Abstract
Investment of limited resources into reproduction is a common feature of plants; coadapted life-history traits function as adaptations that have evolved to maximize fitness. There is currently little known about reproductive allocation (RA) of perennial grasses as a component of a partitioning strategy evolved to optimize sexual reproduction or about how RA may be related allometrically to vegetative size. This study examined variation in RA, biomass partitioning, and allometry in populations of a caespitose perennial grass (Tridens flavus) native to eastern North America. Plants reared from seeds of five populations in central New Jersey, USA, were planted in July 2015 into two undisturbed gardens 30 m apart that differed in availability of sunlight and soil moisture. After 3 years, data were collected on number of seeds (fecundity) and dry mass of seeds, panicles, culms, and leaves. RA (seed mass/vegetative mass) was significantly higher (13.1%) in the garden with higher light and lower soil moisture compared to the other garden in which RA was lower (9.1%) and varied significantly among populations. Fecundity was tightly correlated with vegetative mass in both gardens. A weak, but significant, trade-off between seed mass and number occurred in the garden with lower light and higher soil moisture. RA was positively correlated with relative fitness in both gardens. This research illustrates how population differentiation in reproductive allocation and partitioning may be revealed only in specific environments. Relatively high investment in seed production is important for recruitment and expansion of caespitose perennial grasses throughout successional habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The theory of life-history evolution has long provided a useful framework for the analysis of intraspecific variation (Stearns 1977, 1992; Partridge and Harvey 1988; Reznick 2017; Salguero-Gómez et al. 2018). Life-history traits such as organism size and age at reproductive maturity, and the number and size of offspring produced (Schaffer and Gadgil 1975; Stearns 1992; Morris 2009), are key to understanding how natural selection has led to sets of coadapted features (Stearns 1976) that are considered complex adaptations that evolved to maximize fitness (Braendle et al. 2011; Reznick 2017).
For plants, resources such as available energy or biomass are typically allocated to the competing functions of growth, maintenance, and reproduction (Bazzaz and Ackerly 1992; Kőrner 1994; Kleyer and Minden 2015). In favourable environments, greater vegetative growth results in larger plants able to devote more resources to reproductive functions (Samson and Werk 1986; Hartnett 1990; Bazzaz 1997; Weiner 2004), thereby achieving greater reproductive fitness (review in Younginger et al. 2017). Studies on biomass allocation have shown that differentiation in partitioning patterns can be found among species and habitats (Abrahamson and Gadgil 1973; Harper 1977; Roos and Quinn 1977; Kawano and Miyake 1983; Hara et al. 1988; Kőrner 1994; Mason et al. 2017).
The concept of reproductive allocation (RA) can be used as a relative term to express the extent to which plants of variable size invest limited resources in flowers, fruits, and seeds (Bazzaz 1997; Reekie 1999; Cheplick 2005; Wenk and Falster 2015). RA is a key component of a species’ reproductive strategy (Harper and Ogden 1970; Lovett Doust 1989; de Jong and Klinkhamer 2005) and has a lengthy history of study by evolutionary ecologists (Karlsson and Méndoz 2005), although it is generally agreed that allocation will often change over development as plants increase in size (Welham and Setter 1998; Weiner et al. 2009). However, even when size is accounted for statistically, allocation parameters within a plant species may be partly explained by environmental factors in a way that is consistent with optimal partitioning (McCarthy and Enquist 2007) as a strategy evolved by natural selection to maximize reproductive fitness (Bonser and Aarssen 2009). It is important to emphasize that for RA to have evolved as a significant component of a species’ life history, it should show an association with individual reproductive fitness (Reekie 1999; Cheplick 2005).
Typically expressed as a ratio of reproductive mass to vegetative biomass (or sometimes total biomass, especially in earlier literature, e.g. Harper and Ogden 1970; Abrahamson and Gadgil 1973), RA varies greatly among species with different life forms and among populations of a species growing in different environmental conditions, especially those that affect plant size (Hancock and Pritts 1987; Wilson and Thompson 1989; Hartnett 1990; Bazzaz and Ackerly 1992; Reekie 1998; Sugiyama and Bazzaz 1998; Weiner 2004; Karlsson and Méndoz 2005). Rearing plants under common conditions can reveal differences in RA among species (e.g. Wilson and Thompson 1989; Mason et al. 2017) and among populations within a species (e.g. Quinn and Hodgkinson 1984).
In caespitose perennial grasses, biomass is typically partitioned into vegetative organs such as leaves and culms that form tillers that can produce terminal inflorescences with spikelets during flowering and later, caryopses during seed maturation. The perennial bunchgrass life history is highly successful ecologically and caespitose species have become dominant in grasslands and successional fields worldwide (Pyke 1990; Briske and Derner 1998; Guàrdia et al. 2000). Bunchgrass species tend to show variable RA, although it is usually greater than that of rhizomatous grass species (Wilson and Thompson 1989), perhaps because the former do not allocate resources to rhizomes or stolons which sometimes trade-off with sexual reproduction (Cheplick 1995a; Herben et al. 2015). However, despite their ecological importance in many habitats, there is currently little known about the RA of perennial grasses as a component of a reproductive strategy evolved to optimize sexual reproduction or about how RA is related allometrically to vegetative size. Also, it is not known how the biomass of perennial grasses is partitioned into vegetative and reproductive structures, whether there are trade-offs among competing functions (Stearns 1989), and whether partitioning patterns vary among populations from different habitats when grown under common conditions.
