Abstract
Purpose
Diabetic nephropathy (DN) is a serious microvascular complication of diabetes mellitus. Significantly reduced levels of autophagy in diabetic kidneys play an important role in the development of DN. The present study investigated the effects of dapagliflozin (DAP) on renal autophagy and AMP-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR) pathway in vivo and in vitro.
Methods
We explored the effect of DAP in streptozotocin (STZ)-induced DN rats. The anti-DN effect of DAP was assessed by body weight, kidney weight/body weight ratio, blood and urine biochemical parameters, and pathological changes of kidney tissue. Number of autophagosomes in the kidney was investigated through Transmission electron microscopy. Besides, cell viability and apoptosis of DAP alone or combined with Compound C (CC, a selective AMPK inhibitor)-treated high glucose (HG)-induced HK-2 cells were detected by Cell Counting Kit-8 (CCK-8) and flow cytometry assays. Immunohistochemistry, Western blot, Enzyme-linked immunosorbent assay (ELISA), and immunofluorescence were employed to detect the expression levels of extracellular matrix (ECM) deposition, autophagy, apoptosis, and AMPK/mTOR pathway-associated targets in vivo and in vitro.
Results
The results showed that DAP ameliorated the body weight and decreased kidney weight, fasting blood glucose, and serum/urine biochemical parameters of renal damage, as well as renal pathological changes. Moreover, DAP significantly ameliorated HG-induced cell apoptosis and ECM deposition in HK-2 cells. However, these favorable effects of DAP could be abolished by co-treatment with CC in HG-induced HK-2 cells. Mechanistically, DAP can enhance autophagy in DN including increased LC3-II/I ratio, Beclin-1, p-AMPK protein levels, and decreased p62 and p-mTOR protein expressions, as well as inhibited renal fibrosis and apoptosis.
Conclusion
In summary, DAP alleviated fibrosis, apoptosis, and autophagy in DN rats and HG-induced HK-2 cells by regulating the AMPK/mTOR pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic kidney disease (DKD) is one of the most common chronic complications of diabetes mellitus and one of the leading causes of end-stage renal disease. The prevalence of diabetes mellitus is increasing year by year and has become the leading cause of chronic kidney disease (CKD) worldwide [1]. As of 2017, the number of people with diabetes worldwide is about 451 million, and by 2045, the number of people with diabetes worldwide will reach 693 million [2]. About 40% of these diabetic patients will develop diabetic nephropathy (DN), and end-stage renal disease (ESRD) is the final outcome of DN progression, which requires renal replacement therapy, such as renal transplantation and dialysis treatment [3]. It has been shown that diabetic patients with DN have a 3- to 12-fold higher mortality rate than diabetic patients alone [4]. Finding ways to delay or treat DN has been a hot topic in the field of DN research. Current treatments for DN are mainly through controlling risk factors such as blood glucose, blood pressure, and lipids, as well as the use of renin-angiotensin system (RAS) blockers to reduce urinary protein and delay the deterioration of renal function, but more than 40% of treated patients still face a high risk of progressing to ESRD [5].
In recent years, sodium-dependent glucose transporters 2 (SGLT-2) inhibitors have been shown to be renoprotective and have been used in clinical practice, leading to a new dawn in the treatment of DN [6]. SGLT-2 inhibitors are a new type of non-insulin-dependent hypoglycemic agents, which mainly exert hypoglycemic effects by blocking glucose reabsorption in proximal tubules and promoting urinary sugar excretion [7, 8]. In addition to lowering blood glucose, SGLT-2 inhibitors have been shown to reduce cardiovascular events in previous clinical trials [9,10,11]. Meanwhile, secondary outcomes and exploratory analyses of these clinical trials showed that SGLT-2 inhibitors could improve renal regression [9,10,11], but there are some uncertainties about the renoprotective effects of SGLT-2 inhibitors, and further in-depth studies on their mechanisms of action are needed.
A large number of studies have shown that the pathogenesis of DN is closely related to the inhibition of autophagy and the deposition of extracellular matrix (ECM) in renal cells [12, 13]. Studies have shown that cellular autophagy is a self-protective mechanism to maintain the self-stabilization of the intracellular environment, and its main role is to remove and degrade damaged organelles and excess biomolecules [14, 15]. In diabetes mellitus, the autophagy function of renal cells is inhibited due to the high glucose environment, which leads to damaged organelles and cytotoxic substances in renal cells that can not be removed in a timely and effective manner, which affects the normal function of the cells, and then causes renal injury [16, 17]. Therefore, autophagy has become a new target for the treatment of diabetic nephropathy [18, 19].
