Abstract
Understanding which plant traits confer invasiveness has been a central question in biological invasions research. Heterotheca subaxillaris (camphor-weed), an American plant, is an important invader of coastal sand dunes in Israel. Nevertheless, there has been no comprehensive comparative analysis of H. subaxillaris plant traits from native versus introduced habitats that sheds light on the invasion success of this species in Israel. I studied H. subaxillaris from native (US) versus introduced (Israel) populations to identify functional traits that accorded this species invasion success in Israel. Plant traits considered were shoot and root biomass production, root–shoot ratio, shoot height, root length, number of inflorescences, achene number and mass, and life span. Achenes (seeds) of all populations were germinated under common growing conditions to produce F1 achenes. F1 seedlings were grown in a large-scale common garden aeroponic system until flowering and then harvested. Introduced populations exhibited marked differences in measured parameters than native populations. Notably, root length of introduced populations exceeded 5 m, almost fourfold greater than that of native populations, allowing access to soil moisture and nutrients from deep sand layers and late-summer flowering. Life span of introduced populations almost doubled that of American populations. To the best of my knowledge, this is the first documentation of adaptive micro-evolutionary change favoring deep root allocation and phenological change in an invasive species in sand dunes. Seemingly, a rapid evolutionary change favoring root resource allocation occurred within introduced populations, allowing establishment, expansion, and successful invasion in the harsh ecosystem of Israel’s coastal sand dunes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The spread of organisms into novel habitats and the widespread effects of invasive species on native species have prompted growing international concern (van Kleunen et al. 2015). An increasingly mobile human population enhances the potential for movement of propagules of nonnative species to new habitats, providing multiple opportunities for new invaders to become established (Mack et al. 2000).
Although there is a long tradition of research on invasive species, there is still a lack of consensus as to what predisposes a species to be a successful invader (Kolar and Lodge 2001; Sakai et al. 2001; Mack et al. 2000). Given all these concerns regarding the process of invasion, Sakai et al. (2001) argue that one key to developing a fuller understanding of the elements behind successful invasion is to study the biology of species that have already successfully invaded. This approach may provide insights that will allow us to generalize more effectively about what traits predispose a species toward being a successful invader, especially if we also consider the population biology of the invader in its native range. This is particularly important in the case of aggressive species that have come to dominate their new habitats, threatening the survival of native species and degrading the integrity of local plant communities. By gaining a better understanding of the ecology of an invading species, we will not only improve our understanding of how to control its spread and eliminate it from areas where it has already established a foothold, but also gain insights into the mechanisms of invasions in general and thereby be better able to predict which nonindigenous species pose the greatest risk of becoming invasive (Callaway and Maron 2006).
Although coastal sand dune ecosystems are reportedly among the most severely threatened by plant invasions, very few studies have analyzed the impact of alien species (Rodgers and Parker 2003; Campos et al. 2004). Coastal sandy soil habitats are exposed to high radiation, a high temperature range, and sand mobility. The Israeli Mediterranean coastal dune, which extends lengthwise 190 km, is rich in rare plants alongside psammophile (sand-loving) species (Kutiel and Danin 1987). This ‘corridor’ is rich with a diversity of flora and fauna, including 26 % of all the endemic plant species of Israel (Kutiel 2001). Coastal sand dunes can provide sensitive niches for alien species invasion, due to the typical sparse plant cover.
Very few species have invaded coastal sand dune ecosystems in Israel, but the few that have succeeded have had a great impact on the landscape and on ecosystem functioning (Bar et al. 2004). One of the key invasive species is Heterotheca subaxillaris (Asteraceae). This species was originally introduced from southwest USA in the early 1970s from one single, discrete population with the purpose of stabilizing mobile sand dunes and preventing sand movement into agricultural areas (Dafni and Heller 1990). Heterotheca subaxillaris quickly escaped its original planting areas and has now colonized extensive areas of the coastal sand dunes in Israel (Dufour-Dror 2012). Its distribution range is expanding rapidly and it has become an important invasive species, present along most of the Israeli coastal plain. This species is also known as “camphor-weed” due to the presence of odorous glandules on its leaves that produce a range of mono- and sesquiterpenes with a strong camphor odor (Morimoto et al. 2009; Lonard et al. 2011) that provide chemical defense against herbivores. All these features apparently facilitate their invasive ability. Nevertheless, there has been no comprehensive comparative analysis of H. subaxillaris plant traits from native versus introduced populations which allows us to understand the invasion success of this species in resource-poor ecosystems such as coastal sand dunes. Here, key plant traits of H. subaxillaris from native (USA) versus introduced (Israel) habitats were compared to identify potential functional traits that have allowed this species to become a successful invader in Israel. Specifically, I asked: which plant traits and mechanisms allowed this species to expand its presence in resource-poor ecosystems such as the coastal sand dunes in Israel?
