Abstract
Aims
Fluid overload is a common manifestation of cardiovascular and kidney disease and a leading cause of hospitalizations. To identify patients at risk of recurrent severe fluid overload, we evaluated the incidence and risk factors associated with early repeat hospitalization for fluid overload among individuals with cardiovascular disease and risks.
Methods
Single-center retrospective cohort study of 3423 consecutive adults with an index hospitalization for fluid overload between January 2015 and December 2017 and had cardiovascular risks (older age, diabetes mellitus, hypertension, dyslipidemia, kidney disease, known cardiovascular disease), but excluded if lost to follow-up or eGFR < 15 ml/min/1.73 m2. The outcome was early repeat hospitalization for fluid overload within 30 days of discharge.
Results
The mean age was 73.9 ± 11.6 years and eGFR was 54.1 ± 24.6 ml/min/1.73 m2 at index hospitalization. Early repeat hospitalization for fluid overload occurred in 291 patients (8.5%). After adjusting for demographics, comorbidities, clinical parameters during index hospitalization and medications at discharge, cardiovascular disease (adjusted odds ratio, OR 1.66, 95% CI 1.27–2.17), prior hospitalization for fluid overload within 3 months (OR 2.52, 95% CI 1.17–5.44), prior hospitalization for any cause in within 6 months (OR 1.33, 95% CI 1.02–1.73) and intravenous furosemide use (OR 1.58, 95% CI 1.10–2.28) were associated with early repeat hospitalization for fluid overload. Higher systolic BP on admission (OR 0.992, 95% 0.986–0.998) and diuretic at discharge (OR 0.50, 95% CI 0.26–0.98) reduced early hospitalization for fluid overload.
Conclusion
Patients at-risk of early repeat hospitalization for fluid overload may be identified using these risk factors for targeted interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fluid overload is a common manifestation of cardiovascular and kidney disease [1]. It is associated with increased mortality [1,2,3], cardiovascular complications [1, 2], progressive kidney failure [1, 2], and poorer quality of life for the patients. Fluid overload is also a leading cause of heart failure emergency department visits [4, 5] and hospitalizations [6], thereby contributing to greater health services utilization and economic burden for the healthcare system [7]. In real-world clinical practice, the term “fluid overload” may be used loosely or interchangeably where heart failure with hemodynamic congestion is present because these conditions cannot be easily distinguished as isolated entities [3]. Indeed, fluid overload and heart failure are closely interlinked as they share similar pathogenic mechanisms of sodium and water retention [2, 3]. However, fluid overload extends beyond the conventional “heart failure” associated with reduced left ventricular function. Fluid overload may be diagnosed where heart failure with preserved ejection fraction (HFpEF) is under-recognized among patients with cardiovascular risks such as kidney disease [8], diabetes [9], and hypertension [10]. There is also growing recognition of the adverse health outcomes of fluid overload as an entity in settings other than heart failure, such as in critical illness [3].
Although some studies had described health service utilization and readmissions in heart failure, few studies focused on the wider cohort with fluid overload; even fewer studies specifically examined preventable readmissions [11]. Yet, the recurrence of severe fluid overload is a potentially preventable readmission that deserves greater attention. Successful strategies that focused on early recognition and timely interventions for fluid overload in the community or clinic settings reduced hospitalizations and improved cardiorenal outcomes and health-related quality of life [12, 13]. It is, therefore, important to identify patients at risk of repeat hospitalizations for recurrent severe fluid overload who can benefit from proven strategies to reduce readmissions. In this study, we aimed to (1) evaluate the healthcare utilization and (2) identify risk factors associated with early readmission for fluid overload recurrence among individuals with cardiovascular disease and cardiovascular risks hospitalized for fluid overload.
Methods
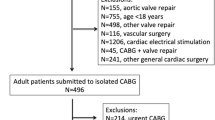
This was a retrospective cohort study of all adults hospitalized for fluid overload at the Singapore General Hospital, an academic medical center and tertiary care hospital, between 1st January 2015 and 31st December 2017. Fluid overload conditions (“fluid overload”, “heart failure”, “congestive heart failure”, “pulmonary edema” and “generalized edema”) were identified from discharge codes based on the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10). The index hospitalization was defined as the first hospitalization for fluid overload during the study period. Patients were included if they had cardiovascular risks (older age, diabetes mellitus, hypertension, dyslipidemia, kidney disease) and/or cardiovascular disease. Supplementary Table S1 showed that among 5121 patients who had an index hospitalization for fluid overload, the most common diagnosis code was congestive heart failure (I500), followed by fluid overload (E877). Figure 1 shows the cohort selection: 767 patients were excluded because they were not on active follow-up (did not have any laboratory visits or prescriptions up to 12 months after discharge and did not have any hospital visits up to 30th December 2018). Among the remaining 4354 patients who were on follow-up, 75 were excluded because they did not satisfy the inclusion criteria and 856 were excluded because they had kidney failure (defined as estimated glomerular filtration rate [eGFR] < 15 ml/min/1.73 m2).