AMP-activated protein kinase (AMPK) is a nutrient-sensitive kinase activated under conditions of energy depletion, and the mammalian target of rapamycin (mTOR) is a key regulatory molecule in the process of autophagy, and the activation of mTOR blocks autophagy under physiological conditions [20, 21]. The activation of AMPK has an inhibitory effect on mTOR, which is one of the classical pathways activated by autophagy signaling, and the AMPK/mTOR signaling pathway is believed to play an important role in the regulation of cellular autophagy and senescence [12, 22]. Among SGLT-2 inhibitors, Dapagliflozin (DAP) has a variety of pharmacological effects. Recent hotspot studies have found that DAP has a good renoprotective effect [23, 24], but whether it exerts its DN prevention through regulating the autophagy signaling pathway mediated by the AMPK-mTOR axis has not been investigated. In the present study, to address these issues, an animal model of DN established by a high-fat diet (HFD) combined with streptozotocin (STZ) injection and also the high glucose-induced human renal tubular epithelial cell line (HK-2) were used to explore whether the AMPK-mTOR pathway is involved in the protective effect of DAP in vitro and in vivo.
Materials and methods
Animal experiments
A total of 70 male SD rats (Aged: 5–6 weeks, weighing 180–200 g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd (Shanghai, China) and housed in the SPF-level standard environment with a 12-h light/dark cycle. All rats were allowed free access to food and water ad libitum. After a week of adaptive feeding, the rats were divided into two groups (n = 10 in each group): the normal group rats received a normal diet and the HFD group (n = 60) received a high-sugar-fat diet for 4 weeks. After that, the rats in the HFD group were intraperitoneally injected with 100 mg/kg STZ (Sigma-Aldrich, St. Louis, MO, USA). After 72 h, the fasting blood glucose (FBG) levels of rats in the HFD group more than 16.7 mmol/L were used. After STZ injection for 4 weeks, the DN rat was established, as accompanied by the uromicroprotein exceeding 30 mg/day. Subsequently, the DN rats (n = 50) were randomly divided into five groups (n = 10 in each group): model group, gliquidone (positive group, GLI, Beijing Wanhui Shuanghe Pharmaceutical Co., LTD, China), DAP low-dose (DAP-L) group, DAP medium-dose (DAP-M) group, DAP high-dose (DAP-H) group. Rats in the control and model groups received an equal amount of distilled water. GLI group rats received gliquidone at a dose of 10 mg/kg body weight per day by gavage. Meanwhile, the rats in the DAP groups were orally treated with DAP (AstraZeneca Pharmaceuticals Co., LTD, London, England) dissolved in distilled water at a dose of 1, 5, or 10 mg/kg every day, respectively for 12 weeks. The body weights were recorded weekly for each group.
Sample collection
After 12 weeks of GLI and DAP administration, the rats were maintained in metabolic cages for 24 h to collect urine samples. Next, all rats were anesthetized and sacrificed through cardiac puncture. Then, the kidneys were collected and weighted to calculate the ratio of kidney weight/body weight (also known as the renal index). The blood samples from rat heart were collected and centrifuged for further examination. The level of FBG, urea nitrogen (BUN), and creatinine (Scr) in serum samples, as well as the level of urine protein, urinary albumin, and urinary creatinine were measured by automatic biochemical analyzer (Hitachi High-Tech Group, Japan).
Histopathological examination
Kidney tissues were fixed in 4% paraformaldehyde for 48 h at room temperature. After being embedded in paraffin, the kidney tissues were sectioned into 5 μm thick sections. Then, the sections were used for hematoxylin and eosin (H&E) staining to observe kidney structure, and periodic Acid-Schiff (PAS) to examine ECM accumulation in glomerular, Masson’s trichrome to detect fibrosis. The photographs were pictured under light microscopy (Olympus, Tokyo, Japan).
Immunohistochemistry assay
The expression levels of the ECM protein, Type I collagen (COL I) and fibronectin (FN) in the renal tissues were detected by the immunohistochemistry assay. Briefly, the paraffin-embedded sections were dewaxed and underwent antigen retrieval and blocked with 5% BSA. The slices were then incubated with an anti-COL I antibody (1:500, ab270993, Abcam) or an anti-FN antibody (1:500, ab268020, Abcam) overnight at 4 °C. The sections were then incubated with the secondary goat anti-rabbit antibody (1:5000, ab97080, Abcam) for 30 min at room temperature, followed by incubating with DAB for 2 min and counterstaining with hematoxylin. At last, the sections were imaged with a light microscope (DS-U3, Nikon Corporation, Tokyo, Japan).
TUNEL assay
Paraffin sections were subjected to dewaxing, hydration, and proteinase K treatment without DNase for a duration of 30 min at 37 °C. Subsequently, the tissues were coated with a 0.5% Triton X-100 solution for a period of 15 min. The extent of apoptosis was assessed using the One-Step TUNEL Cell Apoptosis Detection Kit (C1089, Beyotime, China) according to the manufacturer’s instructions. The nuclei were stained with DAPI (Beyotime) and visualized using a fluorescence microscope (Olympus, Japan).