The present study involves a common garden experiment using a robust perennial bunchgrass native to eastern North America, Tridens flavus (L.) Hitchc. (Valdés-Reyna 2003). Life-history variation was examined in five populations over 3 years in two common gardens that differed in the availability of sunlight and soil moisture. In another report, four components of reproductive fitness were analysed over the 3 years in which flowering and seed maturation occurred in relation to population source and garden (Cheplick ms). In the present report, RA, biomass partitioning to vegetative and reproductive organs, and allometry are analysed for the third and final year when all aboveground biomass was harvested. Specific questions addressed were as follows: (1) Are populations of T. flavus from distinct habitats differentiated with respect to RA and biomass partitioning? (2) How does garden environment affect patterns in RA and biomass partitioning among the populations? (3) Are there allometric scaling relationships between seed production (fecundity) or RA and vegetative biomass? (4) Are there life-history trade-offs between seed number and mass in the two gardens? (5) Is relative fitness, based on the number of seeds per individual (fecundity), positively related to RA in both garden environments? Answers to these questions will provide a framework for understanding how biomass allocation and partitioning interact with vegetative size and environment to determine the life-history strategy of an ecologically widespread, native perennial grass.
Materials and methods
Study species
Tridens flavus [L.] Hitchc. is a warm-season, caespitose perennial grass with C4 photosynthesis native to eastern North America, ranging from southern Canada to northern Mexico, and extending westward to eastern Texas and Nebraska, USA (United States Department of Agriculture 2002; Valdés-Reyna 2003). Typical habitats where it occurs include successional old fields, young woodlands, and disturbed roadsides. It appears to grow well in dry, infertile sandy or rocky soils (Foote and Jackobs 1966; Boggess and Baker 1983; Honu et al. 2006).
Casual observations suggest that the perennial is long-lived, with populations persisting for at least 6–8 years in early-successional fields (Collins et al. 2001; Honu et al. 2006) and probably much longer within young, open woodlands in the eastern United States (Cheplick pers. obs.).
The life cycle of T. flavus in New Jersey is similar to most temperate perennial C4 grasses (Sarath et al. 2014): ground-level tiller buds of established plants are dormant overwinter until regrowth begins again in spring (generally in April). Plants grow rapidly by tiller elongation and leaf extension during the warm summer months. During flowering in August, terminal, highly branched, diffuse panicles on tall culms (up to 2 m) are produced. As in most warm-season, C4 grasses (Moser et al. 2004), the numerous spikelets on these panicles exhibit anthesis and are likely to be outcrossing. For example, an isolated flowering individual far away from other flowering conspecifics does not usually set seed (Cheplick pers. obs.). However, after successful wind-pollination, many viable seeds will mature along multiple branches of each aerial panicle. There does not appear to be any obvious mode of dispersal. In nature, seeds are likely stratified by cold winter temperatures and subsequently germinate in late spring when temperatures are warmer. Following cold treatment, T. flavus seeds can germinate to high percentages (> 90%) in alternating 20/30 °C temperatures that simulate late spring conditions (Olszewski and Folin 2009).
Population seed sources
To represent a diverse array of habitats, five large populations of T. flavus in central New Jersey, USA, were identified for seed collection in Autumn 2014. The populations and their habitats are described in detail in Cheplick (ms), so only an overview is given here. Populations 1–3 (P1–P3) were within Perrineville Lake Park, Monmouth County (40° 14′ 3.4″ N, 74° 25′ 50.5″ W), which consists of 500-ha of successional fields and woodlands. The three populations were only separated by 300–500 m. Population size was estimated based on the density of panicles per m2 recorded in October 2014 and the area of the field or woodland site. The largest population (~ 12,000 plants) was P1 which occupied an early-successional field (0.68 ha) dominated by perennial herbs. The second population (P2) with ~ 6,000 plants was in a successional woodland (0.58 ha) with Elaeagnus umbellata Thunb. shrubs and Liquidambar styraciflua L. saplings (1–2.5 m) scattered among large perennial herbs (Solidago sp., Lespedeza cuneata [Dum. Cours] G. Don). The third population (P3) with ~ 8,000 plants had significantly lower soil moisture than the other two (volumetric water content [VWC] of P3 = ~ 6%, P1 = ~ 17%, P2 = ~ 11%; Cheplick ms) and occupied a dry sunny ridge (0.84 ha) dominated by perennial grasses and scattered Quercus marilandica Münchh. trees (1–2 m). Note that botanical nomenclature follows the PLANTS database of the United States Department of Agriculture (https://plants.usda.gov).
The fourth population (P4) with ~ 300 plants was on a sloping roadside (40° 14′ 36.6″ N, 74° 27′ 7.9″ W) about 6 m wide and 50 m long (300 m2), and was ~ 2.2 km from P1–P3; the site was dominated by perennial grasses and weedy annuals. The habitat had relatively low soil moisture (4.3% VWC). The fifth population (P5) with ~ 450 plants was on a rocky slope along Rising Sun Lake (40° 12′ 26.4″ N, 74° 27′ 52.1″ W) about 4 m wide and 120 m long (480 m2). It was dominated by a thick cover of the perennial grass Deschampsia flexuosa [L.] Trin. and T. flavus at high density (12–15 m−2). Soil moisture was relatively low (~ 5% VWC). P5 was 4.6 km from P4 and ~ 4.3 km from P1 to P3.
Mature seeds were manually stripped from 20 panicles per population on 30 October 2014 and retained in paper envelopes. A sampled panicle was always at least 5 m from the panicle sampled previously to ensure a diverse seed pool from each population. Note that these “seeds” were technically caryopses (fruits of the grass family) but are functionally comparable to the seeds of other plant families.
Common garden experiment
In late January 2015, 20 seeds were added onto moistened filter paper in each of 12 plastic petri dishes (9 cm diameter) per population. Dishes were sealed with Parafilm® and placed into cold, moist stratification (4 °C) for 6 weeks.