Materials and methods
Plant material collection
Heterotheca subaxillaris produces two types of achenes according to their position in the inflorescence: (a) seeds with a pappus from the tubular (disk) flowers and (b) seeds without a pappus from lateral (ray) flowers (Venable and Levin 1985; Gibson and Tomlinson 2002). The two types of seeds differ in their dormancy and dispersal traits (Lonard et al. 2011). Seeds from tubular flowers have no physiological dormancy and are capable of long-distance dispersal, while seeds from lateral flowers show the opposite trend (Baskin and Baskin 1976).
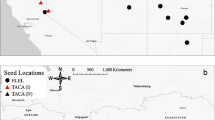
Achenes from tubular flowers were collected in summer (August) 2009 from three American populations (Table 1). These populations are considered to characterize the native origin of the populations that later invaded the Israeli coastal plain. In autumn (October) 2009, achenes from four populations in Israel were collected (Table 1). Each introduced population was separated from the others by at least 20 km. At each sampling site, inflorescences with fruits originating from at least ten different individuals were collected and mixed together to create a representative sample of the population.
Plant growth and sampling
In November 2009, 50 achenes from each population were sorted and put in plastic germination trays filled with washed sand. Achenes (seeds) were germinated in a net house at the Botanical Garden at Tel Aviv University under optimal watering conditions (i.e., constant soil moisture) with drizzle sprinklers above each tray. One-month-old seedlings were transplanted to 15-l plastic plant pots filled with sand (25 cm) over a gravel base layer (5 cm), representing soil conditions similar to those found in the sand dune areas. Native and introduced populations were grown under optimal watering conditions at two separate and distant net houses within the Botanical Garden in order to prevent any potential pollen contact between them, particularly considering that this species was described as self-incompatible (Olsen 1997). The aim of growing the different populations of H. subaxillaris under uniform, standard conditions was to obtain a homogeneous F1 generation of achenes to focus on, while minimizing potential maternal effects arising from the original habitat in which the mother plants developed (for maternal effects, see Gutterman 1992; Donohue and Schmitt 1998). The American populations developed and flowered in July 2010. Newly produced F1 achenes were collected in September 2010 and kept under dry conditions. The introduced populations remained with rosette leaves during the entire first year of growth and produced shoots with flowers only in August 2011. Achenes from the introduced populations were collected in September 2011. This means that the life span from germination to flowering for the American populations was 9 months, while for the Israeli populations it was 21 months.
In November 2011, twenty F1 tubular achenes of each of the native and introduced populations were put in plastic germination trays filled with washed sand and germinated in an ambient net house under optimal watering conditions. In January 2012, ten 2-month-old seedlings of each population were transplanted and moved to an aeroponic chamber (rhizotron) in the Sarah Racine Root Research Laboratory at the Botanical Garden of Tel Aviv University (Waisel 2002; http://www.tau.ac.il/lifesci/units/Gardens/roots.htm). Seedling roots were washed with tap water to remove attached sand grains. A total of 70 seedlings (ten seedlings from each population) were grown in a common garden experiment in this unique infrastructure where the roots stood in the air, while the shoots were kept attached to the floor through a perforated plastic pot (Fig. 1a). The roots were sprayed intermittently with a tap-water-based nutrient solution containing 0.75 g L−1 compound 20:20:20 NPK fertilizer (containing 20 % N compounds, 20 % P2O5, and 20 % K2O and microelements; Haifa Fertilizers, Israel), 0.11 g L−1 CaCl2·2H2O, and 0.14 g·L−1 MgSO4·7H2O. The shoots were in an air-conditioned greenhouse (Eshel and Grünzweig 2013). American populations (Table 1) flowered in July 2012 and were harvested in October 2012 after fruit set and no evidence of additional new inflorescences. Israeli populations started flowering only in June 2013 and were harvested in October 2013. Shoots and roots were sorted, measured, and dried to constant dry weight to estimate biomass production. The number of inflorescences per plant was estimated by counting the number of inflorescences on each of three random shoots and multiplying the average number by the number of shoots developed on each individual. Achene mass was estimated by randomly selecting 100 tubular achenes from inflorescences of each individual. Achene mass was determined using a microbalance with an accuracy of 0.001 mg.
a Rhizotron growth chamber at the Sarah Racine Root Research Laboratory. Plants are grown in perforated flowering pots attached to the chamber floor, while roots are grown in aeroponic conditions. b Individual of H. subaxillaris removed from the aeroponic chamber with developed root system exposed. Metric ruler indicates root length >6 m
Data presented here represent the individuals of different populations grown in the rhizotron infrastructure only.