Transmission electron microscopy
Renal tissues were washed using physiological saline and fixed in 2.5% glutaraldehyde at 4 °C overnight. Then, renal tissues were washed with PBS and post-fixed with 1% osmium tetroxide. Next, the samples were embedded in epoxy resin, and the ultrathin sections (70 nm) were collected using an ultramicrotome (Leica, Germany). After counterstaining with uranyl acetate and lead citrate, the sample sections were examined by using a JEM-1400 Plus transmission electron microscope (Japan).
Cell treatment and Cell counting kit-8 (CCK-8) assay
Human kidney proximal tubular cells (HK-2) were purchased from the Cellverse Bioscience Technology Co., Ltd. (Shanghai, China) and cultured in DMEM/F12 containing 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin solution at 37℃ in a humidified 5% CO2 atmosphere. HK-2 cells were exposed to normal glucose (5.5 mM D-glucose) or high glucose (HG, 30 mM D-glucose) for 48 h. Also, cells cultured in 5.5 mM glucose-containing medium supplemented with 24.5 mM mannitol were used as HG controls. After that, the HG-induced HK-2 cells were treated with 25, 50, 100 μmol/L DAP for 24 h, respectively. Besides, the HG-induced HK-2 cells were pre-treated with 10 μmol/L Compound C (CC), an AMPK inhibitor for 3 h, followed by DAP treatment at a dose of 100 μmol/L for 24 h. After that, 10 µl CCK-8 (Sangon, Shanghai, China) was added to each well for an another 4 h. Finally, the absorbance was measured at 450 nm using a microplate reader (Molecular Devices).
Flow cytometry assay
The apoptosis of DAP alone and CC in combination-treated HK-2 cells was measured with an Annexin V-FITC/PI apoptosis kit (#556,547, BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instructions. Briefly, the treated HK-2 cells were collected and washed with PBS. And then, the cells were incubated with 5 µl Annexin V-fluorescein isothiocyanate (FITC) and 10 µl propidium iodide (PI) in the dark for 15 min. Finally, the cell apoptosis of treated HK-2 cells was estimated using a flow cytometer (BD Biosciences) and FlowJo software.
Enzyme-linked immunosorbent assay (ELISA)
After the treatment of DAP alone and CC in combination in HG-induced HK-2 cells, the cell culture medium were collected and centrifuged at 3000 rpm for 10 min 4 °C. Then, the supernatant was collected. The concentrations of collagen I and fibronectin secreted from treated HK-2 cells in the supernatant were detected using commercial ELISA kits (ml025998 and ml023933, mlbio, Shanghai, China) following the manufacturer’s instructions.
Immunofluorescence assay
The paraffin sections of kidney tissues were de-paraffinised, retrieved, washed with PBS, and then blocked with 5% goat serum for 1 h at room temperature. Subsequently, the sections were incubated with anti-LC3 (1:100, AF5402, Affinity) antibody, anti-p-AMPK (1:100, AF6423, Affinity), and anti-p-mTOR (1:100, AF3308, Affinity) in a wet box overnight at 4 °C. After washing with PBS for three times, the sections were incubated with rabbit 488 fluorescent (green) secondary antibody (1:500, ab150077, Abcam) for 1 h in the dark at room temperature. Next, the cell nuclei were stained using DAPI for 15 min. Then, the sections were covered with an anti-fluorescence quenching mounting plate and observed by a fluorescence microscope (Nikon, Japan). To determine the expression of LC3 and p-AMPK in treated HK-2 cells, the immunofluorescence staining was also performed. Briefly, the treated HK-2 cells were fixed with 4% paraformaldehyde and blocked with 1% BSA containing 0.3% Triton X-100 for 30 min at room temperature. Next, the treated HK-2 cells were incubated with primary anti-LC3 (1:200, ab192890, Abcam) and anti-p-AMPK (1:200, ab92701, Abcam) at 4 °C overnight, followed by being stained with Alexa Fluor 594 (1:500, ab150080, Abcam) for 1 h in the dark. The nuclei were then stained with DAPI. Finally, images were taken on a Ts2-FC fluorescence microscope (Nikon Corporation).