Cold-treated seed dishes were then placed into an incubator set at conditions suitable for T. flavus germination (Olszewski and Folin 2009): 25 °C, 12-h light and 15 °C, 12 h dark. Germination after 32 d was high in all populations (69–90%) and the resulting seedlings were randomly selected for use in the common garden experiment. The seedlings were first cultured in narrow, tubular pots (3.7 cm diameter × 13.5 cm depth) placed outdoors at the garden site on 22 May 2015 to allow acclimation to local weather conditions.
Seedling height recorded several weeks before transplanting to the gardens did not differ significantly among populations (ANOVA, F4, 121 = 2.19, P = 0.074). Mean (± SE) height was 42.5 ± 4.6 mm for P1, 37.7 ± 3.6 mm for P2, 45.5 ± 4.4 mm for P3, 39.5 ± 3.1 mm for P4, and 30.1 ± 3.4 mm for P5.
From 17 to 21 July 2015, 12–13 seedlings per population were planted into two experimental gardens that differed in the availability of sunlight and soil moisture. The original plan was to obtain 12 adult plants per population per garden, but the experienced plant ecologist always plants “extras” and these were also planted, resulting in P1 and P2 with 13 seedlings in Garden A and P1–P4 with 13 seedlings in Garden B. Gardens were 4.5 m × 1.2 m, separated by 30 m, and in central New Jersey, USA (40° 15′ 7.4″ N, 74° 28′ 3.9″ W). The garden site was 3.6 km from P1 to P3, 1.5 km from P4, and 5.0 km from P5. This area of New Jersey has a monthly temperature ranging from − 1.8 °C in January to 23.3 °C in July (30-year averages, 4.5 km from the garden; climate data from https://www.worldclimate.com). Mean annual precipitation is 114 cm and rainfall is greatest in July (12 cm) and August (11 cm). The frost-free growing season averages 170–180 days.
Garden A received 4–5 h of direct sunlight day−1 and had significantly lower soil moisture (VWC = 10–14%) in the first two years of this study compared to Garden B which received 2–3 h of direct sunlight day−1 and was at the base of gently sloping terrain and therefore had greater soil moisture (VWC = 15–20%). Additional light in the form of sunflecks filtered through adjacent woodland into both gardens during each day.
In each garden, seedlings were planted at 30 cm apart in four rows spaced 40 cm apart. A 5-cm diameter hole was excavated using a garden borer to 5 cm depth and a seedling placed into the hole. Soil removed by the borer was replaced. To aid establishment, water was provided as a mist from a garden hose every 2–4 d for 2 weeks. There were low-stature annual weeds and scattered perennial grasses within each garden throughout the 3 years that transplants grew, but soil was not disturbed. During the 3-year study, there was almost no overwinter mortality, assessed as plant regrowth each spring: 7 plants died in Garden A (1–2 per population), while no plants died in Garden B over the entire 3-year experiment.
Several non-destructive growth measures were recorded during the first summer to determine whether early growth differed among populations and gardens. Although minor differences in seedling height among populations before planting approached statistical significance (P = 0.074), height in the common gardens 1 month after transplanting did not vary significantly among populations (F4, 115 = 1.88, P = 0.12). In addition, the log-transformed number of tillers did not differ among populations 2 months (F4, 115 = 1.49, P = 0.21) and 3 months after transplanting (F4, 115 = 0.95, P = 0.44). There was also no significant interaction of population and garden for tiller number at 2 months (F4, 115 = 0.84, P = 0.50) or 3 months (F4, 115 = 0.99, P = 0.42), indicating that no population had a growth advantage in either garden during the first year of the experiment. However, seedlings of all populations did show greater first-year growth in Garden B (data not shown).
Detailed data were recorded on flowering tiller height, seed production, seed set proportions, and seed mass each year as part of an investigation of fitness components in this species. In addition, to be assured that the seeds collected were alive and capable of producing seedlings, a germination trial was done for a subset of plants in the final year (seven individuals per population per garden). Seeds collected in September 2018 were retained separately by plant and stored dry in paper envelopes at ambient temperatures over winter in an unheated outdoor structure. On 17 Feb 2019, subsamples of ~ 100 seeds, each from one individual, were placed into Petri dishes (9 cm diameter) containing moistened filter paper. Dishes were sealed with Parafilm® and maintained at ambient temperatures outdoors until germination began in late May; germinated seeds were counted until no further germination occurred (by 30 June 2019). Mean (± SE) germination ranged from 65.5 ± 10.3% for P2 in Garden B to 81.2 ± 2.5% for P1 in Garden A, but was not significantly different between gardens (F1, 52 = 2.35, P = 0.13) or among populations (F4, 52 = 0.62, P = 0.65).
Following seed collection from panicles in the third year of flowering, all aboveground biomass was harvested on 25 Sept 2018. Each plant was clipped to 2 cm and placed into a paper bag. Plants were dried in a convection oven at 60 °C to constant mass. Then plants were separated into four components for dry mass determination: (1) seeds, (2) panicles (from the flag leaf upward), (3) leaves (blade plus sheath), and (4) culms.
Calculated variables and data analysis
Reproductive allocation (RA) was calculated in two ways. In the first (RA1), total seed mass per plant was the reproductive component that was divided by vegetative mass (i.e. the sum of leaf and culm mass). The second (RA2) included panicle mass with total seed mass as the complete reproductive component as suggested by some workers (Thompson and Stewart 1981; Reekie 1999) who maintain that the “effort” devoted to reproduction should include structures whose only function is to support flowers, fruits, and seeds. Thus, the grass culm which extends beyond the flag leaf and divides into the branches of the panicle can be considered a reproductive structure (Wilson and Thompson 1989; Kőrner 1994) whose function is to support spikelets and developing seeds. Therefore, RA2 = (seed mass + panicle mass)/vegetative mass. Note that both RA1 and RA2 represent “standing RA” (Bazzaz and Ackerly 1992, p. 5) which is the proportion of resources contained in reproductive structures at one point in time, which in this study is for plants that were all 3 years old.
