Abstract
Seed predation and dispersal play key roles in the regeneration of tropical trees. Pre-dispersal predation may cause high mortality in seed crops. Seeds may escape pre-dispersal predation when ingested with the fruit pulp and moved away from the parent trees by frugivores. In southern Mexico, we investigated if seed traits (i.e., length, width, and mass) and seed damage by insects on Dialium guianense differed according to seed source: from the tree crowns, the ground, and from howler (Alouatta pigra) and spider monkey (Ateles geoffroyi) feces. We counted the number of seeds with circular entrance and/or exit holes in their tegument. Ingested seeds were larger, heavier, and wider than non-ingested seeds. Seeds ingested by the howler were, however, significantly larger than those ingested by the spider monkey. Damaged seeds showed the lowest values for all seed traits. The proportion of damage declined significantly from seeds on the ground (37 %), to seeds in spider monkey feces (29 %), to seeds from tree crowns (11 %), and finally to seeds in howler monkey feces (7 %). Fruit selection by primates influences dispersal quality differently, even when feeding on the same plant species. The howler monkey may increase the reproductive success of D. guianense by selecting larger and predation-free seeds/fruits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seed predation and dispersal by animals play important roles in the population dynamics and regeneration of tropical trees, as well as in the maintenance of tropical rain forest biodiversity (Howe and Smallwood 1986; Schupp et al. 2010). Seeds are prone to predation by animals and infected by pathogens from the early stages of development into full maturity, and such predation is a key factor affecting seed mortality, with consequences for plant demography and evolution (Howe and Smallwood 1986; Crawley 1992; Maron and Crone 2006; Beckman and Muller-Landau 2011). There are two main types of seed predation: (1) pre-dispersal seed predation, which occurs while seeds are still attached to their parent plant, and (2) post-dispersal seed predation, which occurs once seeds have been dispersed or detached from their parent plant. Commonly, pre-dispersal seed predation is largely carried out by insects many of which develop inside the seeds (Crawley 1992). In contrast, post-dispersal seed predation is largely carried out by vertebrates such as rodents, but ants and beetles are also important post-dispersal seed pests; moreover, fungal pathogens cause important seed loss (Janzen 1970, 1980; Crawley 1992; Shiels and Drake 2011; Fricke et al. 2014). Nevertheless, vertebrates and fungi can be important pre-dispersal seed predators during fruit development, whereas many mammals thought to be post-dispersal seed predators are described as secondary seed dispersers (Peres 1991; Shiels and Drake 2011; Jansen et al. 2012). The relative importance of both insects and vertebrates as pre- and post-dispersal seed predators however, has been understudied in tropical forest (Nakagawa et al. 2005; Beckman and Muller-Landau 2011; Fricke et al. 2014).
Pre-dispersal predation by animals and pathogens on tropical trees may cause mortality in up to 80 % of the seeds or more (Janzen 1980; Peres 1991; Sallabanks and Courtney 1992; Silvius and Fragoso 2002; Beckman and Muller-Landau 2011) and occurs mainly between seed formation and seed maturity (Moles et al. 2003; Beckman and Muller-Landau 2011). In addition, when fruits are shed from the parent trees, seeds are further exposed to soil-living pests and pathogens (post-dispersal seed predation). Predation before and after fruit shedding and dispersal makes attacked seeds more prone to fungal infection and the production-less vigorous seedlings (Janzen 1970; Nakagawa et al. 2005; Vallejo-Marín et al. 2006; Beckman and Muller-Landau 2011; Fricke et al. 2014). Insects, however, may increase the protein, lipid, and other nutrient contents of the fruits and seeds that may supplement the diets of several frugivore species (Silvius and Fragoso 2002; Bravo 2008; Felton et al. 2009a) and may also scarify the seed coat which improves germination (Vallejo-Marín et al. 2006; Fox et al. 2012).
Pre- and post-dispersal seed predation within and among plant species, however, might depend on seed traits, including length, weight, width, hardness, and dispersal timing, among others (Bravo 2008; Moles et al. 2003; Vallejo-Marín et al. 2006; Baraloto and Forget 2007; Beckman and Muller-Landau 2011). For instance, large seed size might be a potential disadvantage as large seeds are easier to locate and more attractive to seed predators than are small seeds. In contrast, large seed size provides the advantage of greater reserves for the embryo, greater tolerance to attack, and also greater probability of seedling establishment (Paz and Martínez-Ramos 2003; Moles et al. 2003; Baraloto and Forget 2007; Beckman and Muller-Landau 2011). In addition, dispersal efficiency is frequently correlated with both fruit and seed size (Jordano 2014).
In Neotropical rain forests, natural regeneration, and especially that of large-seeded forest trees, is strongly dependent upon animal-mediated seed dispersal (Howe and Smallwood 1986; Jordano 2014). Arboreal primates play a critical role in enhancing the recruitment of several tree species by dispersing their seeds. The manner in which fruits are searched for, handled, and efficiently processed by primates has consequences for plant populations (Julliot 1996; Milton 1998; Link and Di Fiore 2006; Martins 2006; Dew 2008; Russo and Chapman 2011). The most common means of primate dispersal in the Neotropics is seed swallowing (Russo and Chapman 2011). In the tropical rain forest of the Montes Azules Biosphere Reserve (MABR), Chiapas, Mexico, the black howler monkey, Alouatta pigra, and the black-handed spider monkey, Ateles geoffroyi, coexist in the forest and share in their diets at least 35 tree species (Estrada et al. 2004; Benítez-Malvido et al. 2014). One of such species is the legume tree Dialium guianense (Aubl.) Sandw. (Caesalpinioideae) that is codominant in the forest canopy of the region (Boege and Dirzo 2004). Howler and spider monkeys differ in their morphological (hindgut length), physiological (digestion time and color vision), and food-handling behavior (Milton 1981; Amato and Garber 2014; Benítez-Malvido et al. 2014), which may result in different treatment of swallowed (ingested) seeds, even when feeding on the same plant species (Lambert 1998; Milton 1998; Martins 2006; Traveset et al. 2007; Benítez-Malvido et al. 2014).