Western blot analysis
Total protein samples from the kidney tissues and the treated HK-2 cells were prepared by using cooled RIPA buffer (Beyotime, China) and the total protein concentration was detected by BCA Protein Assay Kit (pc0020, Beyotime). An equal amounts of protein (30 μg) samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes (GE Healthcare Life). The membranes were then blocked with 5% nonfat milk in Tris buffered saline (TBST) for 1 h. After washed with TBST, the membranes were incubated with primary antibody of TGF-β1 (1:1000, BF8012, Affinity), α-SMA (1:1000, AF1032, Affinity), p62 (1:1000, AF5384, Affinity), LC3 (1:1000, 4108 s, Cell Signaling Technology, Danvers, MA, USA), Bax (1:1000, AF0120, Affinity), Bcl-2 (1:1000, AF6139, Affinity), p-AMPK (1:1000, AF3423, Affinity), AMPK (1:1000, AF6423, Affinity), p-mTOR (1:1000, AF3308, Affinity), mTOR (1:1000, AF6308, Affinity), E-cadherin (1:1000, 3195 T, Cell Signaling Technology), Vimentin (1:1000, R22775, Zenbio, Suzhou, China), Beclin-1 (1:1000, AF5128, Affinity), and GAPDH (1:5000, 10494-1-AP, Proteintech) overnight at 4 °C, followed by incubation with HRP-conjugated secondary antibodies (1:5000, 7074, Cell Signaling Technology). Then, the membranes were exposed to BeyoECL Plus chemiluminescence reagent (P0018S, Beyotime) and visualized using a ChemiScope6100 instrument (Clinx Science instrument Co. Ltd., Shanghai, China). Finally, the semi-quantitative analysis of protein bands was performed using ImageJ software (National Institutes of Health, USA).
Statistical analysis
All data are shown as the mean ± standard deviation (SD). Statistical significance was estimated using Student’s t test between two groups. Additionally, one-way analysis of variance (ANOVA) followed by Dunnet’s multiple comparison tests was used among multiple groups. P < 0.05 was considered significant.
Results
DAP treatment ameliorated renal function of DN rats
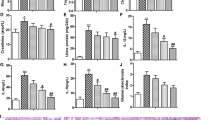
To clarify the roles of DAP on DN, a rat model of DN was established through STZ injection. As shown in Fig. 1A, B, we observed that the body weight in DAP and GLI groups was significantly increased than those of the model group, while the ratio of kidney weight/body weight was greatly reduced in DAP and GLI groups when compared with the model group. Compared with the model group, DAP and GLI treatment significantly suppressed the increase in FBG (Fig. 2A), serum BUN (Fig. 2B), Scr (Fig. 2C), 24-h urinary protein (Fig. 2D), urinary albumin (Fig. 2E), and urinary creatinine (Fig. 2F). These results suggested that DAP effectively ameliorated renal function in STZ-induced DN rats.
Effect of DAP treatment on body weight and kidney index in HFD/STZ-induced DN rats. A Body weight of six groups of rats at 12 weeks after DAP and GLI treatment (n = 10). B The kidney weight/body weight (kidney index) of six groups of rats at 12 weeks after DAP treatment (n = 10). ▲▲P < 0.01 vs. control group. **P < 0.01 vs. model group
Effect of DAP treatment on biochemical index in HFD/STZ-induced DN rats. A–C Serum FBG, BUN, and Cr levels of six groups of rats at 12 weeks after DAP and GLI treatment (n = 10). (D-F) Urine protein, urine albumin, and urinary creatinine levels of six groups of rats at 12 weeks after DAP and GLI treatment (n = 10). ▲▲P < 0.01 vs. control group. *P < 0.05, and **P < 0.01 vs. model group
DAP treatment improved histopathological changes in DN rats
To further investigate the histopathological changes in the kidneys from DN rats with DAP treatment, the H&E, PAS, and Masson staining were performed. H&E staining showed that renal tubular epithelial vacuolar degeneration, irregular glomerular morphology, and renal tubular edema with partial necrosis in the model group (Fig. 3A). As illustrated in Fig. 3B, PAS staining showed that the mesangial and glomerular basement membranes were slightly thickened in DN rats. Additionally, the Masson staining revealed a significant increase of collagen fibers in the kidneys of DN rats (Fig. 3C). However, the abnormal pathological changes were restored by GLI and DAP treatments (Fig. 3). Moreover, Immunohistochemical staining and Western blotting showed that COL-I, FN, TGF-β1, and α-SMA were significantly upregulated in the kidneys of DN rats, which were observably downregulated by the treatment of GLI or DAP (Fig. 4A, B). These results collectively suggested that DAP indeed has anti-renal fibrosis effects against DN.
Effect of DAP treatment on ECM accumulation in the kidney of HFD/STZ-induced DN rats. A Immunohistochemical staining of Type I collagen (COL I) and FN (magnification 200×). B Protein expression of TGF-β1 and α-SMA in the kidney tissues was detected by Western blot assay. ▲▲P < 0.01 vs. control group. *P < 0.05, and **P < 0.01 vs. model group
DAP treatment attenuated apoptosis and promoted autophagy in DN rats
The effect of DAP or GLI on cell apoptosis in the kidneys of DN rats was further investigated by Tunel staining. The results showed that, compared with model group, DAP treatment attenuated cell apoptosis levels in the kidneys of DN rats in a dose-dependent manner (Fig. 5A). To investigate the effect of DAP on cell autophagy, the number of autophagosomes in the kidneys of DN rats was detected by transmission electron microscopy. The results showed that DAP treatment remarkedly increased the number of autophagosomes in DAP-treated groups in a dose-dependent manner compared with model group (Fig. 5B). To further confirm the occurrence of cell apoptosis and autophagy, we then detected apoptosis- and autophagy-related proteins. The results of immunofluorescence staining showed that, following DAP treatment, the protein expression of LC3 was increased (Fig. 5C). Similarly, the protein expression levels of P62 and Bax were markedly down-regulated in the DAP-treated DN rats, while the levels of LC3 and Bcl-2 were significantly up-regulated after DAP administration (Fig. 5C). Research has shown that AMPK/mTOR signaling pathway is associated with cell autophagy in the development of DN. To test this, the AMPK and mTOR levels in the kidneys of DN rats were measured after DAP treatment using immunofluorescence staining and Western blotting. The results showed that the phosphorylation levels of AMPK were higher in DAP-treated DN rats than in model rats; however, the phosphorylation levels of mTOR were repressed by DAP treatment (Fig. 6A-6C). These results suggested that DAP inhibited cell apoptosis and promoted cell autophagy in the kidneys of DN rats.