Biomass partitioning into leaves, culms, panicles, and seeds was expressed for each plant component as the proportion of the total aboveground mass (TAM) comprised of the component.
These partitioning components are often referred to as mass fractions (Kőrner 1994; Poorter and Sack 2012; Kleyer and Minden 2015; Mason et al. 2017). For example, leaf fraction is leaf mass/TAM. Culm, panicle, and seed fraction were calculated similarly.
An index of absolute fitness was computed based on the product of seed set proportion, fecundity, and the mass of one seed for each plant. Relative fitness was standardized as the absolute fitness of an individual in a particular garden divided by the mean absolute fitness averaged for all individuals in that garden (Cheplick 2015).
For all data analyses, the Statistical Analysis System® Version 9.4, University Edition, was used (SAS Institute, Cary, North Carolina, USA). RA1, RA2, and each mass fraction was analysed with two-way ANOVA, with source population (df = 4), garden (df = 1), and their interaction (df = 1) as the sources of variation. To comply with ANOVA assumptions, and because all variables were proportions, they were arcsine, square-root transformed before analysis (Gotelli and Ellison 2004) using Proc glm (general linear models). When population was a significant factor in an ANOVA, Tukey’s HSD test was used to compare population means. These analyses address Introduction question (1) as to whether populations from distinct habitats were differentiated with respect to RA and biomass partitioning and question (2) as to how garden environment may affect patterns in RA and partitioning among populations.
For allometric scaling analyses, all variables were log10-transformed and subjected to reduced major axis (Model Type II) regressions (Niklas 1994; Niklas and Enquist 2003; Marbà et al. 2007). Because all variables examined differed significantly between the two gardens (analysed elsewhere: Cheplick ms), and to determine whether allometric relationships differed between the garden environments, regressions were performed separately by garden. Each regression conformed to the relationship logY2 = logβRMA + αRMA logY1, where Y2 and Y1 are the interdependent variables, βRMA is the y-intercept, and αRMA is the slope (Niklas and Enquist 2003). Regressions performed (Y2 onto Y1) were (1) number of seeds per plant onto vegetative mass (fecundity allocation sensu Aarssen and Taylor 1992), (2) reproductive allocation (RA1, RA2) onto vegetative mass, (3) mass of one seed onto number of seeds per plant, and (4) relative fitness onto reproductive allocation (RA1, RA2).
Regressions 1 and 2 address the question (3) as to whether fecundity or RA is coupled to plant size. Regression 3 addresses the question (4) as to whether there is a life-history trade-off between seed number and seed mass in this species. Regression 4 addresses the question (5) as to whether relative fitness was positively related to RA in both garden environments.
Results
Reproductive allocation
Garden had a highly significant effect on both metrics of reproductive allocation (Table 1): plants in Garden A showed greater allocation to seeds (RA1 mean ± SE = 13.1 ± 0.6%) compared to plants in Garden B (9.1 ± 0.3%; Fig. 1). Populations also differed significantly for RA1 (Table 1). Populations 2 and 5 had significantly lower RA1 than two other populations (P1, P4) in Garden B (Fig. 1). When panicle mass was included as part of the reproductive allocation (RA2), plants in Garden A still showed significantly greater allocation to reproduction (RA2 = 21.6 ± 0.8%) compared to plants in Garden B (16.6 ± 0.4%), but the effect of population was not significant (Table 1).
Mean (± SE) allocation to seeds for five populations of Tridens flavus grown for 3 years in two common gardens. Allocation to seeds (RA1) is (total seed mass/vegetative mass) × 100. Within a garden, different letters indicate significant differences between populations (P < 0.05). See Table 1 for statistical summary
Biomass partitioning
The proportion of the aboveground biomass consisting of leaves or seeds depended on which garden the plants were in (Table 2). In Garden A, 43.7 ± 0.9% and 10.7 ± 0.4% of standing biomass was in leaves and seeds, respectively, compared to 46.1 ± 0.7% and 7.8 ± 0.2%, respectively, in Garden B (all percentages are means ± SE; Fig. 2). Seed mass fraction also varied with population in Garden B: the pattern was the same as that of RA1 in that P2 and P5 had significantly lower seed mass fraction than P1 and P4 (Fig. 2). Neither population nor garden affected culm or panicle mass fraction, and the garden by population interaction was not significant for any mass fraction component (Table 2).
Partitioning of the aboveground biomass to seeds, panicles, leaves, and culms in five populations of Tridens flavus grown for 3 years in two common gardens. For seed fraction, different letters indicate significant differences between populations (P < 0.05). See Table 2 for statistical summary
Allometric scaling
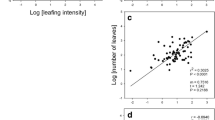
The number of seeds per plant (= fecundity) was tightly correlated with vegetative mass (VM) in both gardens (Table 3; Fig. 3). Slopes were close to unity, indicating the relationship was isometric. The proportion of the variation in fecundity explained by VM was 86% in Garden A and 74% in Garden B (Table 3). In contrast to the positive relationship of fecundity to VM, reproductive allocation to seeds (RA1) showed no significant correlation with VM in either Garden A (F1, 53 = 0.67, P = 0.42) or Garden B (F1, 62 = 0.18, P = 0.67). However, RA2, which included panicle mass as part of the allocation to reproduction, was negatively correlated with VM, but only in Garden B (F1, 62 = 5.59, P = 0.02, R2 = 0.08; αRMA = − 0.53 ± 0.07, logβRMA = − 0.24 ± 0.07).
The classic seed mass-number trade-off was detected, but only in Garden B where the relationship was relatively weak (R2 = 0.08), but statistically significant (Table 3; Fig. 4).