Not much is known, however, about how seed handling by sympatric primate species may affect relative seed dispersal success. For the particular tree species D. guianense, we describe the variation in seed traits (i.e., length, width, and weight) and the proportion of insect-damaged seeds among different seed sources (i.e., pre-dispersed seeds within the fruits attached to the parent trees, freshly fallen seeds within the fruits on the ground below the parent tree, and seeds from spider and howler monkey fecal clumps). We refer to “seed source” as the setting from which seeds were obtained. We predicted that because of differences in fruit handling and choice the number of insect-damaged seeds in feces would differ according to primate species. As a consequence of food choice by primates for large ripe fruits in the tree canopies, which frequently hold the larger seeds within a plant species, we expected ingested seeds to be larger, heavier, and wider than non-ingested seeds (Stevenson et al. 2005; Jordano 2014). In addition, we expected non-ingested seeds to be more exposed to predation risks than seeds ingested by primates, as seeds have remained available to insects and other predators while in the trees and beneath, increasing density-dependence mortality risks (Janzen 1970, 1980; Fricke et al. 2014). In our study, seeds beneath the parent trees have been exposed to post-dispersal predation for several days prior to collection, and therefore we expected a greater proportion of seed damage. Finally, because seed predators select seeds based on their characteristics (e.g., size and hardness), we expected differences in seed traits between intact and damaged seeds with insect-damaged seeds being smaller than intact seeds elsewhere (Cipollini and Stiles 1991; Bravo 2008; Vallejo-Marín et al. 2006; Burgos et al. 2008). We discuss the implications of seed damage on the population ecology of D. guianense and on the dispersal quality of both primates.
Methods
Study area
Fieldwork was conducted in the southern portion of the Montes Azules Biosphere Reserve (MABR), Chiapas, Mexico, which has 330,000 ha (Gómez-Pompa and Dirzo 1995) and is part of the Lacandon rain forest region (16°05′58″N, 90º52′36″W, 10–50 m asl), as well as in the Marqués de Comillas region (MCR). The MCR is an adjacent area separated from the MABR by the Río Lacantún and has been modified by human activity. Human colonization of MCR began about 30–40 years ago, and cattle ranching resulted in rapid deforestation and forest fragmentation (Mariaca-Méndez 2002). The climate is hot and humid with mean annual precipitation and temperature of 2874 mm and 25 °C, respectively (Estrada et al. 2004). The predominant vegetation in the area is lowland tropical rain forest with trees reaching heights of 45 m. The forest is represented by canopy trees including D. guianense, Brosimum alicastrum, and Vatairea lundellii, among others (Boege and Dirzo 2004).
Primate species
Two primate species are present in the region: the black howler (Alouatta pigra) and the black-handed spider monkey (Ateles geoffroyi). Both primate species often feed on the fruits, leaves, and flowers from the same plant species (Estrada et al. 2004; Benítez-Malvido et al. 2014). These primates are recognized as important seed dispersers for numerous tree species in Neotropical forests (Stevenson et al. 2002; Link and Di Fiore 2006; Felton et al. 2009a). Overall, the howler monkey is characterized by having a folivorous diet (Di Fiore and Campbell 2007). By contrast, the spider monkey has been described as a fruit specialist (Di Fiore et al. 2008; González-Zamora et al. 2009). Howler population density within the MABR is 0.13 individuals ha−1 (Estrada et al. 2004). Average home-range size of the black howler in continuous forest is <25 ha (Benítez-Malvido et al. 2014). In MABR, the population density of A. geoffroyi has been estimated as 0.03 individuals ha−1 (Estrada et al. 2004), and average home-range size of the spider monkey in continuous forest is <90 ha (Benítez-Malvido et al. 2014).
Howler and spider monkeys have different transit times of ingesta in their guts, about 21 h and 4–5 h, respectively (Milton 1981); they also differ in the dispersal distances of the seeds they ingest, which in the howler monkey may not exceed 300 m, but in the spider monkey these can exceed 1000 m (Link and Di Fiore 2006; Amato and Garber 2014). These differences affect seed dispersal quality (Schupp et al. 2010; Benítez-Malvido et al. 2014). A previous study on the assessment of ingested seeds of D. guianense by howler and spider monkeys with a different seed crop (seeds collected in 2011) indicated that seeds remained intact and viable after ingestion and that the germination rate (speed) and germinability (percentage of cumulative germination) of seeds ingested by the howler monkey are greater than those of non-ingested seeds and seeds ingested by the spider monkey (Table 1 and Benítez-Malvido et al. 2014).