Statistical analysis
Measured parameters were analyzed using a linear mixed-effects model with origin (native vs. introduced) and population origin as fixed effects and measured plant traits as random effects. The Tukey’s HSD test was applied for pairwise comparisons. Plant biomass production, shoot height, root length, number of inflorescences, and achene mass data were log-transformed prior to analysis to achieve homoscedasticity (Sokal and Rohlf 1995). All statistical analyses were conducted using JMP Pro 12.2 software (SAS Institute Inc., Cary, NC, USA 2015).
Results
The results presented here were obtained from H. subaxillaris individuals of native and introduced populations that were grown at the rhizotron only.
Plant biomass production of the introduced populations was significantly higher than that of the native ones (Table 2; Fig. 2a). Shoot and root biomass production of the introduced populations was double of that of the native ones, indicating a significant change in size since the arrival of this species to Israel some 40 years ago. Similarly, plant height of the invasive populations was double that of native populations, while root length was almost four times greater in introduced populations than in native ones (Table 2; Fig. 2b). The root–shoot ratio also differed significantly between the introduced and native populations. The introduced populations exhibited a ratio greater than 1, indicating greater investment in roots, while the mean ratio for American populations was 1, indicating equal investment in roots and shoots. Reproductive investment was also significantly higher in the introduced populations than the native ones, as the number of inflorescences per shoot and per plant was significantly higher in the introduced populations (Table 2). Considering that each inflorescence holds at least 60 disk achenes (Venable and Levin 1985), and a mean invasive individual can produce on average more than 2300 inflorescences, the amount of achenes produced by one individual can be estimated to reach 138,000 achenes. This production is almost seven times greater than that of native American populations. Mean achene mass of the introduced populations was also significantly higher (Table 2).
The life span of plants from the introduced populations was much greater than that of plants from the native ones. Individuals of the invasive populations remained with rosette leaves for 12 months before producing aerial shoots. On the contrary, native populations of H. subaxillaris started producing aerial shoots 6 months after germination, showing much more rapid development.
Discussion
Alien species with the ability to take advantage of an unexploited resource have the capacity to evolve and become invasive (Davidson et al. 2011; Drenovsky et al. 2012). This is what occurred in the case of H. subaxillaris individuals that escaped from the sand dune stabilization experiment and underwent rapid trait evolution that favored root resource allocation, extended life span, and high seed production, leading to invasion. Schlaepfer et al. (2010) found similar patterns of adaptation among 14 different species in a common garden study comparing invasive and noninvasive plants. These changes are reflected by the clear trait differentiation between native and introduced populations in the present study. The fact that H. subaxillaris in Israel flowers in September, after a long, hot and dry summer (following at least 5 rainless months), poses questions about allocation of reproductive resources during this most stressful period. The results obtained here shed new light on this mystery, showing that H. subaxillaris individuals developed a root system that enables them to reach water in deep sand layers and use unexploited resources. Nevertheless, it seems that the introduced population shared a genetic pre-disposition for deeper roots that was the recipe for a successful dune genotype to be selected for. Additionally, this differential investment to deep root development is supported by the balance-growth hypothesis (Shipley and Meziane 2002). This idea supports that plants will preferentially allocate biomass to the plant organ that is harvesting the resource limiting growth. Considering that light in these opened ecosystems is not a limiting factor, while water and mineral nutrients are scare, higher biomass allocation to root production is favored. Development of deeper root systems was also found for the South African invasive species, Senecio inaequidens (Bossdorf et al. 2008). The large amount of achenes produced by each individual of the introduced populations, regardless of its identity, indicates the potential for colonization and