A positive correlation was found between relative fitness and allocation to seeds (RA1) within each garden (Fig. 5), but the relationship was tighter in Garden B where RA1 explained 25% of the variation in relative fitness compared to only 7% in Garden A (Table 3). However, when panicle mass was included as part of the reproductive allocation (RA2), there was no longer any correlation between relative fitness and allocation to reproductive tissues (Garden A: F1, 53 = 1.36, P = 0.25, R2 = 0.03; Garden B: F1, 62 = 2.58, P = 0.11, R2 = 0.04).
Log–log plots of the relationship between relative fitness and reproductive allocation to seeds (RA1) for Tridens flavus grown for 3 years in two common gardens: a Garden A and b Garden B. Relative fitness was based on a multiplicative index that included fecundity, seed set, and mean seed mass. RA1 is total seed mass/vegetative mass
Discussion
As a perennial, warm-season grass, Tridens flavus tends to grow in successional fields and young, open woodlands (Valdés-Reyna 2003; Honu et al. 2007) where sunlight is direct and readily available for many hours each day. Garden A, with greater daily hours of sunlight and drier soil, was shown to be more favourable to panicle production and fecundity than Garden B (Cheplick ms). In the present analysis, whether reproductive allocation was based solely on seed mass (RA1; Fig. 1) or also included reproductive supporting tissues (RA2), allocation to reproduction was consistently greater in Garden A. Higher RA is often associated with greater light availability in perennial herbs (Pitelka et al. 1980; Gross et al. 1983; Jurik 1983).
Variable performance of plants across multiple common gardens is a frequent result in studies that use this approach (Williams et al. 2008; Cheplick 2015). Although garden by population interactions were not detected for reproductive allocation or biomass partitioning in T. flavus in the present research (Tables 1, 2), population differences were apparent for RA1 (Fig. 1) and seed mass fraction (Fig. 2) but only in Garden B. This illustrates the ecological importance of population differentiation that might be revealed only in specific environments, to the expression of intraspecific variation in phenotypic traits (Roybal and Butterfield 2019).
The differences in RA1 and seed fraction among populations in the garden with reduced light availability and higher soil moisture (i.e. Garden B) are not easily explained. Populations 2 and 5 had the lowest allocation and partitioning to seeds (Figs. 1,2). P5 was from the source population furthest from the garden site (5 km) and the P5 site was a very sunny, rocky slope with low soil moisture and a high density of competitors. Thus, the P5 source population was possibly not well-adapted to the conditions of Garden B (low sun, high moisture, low competition) and unable to devote extensive resources to sexual reproduction. The population (P4) from the site closest to the garden showed the greatest RA1 in both gardens, suggesting that it was better adapted to garden conditions. Like P5, P2 also showed low RA in Garden B; P2 was from a sunny, early-successional woodland about 3.6 km from the gardens. However, P1 and P3 were also from sunny, successional sites that were about the same distance from the gardens, but had higher RA than P2. Therefore, the reduced RA of P2 defies any simple explanation based on the recorded data.
Reproductive allocation that included supporting tissues (RA2) in T. flavus (16.6–21.6%) was within the range (11.4–30.5%) reported for ten perennial grass species with the caespitose growth habit in Britain (Wilson and Thompson 1989). It should be noted that RA2 in the present study is comparable to Wilson and Thompson’s (1989) metric of RA because they included “the entire mature inflorescence including the stem above the highest culm leaf” and expressed total reproductive mass as a percentage of vegetative mass. The values for RA2 in T. flavus are also similar to reproductive allocation of herbaceous perennials in old-field habitats which ranged from 11 to 32% (Gross et al. 1983; Hancock and Pritts 1987). However, allocation to seeds can be quite low in perennial grasses, especially those that are rhizomatous (2–3%; Adams and Wallace 1985; Cheplick 1995b). Although RA in caespitose perennial grasses like T. flavus is lower than that of annual grasses, it is generally higher than that of rhizomatous species which presumably divert some resources to clonal growth (Wilson and Thompson 1989; Cheplick 1995a; Hutchings 1997; Herben et al. 2015). In T. flavus, seed allocation (RA1) averaged 9% in Garden B and 13% in Garden A. In another species with a similar life habit, the perennial bunchgrass Elymus excelsus, RA averaged 10–15%, but declined with age over a 5-year period (Li et al. 2019). Relatively high investment in seed production is probably important for recruitment and expansion of caespitose perennial grasses throughout successional habitats (Pyke 1990; Aguilera and Lauenroth 1995; Guàrdia et al. 2000).
Like many herbaceous perennials in which fecundity and/or reproductive biomass is positively correlated with vegetative mass (Kawano 1981; Aarssen and Taylor 1992; Schmid et al. 1995; Weiner et al. 2009; Younginger et al. 2017), the estimated number of seeds made per plant (fecundity) was closely coupled to vegetative mass in T. flavus (Fig. 3). The slope of the relationship was close to unity (Table 3). An isometric, linear relationship between fecundity or reproductive biomass and vegetative mass is commonly reported for herbaceous plants (Aarssen and Taylor 1992; Weiner et al. 2009; Kleyer and Minden 2015). This indicates that an increase in vegetative mass as plants grow and age produces a corresponding increase in seed output; thus fecundity per unit plant mass (“fecundity allocation” in Aarssen and Taylor 1992) may be relatively fixed across the range of sizes found in a perennial herb population.