Tree species
Dialium guianense is distributed from southern Mexico to Brazil (Vieira et al. 1996) and is considered to be a slow-growing tree species (Pennington and Sarukhán 1998). Reproductive trees of D. guianense can reach up to 45 m high and measure up to 1.5 m diameter at breast height (dbh). Fruits of D. guianense are indehiscent globose pods, greenish, brownish, with 1–2 seeds (1.5 × 1.5 cm), and have fleshy and/or pulpy structures that offer rewards to frugivores. The density of D. guianense in the study area is of 28.1 trees per ha−1 (> 10 cm dbh), and in continuous forests D. guianense accounts for up to 19 % of all seeds in the seed rain and up to 4 % of the seedling bank (González-Di Pierro, unpubl. data). Furthermore, D. guianense is considered a top food species in the diets of spider and howler monkeys (representing >80 % of their feeding time; González-Zamora et al. 2009; González-Di Pierro et al. 2011). Given the prevalence of this species and the availability and abundance of its fruit and seed crops in the forest, we considered it to be a good candidate for testing the influence of seed source on pre- dispersal seed predation by insects.
Seed dispersal and predation
Fruits of D. guianense are consumed, and their seeds dispersed by a variety of frugivores and granivores throughout its distribution range (Vieira et al. 1996). Among the frugivores, there are several primate species (A. palliata, A. pigra, A. geoffroyi, A. hybridus, and Leontopithecus chrysomelas) and ungulates such as the tapir (Tapirus bairdii) that disperse the seeds (Naranjo 2009). In the study region, on average, the howler monkey (A. pigra) consumed and dispersed more seeds of D. guianense in its feces than the spider monkey (A. geoffroyi): 9 vs. 5.2 seeds, respectively, despite the fact that spider monkeys defecate more times during the day (Benítez-Malvido et al. 2014). Several primate species consume unripe fruits and predate the seeds as well (Lagothrix lagothricha and Chiropotes satanas satanas); furthermore, granivorous birds such as parrots (Brotogeris tirica and Deroptyus accipitrinus) and several orders of insects eat the seeds and foliage (e.g., Lepidopteran larvae and Atta cephalotes) (Simão et al. 1997; Boege and Dirzo 2004; Port-Carvalho and Ferrari 2004).
Within the MABR, several species of insects have been recorded within the seeds and fruits of D. guianense collected during this study (Benítez-Malvido, unpubl. data). These include three species of small Scolytinae (Coleoptera: Curculionidae), one species of Tenebrionidae (Coleoptera), Atrypanius irrorellus (Coleoptera: Cerambycidae), two species of parasitic wasps (Hymenoptera: Bethylidae)—one wingless—and one species of thrips (Thysanoptera: suborder Tubulifera). All these insect orders are known to have seed-feeding species that burrow through and feed within the seeds except for the wasps that parasitize the larvae of other insects. For instance, the beetle A. irrorellus has been reported to infest the seeds of several legume species in tropical Mexico (Cullen et al. 2012). To our knowledge, this is the first report of the insects infesting seeds of D. guianense under natural conditions, and therefore, the mechanisms used by these insects to attack the seeds are not yet known. Nevertheless, the general way beetles infest seeds is as follows: adults lay the eggs on the seeds or fruits, the eggs will hatch into larvae that will tunnel into the seeds, and the larvae will pupate and transform into an adult beetle that would burrow to the outside of the seed to continue the cycle (e.g., Johnson 1981).
Seed collection
Seeds were collected (July through August 2013) from four different sources: (1) pre-dispersed seeds from ripe fruits attached to the parent trees, (2) seeds from ripe fruits on the ground below the parent tree, (3) seeds in howler monkey feces, and (4) seeds in spider monkey feces. Seeds collected from tree crowns were considered as experimental controls as they were not yet dispersed (e.g., by gravity or animals). These were also used to compare with fruit selection preferences by arboreal primates (i.e., seed size and infestation). Seeds from tree crowns and from the ground were collected from five D. guianense trees; the dbh of the trees varied from 110 to 180 cm above the buttresses. A total of 100 seeds per seed source were collected giving a total of 400 seeds. Details on seed collection can be found in Benítez-Malvido et al. (2014). To collect seeds defecated by the primates, we followed three groups of spider monkeys and three groups of howler monkeys from 7h00 am to 17h00. Groups were followed until 100 seeds per seed source were collected. Seeds were collected from 15 fecal clumps of A. geofroyii and from 18 fecal clumps in the case of A. pigra immediately after defecation, and therefore, seeds in feces were not exposed to soil pests and pathogens. In A. geofroyii clumps, the total number of D. guianense seeds per group was 16, 52, and 32, whereas that for A. pigra was 32, 28, and 40 seeds, respectively. Once seeds from primate feces were collected, we proceeded to collect the seeds from ripe fruits in tree crowns and from ripe fruits on the ground, taking care not to collect rotten fruits. Primates fed from the same trees from which seeds were collected. Overall, the seed collection process took less than 1 week to complete. Insect-damaged seeds in fecal clumps were damaged prior to dispersal by primates while damaged seeds on the ground may have been infested prior and/or after shedding.
Seed traits and insect damage
Immediately after collection, all seeds were removed from the fruits and rinsed with water to manually remove the fleshy aril. Some pods had larvae, pupae, and adult insects in their pulp and seed. Seeds in feces were thoroughly washed to remove fecal matter and attached plant material; however, we found neither living nor dead insects therein. Seed cleaning was applied in order to measure seed metrics accurately. For each seed collected, we measured its length, width, and weight. Within each seed source, we counted the number of seeds that sustained insect predation without opening the seeds to search for insect larvae, pupae, or adults. A seed was considered as predated when it presented round entrance and or exit holes in the tegument. Seeds could have one or more holes as several insects could be reared from a single seed (Benítez-Malvido, unpubl. data). While the estimate of infestation we used is not highly accurate, it nonetheless provides an indication of food selection by sympatric primates and insects. For the statistical analysis, we pooled the seeds according to seed source.