Effect of DAP treatment on cell apoptosis and autophagy in the kidney of HFD/STZ induced DN rats. A Apoptotic cells in kidney tissue sections were measured with TUNEL staining, Scale bar, 100 μm. B Representative TEM images of autophagosomes (red) in the kidney tissue sections of DAP-treated DN rats are presented (magnification 10,000×, 25,000×). C Immunofluorescence staining was used to detect LC3 expression in the kidney tissue sections of DAP-treated DN rats (Scale bar, 100 μm). D Western blot was employed to assess the protein level of apoptosis and autophagy-related genes (Bax, Bcl-2; p62, LC3) in DAP-treated DN rats. ▲▲P < 0.01 vs. control group. *P < 0.05, and **P < 0.01 vs. model group
Effect of DAP treatment on AMPK/mTOR pathway in the kidney of HFD/STZ induced DN rats. A, B Immunofluorescence staining was used to detect phosphorylation of AMPK (p-AMPK) and mTOR (p-mTOR) in the kidney tissue sections of DAP-treated DN rats (Scale bar, 100 μm). C Expressions of p-AMPK, AMPK, p-mTOR, and mTOR, as detected by Western blotting. ▲▲P < 0.01 vs. control group. *P < 0.05, and **P < 0.01 vs. model group
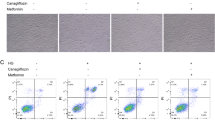
DAP improved the cell viability and inhibited cell apoptosis in HG-induced HK-2 cells
The cell viability and cell apoptosis of HG-induced HK-2 cells were detected by CCK-8 and flow cytometry, respectively, following the administration of DAP at a concentration of 25, 50, and 100 μmol/L alone or in combination with 10 μmol/L of CC for 24 h. The results showed that the cell viability of HG-induced HK-2 cells was increased at both concentrations (Fig. 7A). As demonstrated in Fig. 7B, the cell apoptosis rate of HG-induced HK-2 cells was decreased in the DAP-treated HK-2 cells (Fig. 7B). Meanwhile, the ELISA revealed that DAP treatment led to decreased collagen I and Fibronectin content in the culture supernatant of HG-induced HK-2 cells (Fig. 7C). Consistent with the in vivo results, the protein expression levels of Bcl-2 and E-cadherin were higher in DAP-treated HK-2 cells than in HG-induced HK-2 cells. Conversely, DAP-treated HG-induced HK-2 cells had weakened protein expression levels of Bax, vimentin, and α-SMA (Fig. 7D). More importantly, the results of immunofluorescence staining and Western blotting demonstrated that treatment of DAP could up-regulate the decreased levels of LC3, beclin-1, p-AMPK, while down-regulate the increased levels of p-mTOR in HG-induced HK-2 cells (Fig. 8A, C). However, these effects were partly abolished by CC treatment (Figs. 7, 8). Collectively, the results suggested that the anti-DN effect of DAP involved the activation of autophagy in vitro and in vivo through the AMPK/mTOR pathway.
