Abstract
Background
It is unknown whether five-year overall survival (OS) differs and to what extent between testicular germ-cell tumor (TGCT) patients and age-matched male population-based controls.
Materials
We identified newly diagnosed (2004–2014) TGCT patients within Surveillance Epidemiology and End Results database 2004–2019. We compared OS between non-seminoma (NS-TGCT) and seminoma (S-TGCT) patients relative to age-matched male population-based controls based on Social Security Administration Life-Tables. Smoothed cumulative incidence plots displayed cancer-specific mortality (CSM) vs. other-cause mortality (OCM).
Results
Of all 20,935 TGCT patients, 43% had NS-TGCT and 57% had S-TGCT. Of NS-TGCT patients, 63% were stage I vs. 16% stage II vs. 21% stage III. Of S-TGCT patients, 86% were stage I vs. 8% were stage II vs. 6% stage III. Five-year OS differences between NS-TGCT patients vs age-matched male population-based controls were 97 vs. 99% (Δ = 2%) for stage I, 96 vs. 99% (Δ = 3%) for stage II, 76 vs 98% (Δ = 22%) for stage III. Five-year OS differences between S-TGCT patients vs age-matched male population-based controls were 97 vs. 98% (Δ = 1%) for stage I, 95 vs. 97% (Δ = 2%) for stage II, 87 vs. 98% (Δ = 11%) for stage III. OCM rates ranged from 1 to 3% in NS-TGCT patients and from 2 to 4% in S-TGCT patients.
Conclusion
The OS difference between NS-TGCT patients vs. age-matched male population-based controls was invariably higher across all stages (2–22%) than for S-TGCT patients (1–11%). Reassuringly, OCM rates were marginal in stage I and stage II patients. Conversely, higher OCM rates were recorded in stage III patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Testicular germ-cell tumor (TGCT) is the most common solid malignancy among men aged 20–40 years, enclosing in it two distinct main histological entities: non-seminomas (NS-TGCT) and seminomas (S-TGCT) [1, 2]. Its incidence has risen over the past two decades in Western countries [3]. In 2020, the highest incidence was estimated in western and central Europe (7–11 cases per 100,000), followed by the United States and Canada (5–6 cases per 100,000) [4].
The natural history of TGCT is generally very favorable for stages I and II. Conversely, less favorable survival applies to stage III patients, especially in NS-TGCT rather than seminoma S-TGCT. Indeed, the five-year overall survival (OS) in NS-TGCT patients ranging from 67 to 96%, and in S-TGCT ranging from 88 to 95% [5, 6]. Epidemiological data describing cancer-specific mortality (CSM), as well as other-cause mortality (OCM) rates of TGCT patients are scarce [7].
Currently, it is unknown whether stage I and II NS-TGCT or S-TGCT diagnosis affects future life expectancy. Moreover, it is also unknown to what extent future life expectancy of stage III NS-TGCT and S-TGCT differs from age-matched male population-based controls according to the United States Social Security Administration (SSA) Life-Tables [8]. Moreover, it is also unknown to what extent future life expectancy of different International Germ Cell Cancer Collaborative Group (IGCCCG) prognostic-risk groups differs from age-matched male population-based controls. Additionally, contemporary OCM rates in NS-TGCT as well as S-TGCT patients are unknown.
We addressed these knowledge gaps and tested for differences and quantified their magnitude within the Surveillance Epidemiology and End Results (SEER) database (2004–2019). We hypothesized that no differences exist in stage I and II patients from age-matched male population-based controls. Conversely, we hypothesized that significant differences exist between stage III patients and age-matched male population-based controls. Moreover, we postulated that significant differences exist between different prognostic-risk groups and age-matched male population-based controls. Finally, we hypothesized that OCM rates were marginal at best.
Materials and methods
Study population
The SEER database samples 34.6% of the US population in terms of demographic composition and cancer incidence [9]. Within the SEER database from 2004 to 2019, we selected patients ≥18 years old with newly diagnosed (2004–2014) testis cancer (International Classification of Disease for Oncology [ICD-O-3] site code C62.1 and C62.9) and histologically confirmed S-TGCT (ICD-O histology code 9061-2/3) or NS-TGCT (ICD-O histology code 9065/3, 9070/3, 9071/3, 9080-5/3, 9100-2/3) [2]. We only considered patients treated with orchiectomy with available American Joint Committee on Cancer (AJCC) staging. All autopsy or death certificate-only cases were excluded. Further exclusion criteria consisted of missing follow-up and survival data. Rates of CSM (i.e., death from TGCT) and OCM (i.e., death not attributable to TGCT) were defined according to the SEER mortality code. Owing to the anonymously coded design of the SEER database, study-specific ethics approval was waived by the institutional review board.