Statistical analysis
Overall, a one-way ANOVA test was used to determine whether seed traits (i.e., length, width, and weight; response variables) differed among seed sources (predictor variable). For testing differences in seed traits between intact and damaged seeds within a seed source, we used a nested-ANOVA. For this case, we have two predictor variables: seed source and seed condition (damaged or not) and seed traits as response variables. For the nested-ANOVA, seed condition (sc) was equal to 2, and the number of seed sources (ss) was 4. Hence, the degrees of freedom for “seed condition” were 1 (i.e., sc -1), and 6 for the “seed condition-within-seed source” (i.e., sc X (ss -1); Sokal and Rohlf 1995). In the case of significant ANOVA results, differences were compared with Tukey tests for multiple comparisons (95 % confidence level).
In addition, to detect general trends in seed traits according to seed condition (intact or damaged) in each seed source category, we performed a principal component analysis (PCA). For this analysis, we used the four seed source categories, seed condition (intact or damaged) and the mean values of the three seed traits described above. We constructed a matrix with seed sources as columns and the seed traits for intact and damaged seeds as rows. We obtained two principal components that explained the greatest amount of variance and used them to construct a two-dimensional Euclidean graph (biplot). The level of association of each axis with seed condition was obtained and was represented as a vector in the graph. The length of each vector indicates the level of association between variables and axes, while the direction of the vector indicates how each seed condition is related to the axes. We used a PCA instead of other analytical methods (i.e., hierarchical cluster analysis) because seed metrics were highly correlated, and this method allows for a better assessment of the differences of seed traits and source within seed condition. Finally, to detect fruit selection by primates and differences in pre-dispersal seed predation among seed sources, we used an analysis of deviance based on generalized linear models for count data (GLM, Crawley 1993). All analyses were performed using R version 3.1.0 (R-Core-Team-R 2015).
Results
Seed source, seed traits, and seed predation
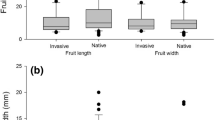
In general, the one-way ANOVA showed a significant difference in seed traits (length, F 3,396 = 57.1; width, F 3,396 = 59.8; and weight, F 3,396 = 56.1; all with P < 0.0001) according to seed source: pre-dispersed seeds attached to the parent trees, seeds on the ground below the parent tree, and seeds from spider and howler monkey fecal clumps. On average, the howler monkey ingested the larger, wider, and heavier seeds of D. guianense, followed by the spider monkey and non-ingested seeds (i.e., those collected from tree crowns and from the ground). Moreover, seeds of D. guianense ingested by the spider monkey were significantly larger, wider, and heavier than non-ingested seeds but consistently smaller than those ingested by the howler monkey, indicating small but consistent differences in fruit choices by primates (Fig. 1). The nested-ANOVA showed that there was a significant effect of seed source on all seed traits between damaged and intact seeds (length, F 1,6 = 19.0; width, F 1,6 = 18.7; and weight, F 1, 6 = 34.2 all with P < 0.0001). The seed condition within seed source showed that for all sources, except for seeds collected from the tree crowns, damaged seeds were consistently smaller, narrower, and lighter than intact seeds (Fig. 1).
Traits (mean + SD) of intact and insect-damaged seeds from Dialium guianense trees (collected in 2013) from different sources: (i) ripe fruits attached to parent trees (tree crowns); (ii) ripe fruits on the ground below parent trees; (iii) howler monkeys´ feces, Alouatta pigra; and (iv) spider monkeys´ feces, Ateles geoffroyi. Different capital letters at the top of the bars indicate significant differences among seed sources, whereas small caps indicate significant difference between intact and damaged seeds within the seed source. The numbers of intact and insect-damaged seeds per seed source (N = 100 per seed source) are indicated in parenthesis
The first two axes of the PCA explained 100 % of total variation in the data. The PCA biplot on the first two principal components formed two clearly distinct groups: (1) large intact seeds predominantly found in primate feces and (2) small and damaged non-ingested seeds (Fig. 2). The PCA differentiated seed weight from the other seed metrics along the first component (PC1), which explained 99.5 % of the total variance. Those damaged seeds collected from tree crowns and from the ground were lighter than ingested seeds. There was a significant positive correlation of intact and damaged (for both cases r = ≥ 0.99, df = 1, P ≤ 0.0001), with PC1. The second principal component PC2, with 0.5 % of total variance, separated damaged non-ingested seeds from those intact seeds found in feces. The correlations, however, were not significant. Lighter, narrower, and smaller seed traits were mainly associated with damaged non-ingested seeds (Fig. 2). The greater difference in seed traits was observed between intact seeds ingested by howler monkeys and seed collected from the tree crowns.