The proportion of the variation in fecundity explained by vegetative dry mass in T. flavus was high: 86% in Garden A and 74% in Garden B (Fig. 3). However, this is not unusual in studies of fecundity allocation in herbaceous plants (e.g. Kawano and Miyake 1983). For nine polycarpic perennial herbs, Aarssen and Taylor (1992) found an average of 72% (range 35–96%) of the variance in fecundity could be explained by aboveground mass. In the caespitose perennial grass Echinochloa crus-galli, 98% of the variance in fecundity was explained by aboveground mass (Martinková and Honĕk 1992). In another analysis of the T. flavus data in which multiple regression models were evaluated to determine which of the measured phenotypic traits best predicted fecundity, vegetative mass still explained 71–81% of fecundity variation, with seed set and seed mass explaining a much smaller proportion (< 5%) (Cheplick ms). These considerations underscore the ecological importance of size to reproductive output in general (Weiner 2004; Bonser and Aarssen 2009), and specifically to perennial grasses like T. flavus in which the growth of a flowering tiller is determinate, but in which whole-plant growth by the addition of vegetative tillers is indeterminate and increases over successive years (Briske and Derner 1998).
In the present analysis, the mass of one seed did not trade-off with fecundity in Garden A, but in Garden B where daily light availability was less, there was evidence of a weak seed mass-number trade-off (Fig. 4). Variation in the seed mass-number relationship within a species is not unexpected as this trade-off can depend on environmental conditions (Samson and Werk 1986; Venable 1992; Paul-Victor and Turnbull 2009). Simplistic models that predict an optimal seed size (e.g. Smith and Fretwell 1974) may not be appropriate when plant resource status is not taken into account (Venable 1992).
In contrast to the close isometric scaling of fecundity to vegetative mass, reproductive allocation to seeds (RA1) in T. flavus was not correlated with vegetative mass in either garden. However, in Garden B when panicle mass was included as part of RA (i.e. RA2), there was a weak negative correlation of RA2 with vegetative mass. In a review of the allometry of RA, Cheplick (2005) noted that the relationship typically varied from none to negative in herbaceous perennial species and summarized several studies (see also Hara et al. 1988). The decline in RA2 with an increase in size could reflect the costs of mechanical support tissues needed for developing floral structures and seeds as plants grow larger (Hartnett 1990; Reekie 1998). Under the reduced light availability in Garden B where seed allocation (RA1) was lower and leaf allocation was higher compared to Garden A, allocation to culms and panicles was the same in Garden B as in the more favourable Garden A environment (Table 2; Fig. 2). This indicates that when resources such as light are limiting fecundity, more energy must be allocated to resource-acquiring tissues (i.e. leaves) to support a given amount of reproductive allocation.
For RA to be an ecologically relevant component of plant life history, it should be positively correlated with relative fitness (Reekie 1999; Cheplick 2005). Using an index of fitness that incorporated fecundity, seed set, and mean seed mass in T. flavus, plants with a greater RA were found to have higher fitness in both garden environments (Fig. 5). This agrees with results for several herbaceous species analysed in Cheplick (2005), including the rhizomatous perennial grass Amphibromus scabrivalvis in which 22% of the variance in fitness was explained by RA. Similarly, for T. flavus in Garden B, 25% of the variance in fitness was explained by RA when based on seed allocation (RA1). However, only 7% of the variance in fitness could be explained by RA1 in Garden A and there was no correlation between fitness and RA2 which included the panicles as reproductive supporting tissues. It is clear from these analyses that the relation of RA to fitness in perennial plants depends on environmental conditions and whether or not supporting tissues are included in the calculation of reproductive allocation. For T. flavus, selection for greater seed allocation may be stronger in environments such as Garden B where resources (such as light availability) are somewhat limiting to growth.
Conclusions
Robust, warm-season, caespitose perennial grasses such as Tridens flavus do not spread laterally via rhizomes and rely strongly on seed production for colonization and persistence in successional habitats. The tight, isometric relationship of fecundity to vegetative mass for 3-year-old T. flavus indicates that conditions favourable to growth, such as sunny, open environments, result in larger plants that produce greater numbers of seeds, potentially with higher rates of seedling establishment, especially as there was no cost manifested as reduced seed mass for highly fecund individuals. Allocation to seeds was higher, but allocation to leaves was lower, in the garden with greater light availability. In the other garden where light availability was lower, plants had lower fecundity and allocated less mass to seeds and more to leaves; there was also a trade-off between seed number and mass, and populations varied significantly in reproductive allocation to seeds. However, selection will tend to favour greater allocation to seeds because relative fitness was positively correlated with reproductive allocation in both gardens. Optimal partitioning of biomass to the competing functions of growth and reproduction is a major part of the life-history strategy that has evolved by natural selection to maximize reproductive fitness in perennial grasses that rely on high fecundity for establishment and persistence.