Effect of DAP on cell viability, apoptosis, and ECM deposition in HG-mediated HK-2 cells. A Cell viability was assessed by CCK-8 assay (n = 6 per group). B The apoptotic rate of HG-mediated HK-2 cells with DAP treatment was determined by flow cytometry using the Annexin-V FITC/PI staining. C The collagen I content and Fibronectin content in the culture supernatant were detected by ELISA. D The protein levels of Bax, Bcl-2, E-cadherin, vimentin, and α-SMA were measured using Western blotting. ▲▲P < 0.01 vs. NG and mannitol group. *P < 0.05, and **P < 0.01 vs. HG group. #P < 0.05, and ##P < 0.01 vs. DAP (100 μmol/L) group
Effect of DAP on autophagy and AMPK/mTOR pathway-associated targets in HG-mediated HK-2 cells. A Immunofluorescence staining was used to detect LC3 expression and B phosphorylation of AMPK in HG-mediated HK-2 cells. C Western blots were conducted to determine LC3, Beclin-1, p-AMPK, AMPK, p-mTOR, and mTOR expression in the HG-mediated HK-2 cells. ▲▲P < 0.01 vs. NG and mannitol group. *P < 0.05, and **P < 0.01 vs. HG group. #P < 0.05, and ##P < 0.01 vs. DAP (100 μmol/L) group
Discussion
DN is a serious microvascular complication of diabetes mellitus, which ultimately leads to the decline of renal function, with a high rate of disability and lethality [25, 26]. Studies have confirmed that the AMPK/mTOR signaling pathway has an important role in regulating renal tissue autophagy during DN and that AMPK/mTOR signaling pathway-mediated renal tissue autophagy can significantly alleviate renal tissue injury in the DN environment [27, 28]. Therefore, in the present study, we established a DN rat model and used the SGLT-2 inhibitor (DAP) to observe the effects of DAP on body weight, renal index, FBG, BUN, Scr, urine protein, urine albumin, urinary creatinine, and renal histopathological changes, as well as on the fibrosis-associated proteins, COL I, FN, TGF-β1, and α-SMA, the autophagy-associated proteins, LC3 II, Beclin 1, and AMPK/mTOR signaling pathway-related protein expressions, to discuss its therapeutic effect, as well as to compare it with GLI, a commonly used drug in the clinical treatment of DN, in order to provide a reference for the degree of efficacy. The results showed that, compared with the model group, the body weight of rats in the DAP groups was increased; renal index, FBG, BUN, Scr, urine protein, urine albumin, urinary creatinine content were decreased; renal histopathological damage was reduced; LC3II, Beclin 1, Bcl-2 and AMPK-related protein expressions were up-regulated, and the expression of COL I, FN, TGF-β1, α-SMA, Bax, mTOR-related proteins were down-regulated, indicating that DAP has an anti-diabetic nephropathy effect by promoting autophagy.
Autophagy is a highly conserved lysosomal degradation pathway, which is an important regulatory mechanism for maintaining intracellular homeostasis [29, 30]. Previous studies have found that persistent high glucose in DN inhibits the expression of autophagy-related protein (LC3, Beclin-1) expression in DN, reducing autophagy levels and impeding the clearance of damaged proteins and cytotoxic products produced by accumulation in the organelles, thus leading to renal injury and renal parenchymal cell dysfunction in DN [31, 32]. Activation of cellular autophagy can significantly inhibit high glucose-induced renal tubular epithelial cell injury and improve cellular function [33]. These studies suggest that the insufficient level of autophagy may be an important mechanism leading to the progression of DN.
The AMPK/mTOR signaling pathway is thought to play an important role in the regulation of cellular autophagy and senescence [34]. AMPK and mTOR via protein phosphorylation can regulate cellular metabolism and autophagy levels, and inhibition of the mTOR kinase signaling pathway and activation of the AMPK signaling pathway can induce cellular autophagy to slow down the aging process and prolong the lifespan of the organism [35]. Ding et al. found that the level of autophagy in renal tubular epithelial cells was significantly increased in animal models of diabetes mellitus of both types 1 and 2 diabetes mellitus [36]. In STZ-induced diabetic rats, metformin increased renal AMPK phosphorylation, reversed mTOR activation, and then inhibited renal hypertrophy. mTOR activity is regulated by growth factors, stress, energy status, and amino acids. mTOR is involved in autophagy, senses nutrient overload, and therefore inhibits autophagy. Rapamycin, a specific mTOR inhibitor, restored the autophagic activity of podocytes cultured under high glucose conditions, suggesting that mTOR may be involved in the inhibition of autophagy in podocytes under diabetic conditions [37]. Kume et al. demonstrated that mTOR signaling was highly activated in podocytes from diabetic kidneys of both humans and animals and that both pharmacological and genetic inactivation of mTOR ameliorated podocyte dysfunction in DKD [38]. Similarly, the results of the present study showed that the p-AMPK/AMPK ratio was significantly decreased and the p-mTOR/mTOR ratio was significantly increased in the model group of rats compared with the control group. Compared with the model group, the p-AMPK/AMPK ratio was significantly higher and the p-mTOR/mTOR ratio was significantly lower in the DAP-treated groups. Collectively, these results preliminarily suggest that DAP could inhibit renal fibrosis in DN rats by regulating cellular autophagy mediated by the AMPK/mTOR signaling pathway.