Biplot of a principal component analysis (PCA) for seed traits of intact and insect-damaged seeds. Seeds were collected from different sources including the following: (i) ripe fruits attached to parent trees (tree crowns); (ii) ripe fruits on the ground below parent trees; (iii) howler monkeys´ feces, Alouatta pigra; and (iv) spider monkeys´ feces, Ateles geoffroyi. Seed sources are indicated as follows: TC tree crown, GR ground, AP A. pigra, and AG A. geoffroyi. On the other hand, seeds are indicated traits by lg length, wd width, and wg weight
Most seeds were intact (ca. 80 %) however, the number of insect-damaged seeds differed significantly among seed source (χ2 = 14.7, df = 3, P < 0.005). The proportion of seeds infested by insects increased from seeds ingested by the howler monkey (7 % of the seeds), to seeds collected from the tree crowns (11 %), to seeds ingested by the spider monkey (29 %), and to seeds collected from the ground beneath the parent trees (37 %). Pre-dispersal predation by insects was four times greater in seeds ingested by the spider monkey than those ingested by the howler monkey.
Discussion
Our results indicate that D. guianense seeds are damaged by insects while still attached to the parent tree, that primates may consume attacked fruits and that seeds are further attacked on the ground when shed from the parent trees; however, overall seed predation by insects, pooling all seed sources (i.e., pre-dispersed seeds attached to the parent trees, seeds on the ground, and seeds from spider and howler monkey fecal clumps), is relatively low (ca. 21 %). On the other hand, seed infestation could have been underestimated when holes produced by very small insects or insects within seeds were not detected by the naked eye (e.g., those of tiny Scolytinae <0.5 mm).
Fruit traits that are relevant to frugivores include design (i.e., color, size, and shape), nutrient content, and secondary metabolites (van der Pijl 1982; Jordano 2014). Howler and spider monkeys, though, have different choices when feeding on the same tree species, as has been shown for the trees Ampelocera hottlei, Brosimum lactescens, and Spondias mombin; these choices are also reflected by the constant differences in seed traits found in different studies and in the consumption of fruits infested by insects in D. guianense (see Benítez-Malvido et al. 2014). These findings may have resulted from anatomical constraints such as gape width (i.e., the maximum size primates can swallow) and in the nutritional goals of each primate species, or both (van der Pijl 1982; Wheelwright 1985; Rey et al. 1997; Stevenson et al. 2005; Norconk et al. 2008; Felton et al. 2009a, b; Amato and Garber 2014).
The primate perspective: fruit choice and nutritional goals
Frugivores may regurgitate, swallow, defecate, spit out, or otherwise drop damaged or undamaged seeds away from the parent plants (Howe and Smallwood 1986; Chaves et al. 2011; Jordano 2014). In the present study, primates differed in their seed-handling behaviors with howler monkeys swallowing and defecating significantly larger intact seeds than spider monkeys. In the study area, when foraging on D. guianense spider monkeys spat out ca. 85 % of the seeds (Chaves et al. 2011) while howler monkeys swallow most of them with the fruit (González-Di Pierro, unpub. data; Benítez-Malvido et al. 2014). These results show that sympatric primates feeding on the same plant species select fruits with different characteristics, which might reduce interspecific competition for the same resource (Robertson et al. 2006; Traveset et al. 2007; Norconk et al. 2008). Furthermore, fruit selection could vary according to sex and age class within primate populations, with adult individuals consuming the larger seeds within a plant species (e.g., Lagothrix lagothricha in Stevenson et al. 2005).
The ability of primates to handle, swallow, and process a given fruit efficiently may depend on fruit size relative to the gape width and mouth size (Wheelwright 1985; Rey et al. 1997; Norconk et al. 2008; Russo and Chapman 2011). Although the main effect of fruit size on handling success, especially in single-seeded fruits such as D. guianense, is given by the seed size and not by fruit size (Jordano 2014). The strong selectivity shown by howler and spider monkeys to ingest seeds of different sizes, suggests that fruit size selection is limited by constraints on fruit handling as in bats, birds, and other primates (Kalko et al. 1996; Rey et al. 1997; Stevenson et al. 2005; Norconk et al. 2008; Benítez-Malvido et al. 2014).
Primates meet their nutritional goals by prioritizing certain nutritional parameters when choosing the types and quantities of different foods and plant items (i.e., fruit, leaves, flowers, petioles, or wood; Felton et al. 2009a, b). Diet selection by primates includes five different nutritional goals (sensu Felton et al. 2009a): (1) energy maximization; (2) nitrogen (protein) maximization; (3) avoidance or regulation of the intake of plant secondary metabolites; (4) limitation in the intake of dietary fiber; and (5) nutrient balancing. Based on anatomical, behavioral, and ecological data, researchers have concluded that Ateles spp. are “energy maximizers.” The spider monkeys, Ateles spp., have short food retention times (4 h), large territories (30–90 ha), a fluid social structure (fusion-fission), and prefer to consume fruits rich in sugars and lipids (Dew 2008; Di Fiore et al. 2008; Felton et al. 2009a, b). In contrast, it has been proposed that howler monkeys are protein “maximizers” as they often select protein-rich plant parts (i.e., young and mature leaves) or supplement their herbivorous diets with insects (Bravo 2008; Amato and Garber 2014). In the study area, however, the spider monkeys might be complementing their protein intake by selecting fruits of D. guianense infested by insects (larvae, pupae, and adults rich in N). Ingestion of insect larvae has been described for other Neotropical primates such as Ateles chamek and Alouatta caraya in Bolivia and Argentina, respectively (Bravo 2008; Felton et al. 2009a, b). Depending on the plant species consumed, the timing of seed dispersal, and on seed traits, ingestion of seeds by primates (i.e., A. caraya) might have different effects on seed predation including the following: kill the larvae but not the seeds, disperse insects undamaged, or destroy infested seeds (Bravo 2008). In this study, damaged seeds of D. guianense were defecated, unbroken, and no insect was observed in them. Furthermore, primate selectivity toward infested seeds and fruits likely contributes to insect dispersal and colonization as they may disperse living insects while moving the seeds (Sallabanks and Courtney 1992; Silvius and Fragoso 2002; Bravo 2008). Another explanation is that although spider monkeys are able to swallow long and wide seeds such as those of Spondias spp. (Chaves et al. 2011; Benítez-Malvido et al.2014), there is a clear tendency to swallow relatively small seeds from the large-seeded species including D. guianense, Spondias mombin, Ampelocera hotlei, etc. Therefore, seed ingestion of D. guianense by spider monkeys might be determined by size and not by the presence of insects (Benítez-Malvido et al. 2014). Infested seeds were smaller than intact seeds for all sources in this study with non-ingested seeds having the lowest values for all seed traits (Fig. 1).