References
Aarssen LW, Taylor DR (1992) Fecundity allocation in herbaceous plants. Oikos 65:225–232
Abrahamson WG, Gadgil M (1973) Growth form and reproductive effort in goldenrods (Solidago, Compositae). Am Nat 107:651–661
Adams DE, Wallace LL (1985) Nutrient and biomass allocation in five grass species in an Oklahoma tallgrass prairie. Am Mid Nat 113:170–181
Aguilera MO, Lauenroth WK (1995) Influence of gap disturbance and type of microsites on seedling establishment in Bouteloua gracilis. J Ecol 83:87–97
Bazzaz FA (1997) Allocation of resources in plants: state of the science and critical questions. In: Bazzaz FA, Grace J (eds) Plant resource allocation. Academic Press, San Diego, pp 1–37
Bazzaz FA, Ackerly DD (1992) Reproductive allocation and reproductive effort in plants. In: Fenner M (ed) The ecology of regeneration in plant communities. CAB International, Wallingford, pp 1–26
Boggess N, Baker BS (1983) Edaphic requirements and characteristics of purpletop. Agron J 75:53–56
Bonser SP, Aarssen LW (2009) Interpreting reproductive allometry: individual strategies of allocation explain size-dependent reproduction in plant populations. Persp Plant Ecol Evol Syst 11:31–40
Braendle C, Heyland A, Flatt T (2011) Integrating mechanistic and evolutionary analysis of life history variation. In: Flatt T, Heyland A (eds) Mechanisms of life history evolution. Oxford University Press, New York, pp 3–10
Briske DD, Derner JD (1998) Clonal biology of caespitose grasses. In: Cheplick GP (ed) Population biology of grasses. Cambridge University Press, Cambridge, pp 106–135
Cheplick GP (1995a) Life-history trade-offs in Amphibromus scabrivalvis (Poaceae): allocation to clonal growth, storage, and cleistogamous reproduction. Amer J Bot 82:621–629
Cheplick GP (1995b) Plasticity of seed number, mass, and allocation in clones of the perennial grass Amphibromus scabrivalvis. Int J Plant Sci 156:522–529
Cheplick GP (2005) The allometry of reproductive allocation. In: Reekie EG, Bazzaz FA (eds) Reproductive allocation in plants. Elsevier Academic Press, Burlington, pp 97–128
Cheplick GP (2015) Approaches to plant evolutionary ecology. Oxford University Press, New York
Cheplick GP (ms) Fitness components in populations of a native perennial grass (Tridens flavus): a three-year common garden experiment. [Unpublished manuscript]
Collins B, Wein G, Philippi T (2001) Effects of disturbance intensity and frequency on early old-field succession. J Veget Sci 12:721–728
de Jong T, Klinkhamer P (2005) Evolutionary ecology of plant reproductive strategies. Cambridge University Press, Cambridge
Foote LE, Jackobs JA (1966) Occurrence and stand density of Tridens flavus (L.) Hitchc. as related to some soil factors. Agron J 58:412–414
Gotelli NJ, Ellison AM (2004) A primer of ecological statistics. Sinauer Associates, Sunderland
Gross KL, Berner T, Marschall E, Tomcko C (1983) Patterns of resource allocation among five herbaceous perennials. Bull Torrey Bot Club 110:345–352
Guàrdia R, Raventós J, Caswell H (2000) Spatial growth and population dynamics of a perennial tussock grass (Achnatherum calamagrostis) in a badland area. J Ecol 88:950–963
Hancock JF, Pritts MP (1987) Does reproductive effort vary across different life forms and seral environments? A review of the literature. Bull Torrey Bot Club 114:53–59
Hara T, Kawano S, Nagai Y (1988) Optimal reproductive strategy of plants, with special reference to the modes of reproductive resource allocation. Plant Sp Biol 3:43–59
Harper JL (1977) Population biology of plants. Academic Press, San Diego
Harper JL, Ogden J (1970) The reproductive strategy of higher plants. I. The concept of strategy with special reference to Senecio vulgaris L. J Ecol 58:681–688
Hartnett DC (1990) Size-dependent allocation to sexual and vegetative reproduction in four clonal composites. Oecologia 84:254–259
Herben T, Šerá B, Klimešová J (2015) Clonal growth and sexual reproduction: tradeoffs and environmental constraints. Oikos 124:469–476
Honu YAK, Gibson DJ, Middleton BA (2006) Response of Tridens flavus (L.) A. S. Hitchc. to soil nutrients and disturbance in an early successional old field. J Torrey Bot Soc 133:421–428
Hutchings MJ (1997) Resource allocation patterns in clonal herbs and their consequences for growth. In: Bazzaz FA, Grace J (eds) Plant resource allocation. Academic Press, San Diego, pp 161–189
Jurik TW (1983) Reproductive effort and CO2 dynamics of wild strawberry populations. Ecology 64:1329–1342
Karlsson PS, Méndez A (2005) The resource economy of plant reproduction. In: Reekie EG, Bazzaz FA (eds) Reproductive allocation in plants. Elsevier Academic Press, Burlington, pp 1–49
Kawano S (1981) Trade-off relationships between some reproductive characteristics in plants with special reference to life history strategy. Bot Mag Tokyo 94:285–294
Kawano S, Miyake S (1983) The productive and reproductive biology of flowering plants. X. Reproductive energy allocation and propagule output of five congeners of the genus Setaria (Gramineae). Oecologia 57:6–13
Kleyer M, Minden V (2015) Why functional ecology should consider all plant organs: an allocation-based perspective. Basic Appl Ecol 16:1–9
Kőrner C (1994) Biomass fractionation in plants: a reconsideration of definitions based on plant functions. In: Roy J, Garnier E (eds) A whole plant perspective on carbon-nitrogen interactions. SPB Academic Press, The Hague, pp 173–185
Li C, Li H, Yang Y (2019) Senescence in growth and reproductive allocation in a bunchgrass. Plant Biol 21:300–306
Lovett Doust J (1989) Plant reproductive strategies and resource allocation. Trends Ecol Evol 4:230–234
Marbà N, Duarte CM, Agusti S (2007) Allometric scaling of plant life history. Proc Nat Acad Sci USA 104:15777–15780
Martinková Z, Honĕk A (1992) Effect of plant size on the number of caryopses in barnyard grass, Echinochloa crus-galli (Poaceae). Preslia Praha 64:171–176
Mason CM, Goolsby EW, Davis KE, Bullock DV, Donovan LA (2017) Importance of whole-plant biomass allocation and reproductive timing to habitat differentiation across the North American sunflowers. Ann Bot 119:1131–1142