Statistical analyses
For each TGCT patient, we simulated an age-matched male control in a one-to-one fashion, according to previously described methodology [10,11,12,13,14,15]. Subsequently, the survival of the control population was computed using a Monte Carlo simulation and a Markov chain of natural progression, according to SSA Life-Tables’ derived probabilities of survival at five years of follow-up. Therefore, derived five-year OS estimates for each age-matched male stage-specific group control will henceforth be referred to as “age-matched male population-based controls”.
Descriptive statistics included frequencies and proportions for categorical variables. Medians and interquartile ranges (IQR) were reported for continuously coded variables.
Kaplan-Meier plots graphically depicted OS of NS-TGCT and S-TGCT patients and of corresponding age-matched male population-based controls in a stage-specific fashion. Subsequently, differences in five-year OS were computed in NS-TGCT and S-TGCT patients relative to age-matched male population-based controls. Finally, smoothed cumulative incidence plots to graphically depicted CSM and OCM rates.
Additionally, we performed subgroup analyses in metastatic NS-TGCT (mNS-CGTT) and in metastatic S-TGCT (mS-TGCT) patients. Here, additional stratification according to IGCCCG prognostic-risk groups (good vs. intermediate vs. poor prognosis mNS-TGCT; good vs. intermediate prognosis mS-TGCT) was applied.
All tests were two-sided, with a significance level set at p < 0.05. In all statistical analyses, R software environment for statistical computing and graphics (R version 4.1.3, R Foundation for Statistical Computing, Vienna Austria, http://www.r-project.org/) was used [16].
Results
Characteristics of NS-TGCT and S-TGCT cohorts
Of 20,935 TGCT patients, 9035 (43%) had NS-TGCT while 11,894 (57%) had S-TGCT. Within NS-TGCT patients, the median age was 28 (IQR: 23–35) and the AJCC stage distribution was as follows: stage I 5722 (63%) vs. stage II 1399 (16%) vs. stage III 1,914 (21%). We identified 2469 mNS-TGCT patients that fulfilled IGCCCG prognostic-risk groups criteria, harbored 1260 (51%) good vs. 425 (17%) intermediate vs. 784 (32%) poor prognosis. (Table 1a)
Conversely, within the S-TGCT, the median age was 37 (IQR: 30–45) and the AJCC stage distribution was as follows: stage I 10,204 (85%) vs. stage II 1008 (8%) vs. stage III 682 (6%). We identified 1690 mS-TGCT patients that fulfilled IGCCCG prognostic-risk groups criteria harbored 1531 (91%) good vs. 159 (9%) intermediate prognosis. (Table 1b)
Overall survival in NS-TGCT and S-TGCT patients versus age-matched male population-based controls
At five years of follow-up, OS differences between NS-TGCT patients vs age-matched male population-based controls ranged from 2 to 22% according to stages (Fig. 1; Table 2). Specifically, the greatest OS difference was recorded in stage III (76 vs 98%, Δ = 22%; Fig. 1c), followed by stage II (96 vs 99%, Δ = 3%; Fig. 1b) and stage I (97 vs 99%, Δ = 2%; Fig. 1a), in that order.
Kaplan-Meier curves comparing five-year overall survival of 20,929 newly diagnosed (2004–2014) testicular germ-cell tumor (TGCT) patients, 9035 harbored nonseminoma (NS-TGCT) (a–c) and 11,894 harbored seminoma (S-TGCT) (d–f) within the Surveillance, Epidemiology, and End Results database 2004–2019 vs their simulated age-matched male population-based controls, according to American Joint Committee on Cancer (AJCC) stage (I vs II vs III). (Legend: absΔ = absolute difference)
Conversely, at five years of follow-up, OS differences between S-TGCT patients vs age-matched male population-based controls ranged from 1 to 11% according to stages (Fig. 1; Table 2). Specifically, the greatest OS difference was recorded in stage III (87 vs 98%, Δ = 11%; Fig. 1f), followed by stage II (95 vs 97%, Δ = 2%; Fig. 1e) and stage I (97 vs 98%, Δ = 1%; Fig. 1d), in that order.