The plant perspective: escaping seed predation
From a plant perspective, dispersal of fully developed intact seeds away from the parent and from a co-specific tree may reduce the loss caused by insects and other seed predators. Although beetles and other insects may deplete seed resources by killing the embryo, they may also scarify seeds, which would improve germination (Vallejo-Marín et al. 2006; Fox et al. 2012). In species with hard seed coats, as in the case of some legumes, insect damage may help in overcoming physical dormancy (Bravo 2008; Burgos et al. 2008; Fox et al. 2012). In the study region, intact seeds of D. guianense showed germination percentages below 60 % even when ingested by these primates, indicating that scarification by insects might be needed to improve germination (Benítez-Malvido et al. 2014). Seeds of D. guianense predated by insects were consistently smaller, narrower, and lighter than intact seeds; however, it is uncertain whether or not insects select smaller seeds with a weaker seed coat or if insects stop the growth and development of the embryo (Cipollini and Stiles 1991; Bravo 2008; Vallejo-Marín et al. 2006; Burgos et al. 2008). In D. guianense, seed weight appears to indicate predation probability. According to the PCA analysis, weight was the main seed trait in segregating seed condition (intact or damaged) according to seed source (Fig. 2).
Endozoochory causes loss of the cuticle protection and seed dormancy, as well as an increase in seed size by water uptake; these new conditions may increase germination and seedling establishment (Robertson et al. 2006; González-Di Pierro et al. 2011). Apparently, seeds ingested by howler monkeys were larger because of the combined effects of both a hydrating process during gut passage (21 h) and selectivity for the larger fruits (Traveset and Verdú 2002; Robertson et al. 2006). Note that despite contrasting gut retention times the seed size difference between the two primate species is not very high indicating that monkeys are selecting larger fruits. As a result of their dietary preference for large, ripe, predation-free fruits, of their slow food passage rate through the digestive tract (ca. 21 h), and of their daily movements, the black howler monkey (Alouatta pigra) seems to act as an effective dispersal agent for the seeds of D. guianense.
While both spider and black howler monkeys act as seed dispersal agents for D. guianense in the study area, the latter primate seems to provide a better seed dispersal service. The black howler monkey may increase the reproductive success of D. guianense by dispersing large seeds free of insects (larvae, pupae, and adults) away from the parent tree and by enhancing germination rates and germinability (Traveset et al. 2007; Benítez-Malvido et al. 2014).
References
Amato KR, Garber PA (2014) Nutrition and foraging strategies of the black howler monkey (Alouatta pigra) in Palenque National Park, Mexico. Am J Primatol 76:774–787. doi:10.1002/ajp.22268
Baraloto C, Forget PM (2007) Seed size, seedling morphology, and response to deep shade and damage in Neotropical rain forest trees. Am J Bot 94:901–911
Beckman NG, Muller-Landau HC (2011) Linking fruit traits to variation in predispersal vertebrate seed predation, insect seed predation, and pathogen attack. Ecology 92:2131–2140
Benítez-Malvido J, González-Di Pierro AM, Lombera R, Guillén S, Estrada A (2014) Seed source, seed traits, and frugivore habits: implications for dispersal quality in two sympatric primates. Am J Bot 101:970–978
Boege K, Dirzo R (2004) Intraspecific variation in growth, defense and herbivory in Dialium guianense (Caesalpiniaceae) mediated by edaphic heterogeneity. Plant Ecol 175:59–69
Bravo SP (2008) Seed dispersal and ingestion of insect-infested seeds by black howler monkeys in flooded forests of the Parana River, Argentina. Biotropica 40:471–476
Burgos A, Grezb AA, Bustamante RO (2008) Seed production, pre-dispersal seed predation and germination of Nothofagus glauca (Nothofagaceae) in a temperate fragmented forest in Chile. For Ecol Manag 255:1226–1233
Chaves OM, Stoner KE, Arroyo-Rodríguez V, Estrada A (2011) Effectiveness of spider monkeys (Ateles geoffroyi vellerosus) as seed dispersers in continuous and fragmented rain forests in Southern Mexico. Int J Prim 1:177–192
Cipollini ML, Stiles EW (1991) Seed predation by the bean weevil Acanthoscelides obtectus on Phaseolus species: consequences for seed size, early growth and reproduction. Oikos 60:205–214
Crawley MJ (1992) Seed predators and plant population dynamics. In: Fenner M (ed) The ecology of regeneration in plant communities. Commonwealth Agricultural Bureau International, Wallingford, pp 157–191
Crawley M (1993) GLIM for ecologists. Blackwell, Cambridge
Cullen J, Julien M, Mcfadyen R (2012) Biological control of weeds in Australia. CSIRO Publishing, Melbourne
Dew JL (2008) Spider monkeys as seed dispersers. In: Campbell CJ (ed) Spider monkeys: behavior, ecology and evolution of the genus Ateles. Cambridge University Press, Cambridge, pp 155–182
Di Fiore A, Campbell CJ (2007) The Atelines: variation in ecology, behavior, and social organization. In: Campbell CJ, Fuentes A, Mackinnon KC, Panger M, Bearder S (eds) Primates in perspective. Oxford University Press, Oxford, pp 155–185
