse C, Lim PO (2018) Plant senescence: how plants know when and how to die. J Exp Bot 69:715–718
Acknowledgements
I thank two anonymous reviewers for their helpful comments that have improved the reporting of this work. Funding for this research was provided in part by JSPS KAKENHI Grant No. 20380093 and by Scientific Collaboration Research Grant of Ryukoku University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Daniel L Potts.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file2 (MOV 1111 kb)
Rights and permissions
About this article
Cite this article
Lei, T. The summer-deciduous habit of Daphne pseudomezereum is a response to warm summer as cooling converts it to an evergreen. Plant Ecol 221, 431–440 (2020). https://doi.org/10.1007/s11258-020-01023-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-020-01023-2