Cancer‑specific mortality and other‑cause mortality in NS-TGCT and S-TGCT patients
At five years of follow-up, CSM rates in NS-TGCT ranged from 2 to 20%, according to stage (Fig. 2; Table 2). Specifically, the CSM rate was 20% in stage III and 2% in both stage I and stage II. Conversely, at five years of follow-up, CSM rates in S-TGCT ranged from 1 to 9%, according to stage. Specifically, the highest CSM rate was recorded in stage III (9%), followed by stage II (2%) and stage I (1%), in that order.
Smoothed cumulative incidence plots addressing cancer-specific mortality (CSM) and other-cause mortality (OCM) of 20,929 newly diagnosed (2004–2014) testicular germ-cell tumor (TGCT) patients, 9035 harbored non-seminoma (NS-TGCT) (a–c) and 11,894 harbored seminoma (S-TGCT) (d–f), within the Surveillance, Epidemiology, and End Results database 2004–2019 according to American Joint Committee on Cancer (AJCC) stage (I vs II vs III)
At five years of follow-up, OCM rates in NS-TGCT ranged from 1 to 3%, according to stage (Fig. 2; Table 2). Specifically, the OCM rates were 3% in stage III and in 1% in both stage I and II. Conversely, OCM rates in S-TGCT ranged from 2 to 4%, according to stage. Specifically, the OCM rates were 4% in stage III and 2% in both stage I and II.
Subgroup analyses in mNS-TGCT and mS-TGCT patients
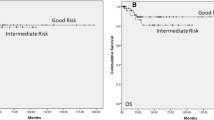
Within mNS-GCT, the greatest OS difference between IGCCCG prognostic-risk group patients and age-matched male population-based controls was recorded in poor prognosis (65 vs 99%, Δ = 34%; Fig. 3c), followed by intermediate prognosis (84 vs 99%, Δ = 15%; Fig. 3b) and good prognosis (93 vs 99%, Δ = 6%; Fig. 3a), in that order.
Kaplan-Meier curves comparing five-year overall survival of 4159 newly diagnosed (2004–2014) with metastatic testicular germ-cell tumor (mTGCT) patients, 2469 harbored nonseminoma (mNS-TGCT) (a–c) and 1,690 (d, e) seminoma (mS-TGCT), within the Surveillance, Epidemiology, and End Results database 2004-2019 vs their simulated age-matched male population-based controls, according to International Germ Cell Cancer Collaborative Group (IGCCCG) prognostic-risk groups (good vs. intermediate vs. poor for mNS-TGCT; good vs. intermediate for mS-TGCT). (Legend: absΔ = absolute difference)
Within mS-TGCT, the greatest OS difference between IGCCCG prognostic-risk group patients and age-matched male population-based controls was recorded in intermediate prognosis (78 vs 98%, Δ = 20%; Fig. 3e), followed by good prognosis (93 vs 97%, Δ = 4%; Fig. 3d).
At five-years of follow-up, CSM rates in mNS-TGCT ranged from 4 to 31%, according to IGCCCG prognostic-risk groups (Fig. 4; Table 3). Specifically, the CSM rate was 31% in poor prognosis patients, 13% in intermediate prognosis patients and 4% in good prognosis patients.
Smoothed cumulative incidence plots addressing cancer-specific mortality (CSM) and other-cause mortality (OCM) of 4159 newly diagnosed (2004–2014) with metastatic germ-cell tumor of the testis (mTGCT) patients, 2469 harbored non-seminoma (mNS-TGCT) (a–c) and 1690 harbored seminoma (mS-TGCT) (d, e), within the Surveillance, Epidemiology, and End Results database 2004–2019 according to International Germ Cell Cancer Collaborative Group (IGCCCG) prognostic-risk groups (good vs. intermediate vs. poor for mNS-TGCT; good vs. intermediate for mS-TGCT)
Conversely, at five-years of follow-up, CSM rates in mS-TGCT ranged from 4 to 18%, according to IGCCCG prognostic-risk groups. Specifically, the CSM rate was 18% in intermediate prognosis patients and 4% in good prognosis patients.