Di Fiore A, Link A, Dew JL (2008) Diets of wild spider monkeys. In: Campbell CJ (ed) Spider monkeys: behaviour, ecology and evolution of the genus Ateles. Cambridge University Press, Cambridge, pp 81–137
Estrada A, Van belle S, García Del Valle Y (2004) Survey of black howler (Alouatta pigra) and spider (Ateles geoffroyi) monkeys along the Río Lacantún, Chiapas, Mexico. Neotrop Primates 12:70–75
Felton AM, Felton A, Raubenheimer D, Simpson SJ, Foley WJ, Wood JT, Lindenmayer DB (2009a) Protein content of diets dictates the daily energy intake of a free-ranging primate. Behav Ecol 20:685–690. doi:10.1093/beheco/arp021
Felton AM, Felton A, Lindenmayer DB, Foley WJ (2009b) Nutritional goals of wild primates. Funct Ecol 23:70–78
Fox CW, Wallin WG, Bush ML, Czesak ME, Messina FJ (2012) Effects of seed beetles on the performance of desert legumes depend on host species, plant stage, and beetle density. J Arid Environ 80:10–16
Fricke E, Tewksbury JJ, Rogers HS (2014) Multiple natural enemies cause distance-dependent mortality at the seed-to-seedling transition. Ecol Lett 17:593–598
Gómez-Pompa A, Dirzo R (1995) Atlas de las áreas naturales protegidas de México. CONABIO-INE, Mexico City
González-Di Pierro AM, Benítez-Malvido J, Méndez-Toribio M, Zermeño I, Arroyo-Rodríguez V, Stoner KE, Estrada A (2011) Effects of the physical environment and primate gut passage on the early establishment of an old-growth forest tree species (Ampelocera hottlei Standley) in tropical rain forest fragments. Biotropica 43:459–466
González-Zamora A, Arroyo-Rodríguez V, Chaves OM, Sánchez-López S, Stoner KE, Riba-Hernández P (2009) Diet of spider monkeys (Ateles geoffroyi) in Mesoamerica: current knowledge and future directions. Am J Primatol 71:8–20
Howe HF, Smallwood J (1986) Ecology of seed dispersal. Annu Rev Ecol Syst 13:201–228
Jansen PA, Hirscha BT, Emsensb W-J, Zamora-Gutiérrez V, Wikelskia M, Kayset R (2012) Thieving rodents as substitute dispersers of megafaunal seeds. PNAS 109:12610–12615
Janzen DH (1970) Herbivores and the number of tree species in tropical forests. Am Nat 104:501–528
Janzen DH (1980) Specificity of seed-attacking beetles in a Costa Rican deciduous forest. J Ecol 68:929–952
Johnson CD (1981) Interactions between bruchid (Coleoptera) feeding guilds and behavioral patterns of pods of the Leguminosae. Environ Entomol 10:249–253
Jordano P (2014) Fruits and frugivory. In: Ghallager RS (ed) Seeds: the ecology of regeneration in plant communities, 3rd edn. CABI Publishing, Wallingford, pp 18–61
Julliot C (1996) Fruit choice by red howler monkeys (Alouatta seniculus) in a tropical rain forest. Am J Primatol 40:261–282
Kalko EKV, Herre EA, Handley CO (1996) Relation of fig fruit characteristics to fruit-eating bats in the new and old world tropics. J Biogeo 23:565–576
Lambert JE (1998) Primate digestion: interaction among anatomy, physiology, and feeding ecology. Evol Anthropol 7:8–20
Link A, Di Fiore A (2006) Seed dispersal by spider monkeys and its importance in the maintenance of neotropical rain-forest diversity. J Trop Ecol 22:235–246
Mariaca-Méndez R (2002) Marqués de Comillas, Chiapas: procesos de inmigración en el trópico húmedo de México. Ph.D. Dissertation, Universidad Iberoamericana, Mexico
Maron JL, Crone E (2006) Herbivory: effects on plant abundance, distribution and population growth. Pro Roy Soc B-Biol Sci 273:2575–2584
Martins MM (2006) Comparative seed dispersal effectiveness of sympatric Alouatta guariba and Brachyteles arachnoides in southeastern Brazil. Biotropica 38:57–63
Milton K (1981) Food choice and digestive strategies by two sympatric primate species. Am Nat 117:496–505
Milton K (1998) Physiological ecology of howlers (Alouatta): energetic and digestive considerations and comparison with the Colobinae. Int J Primatol 19:513–548
Moles AT, Warton DI, Westoby M (2003) Do small-seeded species have higher survival through seed predation than large-seeded species? Ecology 84:3148–3162
Nakagawa M et al (2005) Predispersal seed predation by insects vs. vertebrates in six Dipterocarp species in Sarawak, Malaysia. Biotropica 37:389–396
Naranjo E (2009) Ecology and conservation of Baird’s tapir in Mexico. Trop Conserv Sci 2:140–158
Norconk MA, Wright BW, Conklin-Brittain NL, Vinyard CJ (2008) Mechanical and nutritional properties of foods as factors in Plattirrhyne dietary adaptations. In: Garber PA, Estrada A, Bicca-Marques JC, Heymann EW, Strier KB (eds) South American primates: comparative perspectives in the study of behavior, ecology and conservation. Springer, New York, pp 279–319
Paz H, Martínez-Ramos M (2003) Seed mass and seedling performance within eight species of Psychotria (Rubiaceae). Ecology 84:439–450
Pennington TD, Sarukhán J (1998) Árboles tropicales de México. UNAM-FCE, Mexico
Peres CA (1991) Seed predation of Cariniana micrantha (Lecythidaceae) by brown capuchin monkeys in Central Amazonia. Biotropica 23:262–270
Port-Carvalho M, Ferrari SF (2004) Occurrence and diet of the black bearded saki (Chiropotes satanas satanas) in the fragmented landscape of western Maranhão, Brazil. Neotrop Primates 12:17–21
R-Core-Team-R (2015) R: a language and environment for statistical computing. Foundation for statistical computing, Vienna, Austria. In: computing, R.F.F.S. (ed). URL http://www.r-project.org/.