cover expansion of this species. Phenological aspects underlie this phenomenon. Similar results were also found in a study comparing plant traits of native versus exotic species in sand dunes in Italy (Stanisci et al. 2010). Exotic species were capable of expanding their life cycle, while most native species have already finished their own. The flowering period under field conditions in Israel begins in September and extends until April of the following year. The deep-rooting capabilities enable the use of soil resources in autumn before the onset of the winter rains. Once the rainy season commences and more resources become available, new inflorescence buds are produced continually until spring. It is seems that photoperiod does not have an important role in the production of new flowering buds, but the availability of soil nutrients. Similar results were observed when studying reproductive phenology of the invasive Centaurea solstitialis (yellow starthistle) in western US (Roche et al. 1997). This extended flowering period of H. subaxillaris results in the production of thousands of seeds per individual plant. The native populations in the USA exhibit a much shorter flowering period and life span, as frost or cold conditions in winter in southwestern USA where the plant is found cause plant death. Additionally, our results also showed that H. subaxillaris is currently occupying a temporal and spatial niche in the Israeli coastal sand dune area that has not yet been exploited by native species and, therefore, they are not competing directly for the same resources. To the best of my knowledge, this is the first time that adaptive micro-evolutionary change favoring root allocation and phenological change in an invasive species in sand dunes has been documented. A rapid evolutionary change occurred within the introduced populations favoring root resources’ allocation and allowed this species to evolve, establish, expand its range, and successfully invade the harsh ecosystem of Israel’s coastal sand dunes.
Rapid evolutionary change in invasive species is not a rare event; a large body of literature has found widespread evidence of this phenomenon in invading populations (see Sakai et al. 2001; Mack et al. 2000; Bossdorf et al. 2005; Dlugosch and Parker 2008). Here, I follow Hairston et al. (2005) in defining ‘rapid’ evolutionary change as ‘genetic change occurring rapidly enough to have a measurable impact on simultaneous ecological change.’ The measurable impact of H. subaxillaris is clear during the flowering period as the costal sand dunes turn yellow due to the proliferous flowering shoots of this species. Demonstrating that adaptive evolutionary change in ecologically relevant traits has taken place in an invasive plant species requires careful design of a common garden experiment that combines ecological and genetic factors to test for local adaptation in native vs. introduced populations (Bossdorf et al. 2005; Callaway and Maron 2006). Genetic studies are needed to shed further light on the introduced populations, but traits that have undergone evolutionary change in H. subaxillaris are diverse (i.e., deep rooting, extended phenology, insensitivity to photoperiod), and have allowed this species to colonize great expanses of the coastal sand dunes, becoming one of the most successful invasive species in Israel.
References
Bar P, Cohen O, Shoshany M (2004) The invasion rate of the alien species Acacia saligna within the coastal sand dune habitats of Israel. Isr J Plant Sci 52:115–124
Baskin JM, Baskin CC (1976) Germination dimorphism in Heterotheca subaxillaris var. subaxillaris. B Torrey Bot Club 103:201–206
Bossdorf O, Auge H, Lafuma L, Rogers WE, Siemann E, Prati D (2005) Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia 144:1–11
Bossdorf O, Lipowsky A, Prati D (2008) Selection of preadapted genotypes allowed Senecio inaequidens to invade Central Europe. Divers Distrib 14:676–685
Callaway RM, Maron JL (2006) What have exotic plant invasions taught us over the past 20 years? Trends Ecol Evol 7:369–374
Campos JA, Herrera M, Biurrun I, Loidi J (2004) The role of alien plants in the natural coastal vegetation in central—northern Spain. Biodivers Conserv 13:2275–2293
Dafni A, Heller D (1990) Invasions of adventive plants in Israel. In: Di Castri F, Hansen AJ, Debussche M (eds) Biological invasions in Europe and the Mediterranean basin. Kluwer Academic Publishers, Dordrecht, pp 135–160
Davidson AM, Jennions M, Nicotra AB (2011) Do invasive species show higher phenotypic plasticity than native species and if so, is it adaptive? A meta-analysis. Ecol Lett 14:419–431
Dlugosch KM, Parker IM (2008) Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Mol Ecol 17:431–449