Furthermore, the cell viability of HG-induced HK-2 cells was also strengthened by the treatment of DAP, as demonstrated by CCK-8 assays. Flow cytometry showed that DAP treatment significantly reduced apoptosis of HG-induced HK-2 cells in this study. Besides, Yang and Xu et al. found that DAP effectively mitigated advanced glycation end product-induced podocyte injury or HG-induced HK-2 cells through upregulation of autophagy by targeting AMPK/mTOR pathway [39, 40]. To further clarify the role of cellular autophagy mediated by the AMPK/mTOR signaling pathway in DAP treatment of diabetic kidney injury. In this experiment, we administered the AMPK inhibitor (Compound C, CC) to HG-mediated HK-2 cells in the DAP-treated group, and the results showed that the AMPK inhibitor was able to reverse the effect of DAP in HG-mediated cellular damaging, which was mainly manifested as a decrease in the p-AMPK/AMPK ratio, an increase in the p-mTOR/mTOR ratio, LC3-II/LC3- I ratio, ECM deposition and apoptosis. Therefore, these results also confirm that our results are consistent with the literature reports, indicating that DAP can play a therapeutic role in DN by regulating the AMPK/mTOR signaling pathway.
Conclusions
In summary, DAP was able to alleviate DN renal tissue injury, and its mechanism may be related to the autophagy of renal tissues mediated through the regulation of the AMPK/mTOR signaling pathway, which might provide more objective theoretical support for the use of SGLT-2 inhibitors in the clinical treatment of DN.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Code availability
Not applicable.
References
Xie Y, Bowe B, Mokdad AH et al (2018) Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int 94(3):567–581
Cho NH, Shaw JE, Karuranga S et al (2018) IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 138:271–281
Alicic RZ, Rooney MT, Tuttle KR (2017) Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol 12(12):2032–2045
Alicic RZ, Johnson EJ, Tuttle KR (2018) SGLT2 inhibition for the prevention and treatment of diabetic kidney disease: a review. Am J Kidney Dis 72(2):267–277
Garofalo C, Borrelli S, Liberti ME et al (2019) SGLT2 inhibitors: nephroprotective efficacy and side effects. Medicina (Kaunas) 55(6):268
Sarafidis P, Pella E, Kanbay M, Papagianni A (2023) SGLT-2 inhibitors and nephroprotection in patients with diabetic and non-diabetic chronic kidney disease. Curr Med Chem 30(18):2039–2060
Palmer BF, Clegg DJ (2023) Kidney-protective effects of SGLT2 inhibitors. Clin J Am Soc Nephrol 18(2):279–289
Vallon V, Verma S (2021) Effects of SGLT2 inhibitors on kidney and cardiovascular function. Annu Rev Physiol 83:503–528
Kadowaki T, Nangaku M, Hantel S et al (2019) Empagliflozin and kidney outcomes in Asian patients with type 2 diabetes and established cardiovascular disease: results from the EMPA-REG OUTCOME® trial. J Diabetes Investig 10(3):760–770
Perkovic V, de Zeeuw D, Mahaffey KW et al (2018) Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS program randomised clinical trials. Lancet Diabetes Endocrinol 6(9):691–704
Wiviott SD, Raz I, Bonaca MP et al (2019) Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 380(4):347–357
Han YP, Liu LJ, Yan JL et al (2023) Autophagy and its therapeutic potential in diabetic nephropathy. Front Endocrinol (Lausanne) 14:1139444
Chen B, Li Y, Liu Y, Xu Z (2019) circLRP6 regulates high glucose-induced proliferation, oxidative stress, ECM accumulation, and inflammation in mesangial cells. J Cell Physiol 234(11):21249–21259
Vargas JNS, Hamasaki M, Kawabata T, Youle RJ, Yoshimori T (2023) The mechanisms and roles of selective autophagy in mammals. Nat Rev Mol Cell Biol 24(3):167–185
Cao W, Li J, Yang K, Cao D (2021) An overview of autophagy: mechanism, regulation and research progress. Bull Cancer 108(3):304–322
Shan Z, Fa WH, Tian CR, Yuan CS, Jie N (2022) Mitophagy and mitochondrial dynamics in type 2 diabetes mellitus treatment. Aging (Albany NY) 14(6):2902–2919
Sehrawat A, Mishra J, Mastana SS et al (2023) Dysregulated autophagy: a key player in the pathophysiology of type 2 diabetes and its complications. Biochim Biophys Acta Mol Basis Dis 1869(4):166666
Sheng H, Zhang D, Zhang J et al (2022) Kaempferol attenuated diabetic nephropathy by reducing apoptosis and promoting autophagy through AMPK/mTOR pathways. Front Med (Lausanne) 9:986825
Zhong Y, Luo R, Liu Q et al (2022) Jujuboside A ameliorates high fat diet and streptozotocin induced diabetic nephropathy via suppressing oxidative stress, apoptosis, and enhancing autophagy. Food Chem Toxicol 159:112697