Rey PJ, Gutierrez LE, Alcantara J, Valera F (1997) Fruit size in wild olives: implications for avian seed dispersal. Funct Ecol 11:611–618
Robertson AW, Trass A, Ladley JJ, Kelly D (2006) Assessing the benefits of frugivory for seed germination: the importance of the deinhibition effect. Funct Ecol 20:58–66
Russo SE, Chapman CA (2011) Primate seed dispersal: linking behavioural ecology with forest community structure. In: Campbell CJ, Fuentes A, MacKinnon KC, Bearder SK, Stumpf RM (eds) Primates in perspective 523-534. Oxford University Press, Oxford
Sallabanks R, Courtney SP (1992) Frugivory, seed predation, and insect–vertebrate interactions. Ann Rev Entomol 37:377–400
Schupp EW, Jordano P, Gómez JM (2010) Seed dispersal effectiveness revised: a conceptual review. New Phytol 188:333–353
Shiels AB, Drake DR (2011) Are introduced rats (Rattus rattus) both seed predators and dispersers in Hawaii? Biol Inv 13:883–894
Silvius KM, Fragoso JMV (2002) Pulp handling by vertebrate seed dispersers increases palm seed predation by bruchid beetles in the northern Amazon. J Ecol 90:1024–1032
Simão I, Maës Dos Santos FA, Pizzo MA (1997) Vertical stratification and diet of psittacids in a tropical lowland forest of Brazil. Ararajuba 5:169–174
Sokal RS, Rohlf FJ (1995) Biometry. Freeman and Company, New York
Stevenson PR, Castellanos MC, Pizarro JC, Garavito M (2002) Effects of seed dispersal by three ateline monkey species on seed germination at Tinigua National Park, Colombia. Int J Primatol 23:1187–1204
Stevenson PR, Pineda M, Samper T (2005) Influence of seed size on dispersal patterns of woolly monkeys (Lagothrix lagothricha) at Tinigua Park, Colombia. Oikos 110:435–440
Traveset A, Robertson AW, Rodríguez-Pérez J (2007) A review on the role of endozoochory in seed germination. In: Dennis AJ, Schupp EW, Green RJ, Westcott DA (eds) Seed dispersal: theory and its application in a changing world. CABI Publishing, Wallingford, pp 78–103
Vallejo-Marín M, Domínguez CA, Dirzo R (2006) Simulated predation reveals a variety of germination responses of Neotropical rainforest species. Am J Bot 93:360–376
Van Der Pijl L (1982) Principles of dispersal in higher plants. Springer-Verlag, Berlin 82 pp
Vieira ICG, Gavão N, Rosa NA (1996) Caracterização morfológica de frutos e germinação de sementes de espécies arbóreas nativas da Amazônia. Boletim Paraense Emílio Goeldi, Série Botânica 12:271–288
Wheelwright NT (1985) Fruit size, gape width, and the diets of fruit-eating birds. Ecology 66:808–818
Acknowledgments
This research was supported by grants from the Consejo Nacional de Ciencia y Tecnología, Mexico (CONACyT-79121) and Universidad Nacional Autónoma de México (PAPIIT IN206111 and DGAPA sabbatical grant) to J. Benítez-Malvido. We thank the Comisión Nacional de Áreas Naturales Protegidas (CONANP, permission number SGPA/DGVS/07830)) for granting permits to work in the MABR and the Instituto de Investigaciones en Ecosistemas y Sustentabilidad, UNAM, for providing logistical support. We are grateful to G. Lombera for his valuable assistance in the field and to F. Noguera and J. Romero-Nápoles for identifying the insect taxa. We are grateful for the technical support provided by J. M. Lobato-García, J. Rodríguez-Velázquez, H. Ferreira, and A. Valencia-García. We want to thank L. Culot and an anonymous reviewer whose comments and suggestions improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R.A. Montgomery.
Rights and permissions
About this article
Cite this article
Benítez-Malvido, J., Zermeño-Hernández, I., González-DiPierro, A.M. et al. Frugivore choice and escape from pre-dispersal seed predators: the case of Dialium guianense and two sympatric primate species in southern Mexico. Plant Ecol 217, 923–933 (2016). https://doi.org/10.1007/s11258-016-0617-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-016-0617-6