Donohue K, Schmitt J (1998) Maternal environmental effects: adaptive plasticity? In: Mousseau TA, Fox CW (eds) Maternal effects as adaptations. Oxford University Press, Oxford, pp 137–158
Drenovsky RE, Grewell BJ, D’Antonio CM, Funk JL, James JJ, Molinari N, Parker IM, Richards CL (2012) A functional trait perspective on plant invasion. Ann Bot 110:141–153
Dufour-Dror JM (2012) Alien invasive plants in Israel, 1st edn. Gefen Publishing, Jerusalem
Eshel A, Grünzweig J (2013) Root-shoot allometry of tropical forest trees determined in large-scale aeroponics. Ann Bot 112:291–296
Gibson JP, Tomlinson AD (2002) Genetic diversity and mating system comparisons between ray and disc achene seed pools of the heterocarpic species Heterotheca subaxillaris (Asteraceae). Int J Plant Sci 163:1025–1034
Gutterman Y (1992) Maternal effects on seeds during development. In: Fenner M (ed) Seeds: the ecology of regeneration in plant communities. CAB International, Wallingford, pp 59–84
Hairston NG, Ellner SP, Geber MA, Yoshida T, Fox JA (2005) Rapid evolution and the convergence of ecological and evolutionary time. Ecol Lett 8:1114–1127
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16:199–204
Kutiel P (2001) Conservation and management of the Mediterranean coastal sand dunes in Israel. J Coast Conserv 7:183–192
Kutiel P, Danin A (1987) Annual species diversity and above ground phytomass in relation to some soil properties in the sand dunes of the northern Sharon Plains, Israel. Vegetatio 70:45–49
Lonard RI, Judd FW, Stalter R (2011) Biological flora of coastal dunes and wetlands: Heterotheca subaxillaris (Lam.) Britton & Rusby. J Coast Res 27:1052–1058
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
Morimoto M, Cantrell CL, Libous-Bailey L, Duke SO (2009) Phytotoxicity of constituents of glandular trichomes and the leaf surface of camphorweed, Heterotheca subaxillaris. Phytochemistry 70:69–74
Olsen KM (1997) Pollination effectiveness and pollinator importance in a population of Heterotheca subaxillaris (Asteraceae). Oecologia 109:114–121
Roche CT, Thill DC, Shafii B (1997) Reproductive phenology in yellow starthistle (Centaurea solstitialis). Weed Sci 47:763–770
Rodgers JC III, Parker KC (2003) Distribution of alien plant species in relation to human disturbance on the Georgia Sea Islands. Divers Distrib 9:385–398
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O’Neil P, Parker IM, Thompson JN, Weller SG (2001) The population biology of invasive species. Ann Rev Ecol Syst 32:305–332
Schlaepfer DR, Glättli M, Fischer M, van Kleunen M (2010) A multispecies experiment in their native range indicates pre-adaptation of invasive alien plant species. New Phytol 185:1087–1099
Shipley B, Meziane D (2002) The balanced-growth hypothesis and the allometry of leaf and root biomass allocation. Funct Ecol 16:326–331
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research, 3rd edn. WH Freeman and Company, New York
Stanisci A, Acosta ATR, Di Iorio A, Vergalito M (2010) Leaf and root trait variability of alien and native species along Adriatic coastal dunes (Italy). Plant Biosyst 144:47–52
van Kleunen M, Dawson W, Essl F, Pergl J, Winter M, Weber E et al (2015) Global exchange and accumulation of non-native plants. Nature 525:100–103
Venable DL, Levin DA (1985) Ecology of achene dimorphism in Heterotheca latifolia. 1. Achene structure, germination and dispersal. J Ecol 73:133–145
Waisel Y (2002) Aeroponics: a tool for root research under minimal environmental restrictions. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots: the hidden half, 3rd edn. Marcel Dekker, New York, pp 323–331
Acknowledgments
I thank Claus Holzapfel for the exchange of ideas about comparing native vs. invasive populations of H. subaxillaris. Thanks are also extended to Lauch Fraser for inviting me to be part of this special issue celebrating Roy Turkington’s retirement. I came to know Roy during one of his sabbatical stays in the Holy Land, and very much enjoyed discussing ecology and nature with him. This study was partly supported by internal funds from Tel Aviv University. The support of the Sarah Racine Root Research Laboratory at the Botanical Garden of Tel Aviv University is highly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Prof. Lauchlan Fraser, Dr. Chris Lortie, and Dr. JC Cahill.
Rights and permissions
About this article
Cite this article
Sternberg, M. From America to the Holy Land: disentangling plant traits of the invasive Heterotheca subaxillaris (Lam.) Britton & Rusby. Plant Ecol 217, 1307–1314 (2016). https://doi.org/10.1007/s11258-016-0656-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-016-0656-z