At five-years of follow-up, OCM rates in mNS-TGCT were 2% for good and intermediate patients, while it was 4% for poor prognosis patients. Conversely, at five-years of follow-up, OCM rates in mS-TGCT were 4% in intermediate prognosis patients and 3% in good prognosis patients.
Discussion
It is unknown whether five-year OS differs and to what extent between TGCT patients and age-matched male population-based controls. Our comprehensive analyses of OS between TGCT patients and age-matched male population-based controls revealed several noteworthy observations.
First, we identified 20,935 TGCT patients who underwent orchiectomy within the SEER database over a period of eleven years (2004–2014). This number is comparable to other publications addressing TGCT within SEER and National Cancer Database (NCDB) [17,18,19]. TGCT is the most common type of testicular cancer, however, it accounts for less than 2% of adult malignancies [20]. Therefore, single-institution or even multi-institutional databases may suffer of limited numbers of observations. For example, Yamashita et al. relied on a cohort of only 563 patients (1980–2019) diagnosed with testicular cancer from a single high-volume center [21]. Similarly, Noureldin et al., in a large multi-institutional database identified only 869 patients (2008–2017) diagnosed with testicular cancer [20]. In consequence, only large epidemiological databases such as SEER or NCDB offer the opportunity to study relatively large cohorts of such a rare entity as TGCT.
Second, in stage I and II marginal OS differences were recorded between NS-TGCT and S-TGCT patients relative to age-matched male population-based controls. Expectedly, the lowest difference was recorded in stage I S-TGCT, followed by stage I NS-TGCT, stage II S-TGCT and stage II NS-TGCT, in that order. These observations are consistent with the notion that stage I and II both NS-TGCG and S-TGCT represent curable diseases [4, 22]. Indeed, our observations validate that notion based on OS differences, that were marginal between S-TGCT stage I patients and age-matched male population-based controls (97 vs. 98%; Δ = 1%), as well as between NS-TGCT stage I patients and age-matched male population-based controls (97 vs 99%; Δ = 2%). Moreover, only marginally higher OS differences were recorded in NS-TGCT and S-TGCT patients relative to corresponding age-matched male population-based controls (Δ = 3% in NS-TGCT and Δ = 2% in S-TGCT). Taken together, these observations indicate that future life expectancy is not affected in stage I and II NS-TGCT and S-TGCT patients, relative to age-matched male population-based controls. The above findings addressing stage I and II TGCT cannot be directly compared with previous studies, since no such studies exist. However, the observations made in the current study should ideally be validated within an independent external validation cohort from within a different large-scale data repository.
Third, in stage III we recorded higher OS differences in NS-TGCT and S-TGCT patients relative to age-matched male population-based controls. Specifically, the highest difference was recorded between stage III NS-TGCT and age-matched male population-based controls (76 vs. 98%; Δ = 22%), followed by stage III S-TGCT (87 vs. 98; Δ = 11%). Taken together, these observations indicate that future life expectancy is shortened in stage III NS-TGCT relative to age-matched male population-based controls and to a lesser extent to stage III S-TGCT. These observations cannot be directly compared with absolute OS rates from other studies, since no such studies exist.
Fourth, specific subgroup analyses that tabulated mNS-TGCT and mS-TGCT patients according to IGCCCG prognostic-risk groups revealed a dose-response effect, where future life expectancy was most reduced in poor, followed by intermediate and good prognosis patients. Specifically, these observations were more pronounced in mNS-TGCT patients (poor 65 vs 99%, Δ = 34%; intermediate 84 vs 99%, Δ = 15%; good 93 vs 99%, Δ = 6%). Despite less pronounced differences in absolute values of life expectancy detriments, a similar dose-response effect was recorded in mS-TGCT patients (intermediate 78 vs 98%, Δ = 20%; good 93 vs 97%, Δ = 4%). As for the overall analyses, shortened life expectancy is expected in metastatic patients, relative to age-matched male population-based control. Moreover, the magnitude of life expectancy detriment is also expected to be most pronounced in poor IGCCCG prognostic-risk group patients, followed by intermediate and good. However, no factual data supporting these expectations were previously reported. In consequence, the current study cannot be directly compared.