Ge Y, Zhou M, Chen C, Wu X, Wang X (2022) Role of AMPK mediated pathways in autophagy and aging. Biochimie 195:100–113
Sanchez-Garrido J, Shenoy AR (2021) Regulation and repurposing of nutrient sensing and autophagy in innate immunity. Autophagy 17(7):1571–1591
Sun T, Liu J, Xie C, Yang J, Zhao L, Yang J (2021) Metformin attenuates diabetic renal injury via the AMPK-autophagy axis. Exp Ther Med 21(6):578
Provenzano M, Puchades MJ, Garofalo C et al (2022) Albuminuria-lowering effect of Dapagliflozin, Eplerenone, and their combination in patients with chronic kidney disease: a randomized crossover clinical trial. J Am Soc Nephrol 33(8):1569–1580
Kurata Y, Nangaku M (2022) Dapagliflozin for the treatment of chronic kidney disease. Expert Rev Endocrinol Metab 17(4):275–291
Samsu N (2021) Diabetic nephropathy: challenges in pathogenesis, diagnosis, and treatment. Biomed Res Int 2021:1497449
Hu Q, Chen Y, Deng X et al (2023) Diabetic nephropathy: Focusing on pathological signals, clinical treatment, and dietary regulation. Biomed Pharmacother 159:114252
Liu H, Wang Q, Shi G et al (2021) Emodin ameliorates renal damage and podocyte injury in a rat model of diabetic nephropathy via regulating AMPK/mTOR-mediated autophagy signaling pathway. Diabetes Metab Syndr Obes 14:1253–1266
Liu H, Chen W, Wan S et al (2023) Canagliflozin ameliorates high glucose-induced apoptosis in NRK-52E cells via inhibiting oxidative stress and activating AMPK/mTOR-mediated autophagy. Mol Biol Rep. https://doi.org/10.1007/s11033-023-08855-x
Yang M, Li C, Yang S et al (2021) Mitophagy: a novel therapeutic target for treating DN. Curr Med Chem 28(14):2717–2728
Saad R, Tadmor H, Ertracht O et al (2022) The molecular effects of SGLT2i empagliflozin on the autophagy pathway in diabetes mellitus type 2 and its complications. J Diabetes Res 2022:8337823
Yang C, Chen XC, Li ZH et al (2021) SMAD3 promotes autophagy dysregulation by triggering lysosome depletion in tubular epithelial cells in diabetic nephropathy. Autophagy 17(9):2325–2344
Maiti AK (2021) Development of biomarkers and molecular therapy based on inflammatory genes in diabetic nephropathy. Int J Mol Sci 22(18):9985
Zhuang L, Jin G, Hu X, Yang Q, Shi Z (2019) The inhibition of SGK1 suppresses epithelial-mesenchymal transition and promotes renal tubular epithelial cell autophagy in diabetic nephropathy. Am J Transl Res 11(8):4946–4956
Zheng J, Hu S, Wang J et al (2021) Icariin improves brain function decline in aging rats by enhancing neuronal autophagy through the AMPK/mTOR/ULK1 pathway. Pharm Biol 59(1):183–191
Tabibzadeh S (2021) Signaling pathways and effectors of aging. Front Biosci (Landmark Ed) 26(1):50–96
Ding Y, Choi ME (2015) Autophagy in diabetic nephropathy. J Endocrinol 224(1):R15–R30
Jin J, Hu K, Ye M, Wu D, He Q (2018) Rapamycin reduces podocyte apoptosis and is involved in autophagy and mTOR/ P70S6K/4EBP1 signaling. Cell Physiol Biochem 48(2):765–772
Kume S, Yamahara K, Yasuda M, Maegawa H, Koya D (2014) Autophagy: emerging therapeutic target for diabetic nephropathy. Semin Nephrol 34(1):9–16
Yang L, Liang B, Li J et al (2022) Dapagliflozin alleviates advanced glycation end product induced podocyte injury through AMPK/mTOR mediated autophagy pathway. Cell Signal 90:110206
Xu J, Kitada M, Ogura Y, Liu H, Koya D (2021) Dapagliflozin restores impaired autophagy and suppresses inflammation in high glucose-treated HK-2 cells. Cells 10(6):1457
Funding
This work was supported by the Jinhua Science and Technology Bureau project (Grant No. 2021-4-067).
Author information
Authors and Affiliations
Contributions
Yu-Yan Ye and Yun Chen conceived and designed the study. Yu-Yan Ye, Yun Chen, Jing Yang, Jie Wu, and Peng Wang performed experimental analysis. Yu-Yan Ye supervised all the research and acquired the fund. Yu-Yan Ye wrote the first draft of the manuscript. Yu-Yan Ye and Yun Chen critically revised the manuscript. Yun Chen improved the language the manuscript. All authors have significantly participated in the manuscript preparation and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All experiments were approved by the Experimental Animal Ethical Committee of Zhejiang Eyong Pharmaceutical Research and Development Center and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ye, YY., Chen, Y., Yang, J. et al. Dapagliflozin restores autophagy and attenuates apoptosis via the AMPK/mTOR pathway in diabetic nephropathy rats and high glucose-induced HK-2 cells. Int Urol Nephrol (2024). https://doi.org/10.1007/s11255-024-04172-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11255-024-04172-9