McCarthy MC, Enquist BJ (2007) Consistency between an allometric approach and optimal partitioning theory in global patterns of plant biomass allocation. Funct Ecol 21:713–720
Morris WF (2009) Life history. In: Levin SA (ed) The Princeton guide to ecology. Princeton University Press, Princeton, pp 126–136
Moser LE, Burson BL, Sollenberger LE (2004) Warm-season (C4) grass overview. In: Moser LE, Burson BL, Sollenberger LE (eds) Warm-season (C4) grasses. American Society of Agronomy, Crop Science Society of America, Soil Science Society of America, Madison, pp 1–14
Niklas KJ (1994) Plant allometry. University of Chicago Press, Chicago
Niklas KJ, Enquist BJ (2003) An allometric model for seed plant reproduction. Evol Ecol Res 5:79–88
Olszewski MW, Folin GJ (2009) Germination responses of purpletop and big bluestem caryopses subjected to distilled water or potassium nitrate prechilling, sodium hypochlorite, and storage. Hort Sci 44:1407–1411
Partridge L, Harvey PH (1988) The ecological context of life history evolution. Science 241:1449–1454
Paul-Victor C, Turnbull LA (2009) The effect of growth conditions on the seed size/number trade-off. PLoS ONE 4:e6917. https://doi.org/10.1371/journal.pone.0006917
Pitelka LF, Stanton DS, Peckenham MO (1980) Effects of light and density on resource allocation in a forest herb, Aster acuminatus (Compositae). Am J Bot 67:942–948
Poorter H, Sack L (2012) Pitfalls and possibilities in the analysis of biomass allocation patterns in plants. Front Plant Sci 3:1–10
Pyke DA (1990) Comparative demography of co-occurring introduced and native tussock grasses: persistence and potential expansion. Oecologia 82:537–543
Quinn JA, Hodgkinson KC (1984) Plasticity and population differentiation in reproductive characters and resource allocation in Danthonia caespitosa (Gramineae). Bull Torrey Bot Club 111:19–27
Reekie EG (1998) An explanation for size-dependent reproductive allocation in Plantago major. Can J Bot 76:43–50
Reekie EG (1999) Resource allocation, trade-offs, and reproductive effort in plants. In: Vuorisalo TO, Mutikainen (eds) Life history evolution in plants. Kluwer Academic, Dordrecht, pp 173–193
Reznick D (2017) Evolution of life histories. In: Losos JB (ed) The Princeton guide to evolution. Princeton University Press, Princeton, pp 270–277
Roos FH, Quinn JA (1977) Phenology and reproductive allocation in Andropogon scoparius (Gramineae) populations in communities of different successional stages. Amer J Bot 64:535–540
Roybal CM, Butterfield BJ (2019) Species-specific trait-environment relationships among populations of widespread grass species. Oecologia 189:1017–1026
Salguero-Gómez R, Violle C, Gimenez O, Childs D (2018) Delivering the promises of trait-based approaches to the needs of demographic approaches, and vice versa. Funct Ecol 32:1424–1435
Samson DA, Werk KS (1986) Size-dependent effects in the analysis of reproductive effort in plants. Am Nat 127:667–680
Sarath G, Baird LM, Mitchell RB (2014) Senescence, dormancy and tillering in perennial C4 grasses. Plant Sci 217:140–151
Schaffer WM, Gadgil MD (1975) Selection for optimal life histories in plants. In: Cody ML, Diamond JM (eds) Ecology and evolution of communities. Harvard University Press, Cambridge, pp 142–157
Schmid B, Bazzaz FA, Weiner J (1995) Size dependency of sexual reproduction and of clonal growth in two perennial plants. Can J Bot 73:1831–1837
Smith CC, Fretwell SD (1974) The optimal balance between size and number of offspring. Am Nat 108:499–506
Stearns SC (1976) Life-history tactics: a review of the ideas. Quart Rev Biol 51:3–47
Stearns SC (1977) The evolution of life history traits: A critique of the theory and a review of the data. Ann Rev Ecol Syst 8:145–171
Stearns SC (1989) Trade-offs in life-history evolution. Funct Ecol 3:259–268
Stearns SC (1992) The evolution of life histories. Oxford University Press, New York
Sugiyama S, Bazzaz FA (1998) Size dependence of reproductive allocation: the influence of resource availability, competition and genetic identity. Funct Ecol 12:280–288
Thompson K, Stewart AJA (1981) The measurement and meaning of reproductive effort in plants. Am Nat 117:205–211
United States Department of Agriculture (2002) Purpletop: Tridens flavus (L.) Hitchc. Plant Fact Sheet, Natural Resources Conservation Service, Plant Materials Program (https://plant-materials.nrcs.usda.gov)
Valdés-Reyna J (2003) Tridens Roem. & Schult. In: Barkworth ME, Capels KM, Long S, Piep MB (eds) Flora of North American North of Mexico, Volume 25, Magnoliophyta: Commelinidae (in part): Poaceae, part 2. Oxford University Press, New York, pp 33–40
Venable DL (1992) Size-number trade-offs and the variation of seed size with plant resource status. Am Nat 140:287–304
Weiner J (2004) Allocation, plasticity and allometry in plants. Pesp Plant Ecol Evol Syst 6:2017–2215
Weiner J, Campbell LG, Pino J, Echarte L (2009) The allometry of reproduction within plant populations. J Ecol 97:1220–1233
Welham CVJ, Setter RA (1998) Comparison of size-dependent reproductive effort in two dandelion (Taraxacum officinale) populations. Can J Bot 76:166–173
Wenk EH, Falster DS (2015) Quantifying and understanding reproductive allocation schedules in plants. Ecol Evol 5:5521–5538
Williams JL, Auge H, Maron JL (2008) Different gardens, different results: native and introduced populations exhibit contrasting phenotypes across common gardens. Oecologia 157:239–248
Wilson AM, Thompson K (1989) A comparative study of reproductive allocation in 40 British grasses. Funct Ecol 3:297–302
Younginger BS, Sirová D, Cruzan MB, Ballhorn DJ (2017) Is biomass a reliable estimate of plant fitness? Appl Plant Sci 5:1600094
Acknowledgements
The manuscript benefited greatly from the comments and suggestions of two anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Wayne Polley.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cheplick, G.P. Life-history variation in a native perennial grass (Tridens flavus): reproductive allocation, biomass partitioning, and allometry. Plant Ecol 221, 103–115 (2020). https://doi.org/10.1007/s11258-019-00996-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-019-00996-z