Last but not least, we addressed OCM rates in NS-TGCT and S-TGCT patients. We hypothesized that virtually no OCM should be recorded in this young patient population in whom treatment toxicity should be minimal. Indeed, in stage I we recorded an OCM rate of 1% both in NS-TGCT and S-TGCT patients. Additionally, in stage II we recorded an OCM rate of 1% in NS-TGCT patients and 2% in S-TGCT patients. Conversely, higher OCM rates were recorded both in NS-TGCT and S-TGCT stage III patients. Specifically, the OCM rates were 3 and 4% in NS-TGCT and S-TGCT, respectively. These observations regarding OCM are consistent with expected non-existent or at best marginal OCM in stage I and stage II patients. Conversely, higher OCM rates in stage III patients (3% in NS-TGCT and 4% in S-TGCT) are also consistent with expected higher OCM in those individuals that may originate from systemic therapy.
Taken together, despite the younger age at diagnosis future life expectancy of TGCT patients was lower compared to age-matched male population-based controls. Specifically, we recorded a dose-response effect across the different stages, more pronounced in NS-TGCT than in S-TGCT. Indeed, stage I NS-TGCT patients exhibited worse survival relative to age-matched male population-based controls (97 vs 99%; Δ = 2%) than S-TGCT patients (98 vs 99%; Δ = 1%). Similar direction, but higher magnitude, even if marginal was recorded in stage II patients. Specifically, NS-TGCT patients exhibited worse survival relative to age-matched population-based controls (96 vs 99%; Δ = 3%), than S-TGCT patients (97 vs 99%; Δ = 2). Moreover, in stage III we recorded the largest difference in future life expectancy. Specifically, stage III NS-TGCT patients exhibited worse survival (76 vs 98%; Δ = 22%) relative to age-matched male population-based controls than S-TGCT patients (87 vs 98%; Δ = 11%). Stratification of metastatic patients according to the IGCCCG prognostic-risk group also allowed to identify a dose-response effect. Specifically and expectedly, future life expectancy is most affected in poor, followed by intermediate, and good prognosis, in that order in NS-TGCT patients and to a lesser extent in intermediate and good prognosis in S-TGCT patients. Last but not least, OCM rate is non-existent to marginal in stages I and II NS-TGCT and S-TGCT patients. However, more pronounced OCM rates were recorded in stage III patients. The above results are noteworthy for patient counseling. Indeed, in stage I and stage II, clinicians can reassure patients regarding the low effect of TGCT on future life expectancy after orchiectomy performed. Conversely, in more advanced stage clinicians can encourage patients to undergo post-orchiectomy treatments, such as systemic therapy and/or radiotherapy and/or retroperitoneal lymph node dissection. Additionally, a higher awareness of the disease and its mortality, may be an useful tool to make patients more compliant in attending follow-up visits.
Despite its novelty, our study is not devoid of limitations. First and foremost, our study shares the limitations of all similar studies that were based on the SEER database and relied on a retrospective data design, with potential for selection biases that may apply to prognosis groups in a similar fashion to historical and contemporary multi-institutional retrospective analyses [23, 24]. Second, our findings are applicable to individuals who are identified within the SEER database. Consequently, the observations made within the current study are not generalizable to individuals from outside the United States or even patients who are not comparable to those included in the SEER database. Third, despite a large patient population, the amount of detail is limited. Indeed, only survival rates were available. For example, no data regarding lymphovascular or stomal invasion that may affect TGCT patients’ survival were available. Moreover, the nature of the SEER database did not allow us to address early cancer control outcomes such as relapse rates or metastatic progression rates. Therefore, lack of inclusion of more detailed information and endpoints precludes generalizability of our results. Fourth, baseline comorbidity status of TGCT patients was also not available. However, we partially addressed this limitation by the inclusion of OCM rates in the current analyses. The latter represents a valid surrogate for most significant comorbidities that predispose to mortality. Fifth, the comparison group that consisted of age-matched male controls represented a simulated population in whom OS was defined according to SSA Life-Tables. Although this methodology has been used in various previous publications and is well accepted, it only represents a surrogate for true population controls without the trait of interest, naming TGCT. Moreover, data derived from SSA Life-Tables does not provide a specific cause of death. In consequence, the comparison to the SSA Life Tables only allows for investigating OS. Similarly, further adjustments for patient characteristics other than age could also not be made, since the SSA Life Tables do not provide this type of stratification.
Conclusion
The OS difference between NS-TGCT patients vs. age-matched male population-based controls was invariably higher across all stages (2–22%) than for S-TGCT patients (1–11%). Reassuringly, OCM rates were marginal in stage I and II patients. Conversely, higher OCM rates were recorded in stage III patients.
Data availability
All data generated for this analysis were from the SEER database (https://seer.cancer.gov/data/). The code for the analyses will be made available upon request.
References
Stephenson A, Eggener SE, Bass EB et al (2019) Diagnosis and treatment of early stage testicular cancer guideline: AUA GUIDELINE
Moch H, Cubilla AL, Humphrey PA et al (2016) The 2016 WHO classification of tumours of the urinary system and male genital organs—part A: renal, penile, and testicular tumours. Eur Urol 70:93–105. https://doi.org/10.1016/j.eururo.2016.02.029
Cheng L, Albers P, Berney DM et al (2018) Testicular cancer. Nat Rev Dis Primer 4:29. https://doi.org/10.1038/s41572-018-0029-0
Chovanec M, Cheng L (2022) Advances in diagnosis and treatment of testicular cancer. BMJ. https://doi.org/10.1136/bmj-2022-070499
Gillessen S, Sauvé N, Collette L et al (2021) Predicting outcomes in men with metastatic nonseminomatous germ cell tumors (NSGCT): Results from the IGCCCG Update Consortium. J Clin Oncol 39:1563–1574. https://doi.org/10.1200/JCO.20.03296
Beyer J, Collette L, Sauvé N et al (2021) Survival and new prognosticators in metastatic seminoma: results from the IGCCCG-Update Consortium. J Clin Oncol 39:1553–1562. https://doi.org/10.1200/JCO.20.03292
Park JS, Kim J, Elghiaty A, Ham WS (2018) Recent global trends in testicular cancer incidence and mortality. Medicine (Baltimore) 97:e12390. https://doi.org/10.1097/MD.0000000000012390
Social Security Administration-Actuarial Life Table. https://www.ssa.gov/oact/STATS/table4c6.html. Accessed 13 Jan 2023
SEER Cancer Statistics Review, 1975-2018. In: SEER. https://seer.cancer.gov/csr/1975_2018/index.html. Accessed 14 Jan 2023
Preisser F, Bandini M, Mazzone E et al (2019) Validation of the social security administration life tables (2004–2014) in localized prostate cancer patients within the surveillance, epidemiology, and end results database. Eur Urol Focus 5:807–814. https://doi.org/10.1016/j.euf.2018.05.006
Chierigo F, Borghesi M, Würnschimmel C et al (2022) Life expectancy in metastatic urothelial bladder cancer patients according to race/ethnicity. Int Urol Nephrol 54:1521–1527. https://doi.org/10.1007/s11255-022-03221-5
Würnschimmel C, Nocera L, Wenzel M et al (2022) Race/ethnicity may be an important predictor of life expectancy in localized prostate cancer patients: novel analyses using social security administration life tables. J Racial Ethn Health Disparities. https://doi.org/10.1007/s40615-022-01257-y
Würnschimmel C, Collà Ruvolo C, Nocera L et al (2022) Race/ethnicity determines life expectancy in surgically treated T1aN0M0 renal cell carcinoma patients. Eur Urol Focus 8:191–199. https://doi.org/10.1016/j.euf.2021.02.004
Cano Garcia C, Piccinelli ML, Tappero S et al (2023) Differences in overall survival of T2N0M0 bladder cancer patients vs. population-based controls according to treatment modalities. Int Urol Nephrol 55:1117–1123. https://doi.org/10.1007/s11255-023-03517-0
Garcia CC, Nimer N, Piccinelli ML et al (2023) Differences in overall survival between clear cell metastatic renal cell carcinoma patients versus population-based controls according to race/ethnicity in the United States. Ann Epidemiol. https://doi.org/10.1016/j.annepidem.2023.01.003
R: The R Project for Statistical Computing. https://www.r-project.org/. Accessed 14 Jan 2023
Palumbo C, Mistretta FA, Mazzone E et al (2019) Contemporary incidence and mortality rates in patients with testicular germ cell tumors. Clin Genitourin Cancer 17:e1026–e1035. https://doi.org/10.1016/j.clgc.2019.06.003
Mao W, Wu J, Kong Q et al (2020) Development and validation of prognostic nomogram for germ cell testicular cancer patients. Aging 12:22095–22111. https://doi.org/10.18632/aging.104063
Woldu SL, Moore JA, Ci B et al (2018) Practice patterns and impact of postchemotherapy retroperitoneal lymph node dissection on testicular cancer outcomes. Eur Urol Oncol 1:242–251. https://doi.org/10.1016/j.euo.2018.04.005
Noureldin YA, Alqirnas MQ, Aljarallah MF et al (2022) Testicular cancer among Saudi adults: hands on a nationwide cancer registry over 10 years. Arab J Urol 20:182–188. https://doi.org/10.1080/2090598X.2022.2084902
Yamashita S, Koyama J, Goto T et al (2020) Trends in age and histology of testicular cancer from 1980–2019: a single-center study. Tohoku J Exp Med 252:219–224. https://doi.org/10.1620/tjem.252.219
Facchini G, Rossetti S, Berretta M et al (2019) Prognostic and predictive factors in testicular cancer. Eur Rev Med Pharmacol Sci 23:3885–3891
Cano Garcia C, Panunzio A, Tappero S et al (2023) Survival of testicular pure embryonal carcinoma vs. mixed germ cell tumor patients across all stages. Medicina (Mex) 59:451. https://doi.org/10.3390/medicina59030451
Cano Garcia C, Barletta F, Incesu R-B et al (2023) Survival of testicular pure teratoma vs. mixed germ cell tumor patients in primary tumor specimens across all stages. Cancers 15:694. https://doi.org/10.3390/cancers15030694
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization and Methodology: SM, MLP; Acquisition of the data: SM, CCG, AB; Formal analysis: SM, ZT; Investigation and Data Curation: SM, ST, FB; Writing- Original Draft: SM, MLP, RBI, LS; Visualization: FS, VM, GC, CCR, SS, OdC, GM, FKHC, CT, AB, DT, SA, LC; Funding acquisition: N/A; Supervision: NL, PIK; Project administration: PIK.
Corresponding author
Ethics declarations
Conflict of interest
Shahrokh F. Shariat: Horonraria: Astellas, Astra Zeneca, Bayer, BMS, Cepheid, Ferring, lpsen, Janssen, Lilly, MSD, Olympus, Pfizer, Pierre Fabre, Richard Wolf, Roche, Sanochemia, Sanofi, Takeda, Urogen. Consulting or Advisory Role: Astellas, Astra Zeneca, Bayer, BMS, Cepheid, Ferring, lpsen, Janssen, Lilly, MSD, Olympus, Pfizer, Pierre Fabre, Richard Wolf, Roche, Sanochemia, Sanofi, Takeda, Urogen. Speakers’ Bureau: Astellas, Astra Zeneca, Bayer, BMS, Cepheid, Ferring, lpsen, Janssen, Lilly, MSD, Olympus, Pfizer, Pierre Fabre, Richard Wolf, Roche, Sanochemia, Sanofi, Takeda, Urogen, Movember Foundation. Patents: Method to determine prognosis after therapy for prostate cancer - granted 2002-09-06, Methods to determine prognosis after therapy for bladder cancer - granted 2003-06-19, Prognostic methods for patients with prostatic disease - granted 2004-08-05; Soluble Fas urinary marker for the detection of bladder transitional cell carcinoma - granted 2010-07-20. Our research was conducted without any other potential conflicts of interest.
Ethics statement
All analyses and their reporting followed the SEER reporting guidelines. Due to the anonymously coded design of the SEER database, study‐ specific Institutional Review Board ethics approval as not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Morra, S., Piccinelli, M.L., Cano Garcia, C. et al. Differences in future life expectancy of testicular germ-cell tumor patients vs. age-matched male population-based controls. Int Urol Nephrol 55, 3119–3128 (2023). https://doi.org/10.1007/s11255-023-03763-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03763-2