Data were retrieved from electronic medical records. “Baseline” data included demographic data (age, gender, and ethnicity); comorbidities (hypertension, diabetes, kidney disease, cardiovascular disease, atrial fibrillation, systolic and diastolic blood pressure [BP] and serum creatinine on admission) and hospitalization dates and discharge diagnoses within 6 months before index hospitalization. Hypertension was defined as systolic BP > 140 mmHg or diastolic BP > 90 mmHg (at index hospitalization) or the use of BP-lowering medications. Diabetes mellitus was defined according to diagnosis codes [14], laboratory results (fasting plasma glucose ≥ 7 mmol/L, oral glucose tolerance test plasma glucose ≥ 11.1 mmol/L at 2 h, HbA1c ≥ 7%) and/or the use of glucose-lowering medication. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation. Kidney disease was present if eGFR was < 60 ml/min/1.73 m2. Cardiovascular disease was defined as the presence of ischemic heart disease, congestive cardiac failure, acute myocardial infarct, stroke, and peripheral vascular disease. Prescriptions for BP-lowering medication, renin–angiotensin system (RAS) blockers such as angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers, thiazide or loop diuretics, systemic non-steroidal anti-inflammatory drugs (NSAIDs) and statins (Supplementary Table S2) within 6 months before the index hospitalization and at discharge were retrieved from electronic prescription records.
The primary outcome was early repeat hospitalization for fluid overload within 30 days of discharge of the index hospitalization. A 30-day readmission was the most frequently evaluated outcome in several systematic reviews [11, 15], and also frequently used as a quality indicator for value-driven care practices and financial reimbursements [16]. The secondary outcome was the time to subsequent hospitalization for fluid overload. Outcomes were assessed until 30th December 2018.
This study abided by the Declaration of Helsinki. The SingHealth Centralized Institutional Review Board (2020/3061) determined that the study did not require ethical deliberation for the use of de-identified data. The study was reported according to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement (Supplementary Table S3).
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 26 (IBM Corp., Armonk, New York). Categorical variables were presented as proportions and continuous variables were summarized as means ± standard deviations (SD) or medians with interquartile ranges [IQR (25th percentile, 75th percentile)] as appropriate. Baseline characteristics were compared according to the primary outcome using Pearson Chi-square test or Fisher’s exact test for categorical variables and the Mann–Whitney U test for continuous variables. Multi-variable analysis using binary logistic regression (enter method) was used to obtain odds ratio (OR) and 95% confidence interval (CI) for pre-determined factors (1) associated with all-cause readmissions in the literature (age [17], gender [17], ethnicity [17], length of stay [LOS] during the index hospitalization [17]), (2) has p ≤ 0.25 on univariate analysis (prior hospitalization for fluid overload; prior hospitalization for any cause; cardiovascular disease, atrial fibrillation, NSAID and RAS blocker before index hospitalization; systolic BP, eGFR, intravenous furosemide during index hospitalization; and statin and RAS blocker at discharge), or (3) associated with fluid overload (diabetes, diuretic before and at discharge). Multicollinearity was checked by examining the correlation matrix for coefficient values ≥ 0.80. Sensitivity analysis was performed using Cox regression analysis for factors associated with time to repeat hospitalization for fluid overload, censored at 30 days from discharge. All tests were two-tailed and statistical significance was defined as p < 0.05 unless otherwise stated. To assess the multivariable model for the primary outcome, the C-statistic, the equivalent of the area (AUC) under a receiver operating characteristic (ROC) curve, was computed as a measure of discrimination. A value of 0.5 suggests poor predictive performance while a value of 1.0 suggests perfect ability to differentiate between individuals with and without the outcome. Calibration of the model was evaluated by the Hosmer–Lemeshow goodness-of-fit test, where p < 0.05 suggests poor agreement between predicted and observed risks. In the absence of a validated risk prediction score for repeat hospitalization for fluid overload, the multivariable model was compared with a model that comprised age, gender, race and the LACE index. The LACE index assigns scores according to length of stay, acuity of admission, the Charlson comorbidity index and number of emergency department visits in the past 6 months to identify those at high-risk for repeat hospitalizations [18]. Among the many prediction models for hospital readmissions [15], the LACE index was considered advantageous in real-world clinical applications because of its discriminatory ability, simplicity, replicability and potential generalizability [17]. It was also one of the more frequently evaluated models for readmissions in patients with and without heart disease [11, 19].
Results
The study cohort included 3423 unique patients with cardiovascular disease and/or risks who were hospitalized for fluid overload. Table 1 describes the characteristics of the cohort. The mean age was 73.9 ± 11.6 years. The ethnic composition was generally consistent with the country’s multi-ethnic population according to the population census in 2020 [20], and earlier publications from the same institution [17]. Cardiovascular disease and risk factors for cardiovascular disease were frequent. The majority (3087 patients; 90.2%) were admitted via the emergency department. The median LOS was 4 (2, 9) days. The LOS was not significantly different in patients with older age, diabetes, and kidney disease than in those without (Supplementary Table S4). The median LACE score was 12 (10, 14) and the majority (2737 patients, 80.0%) had “high-risk” LACE scores of 10 or more.
The median follow-up was 17.9 (7.4, 30.3) months. Early repeat hospitalization for fluid overload within 30 days occurred in 291 patients (8.5%). The time to repeat hospitalization for fluid overload was 4.3 (1.2, 11.5) months for 1279 patients (37.4%). Table 1 compares the characteristics of those with the primary outcome with those who did not. Those who had an early repeat hospitalization for fluid overload within 30 days post-discharge were more likely to have cardiovascular disease, atrial fibrillation, prior hospitalization for fluid overload, prior hospitalization for any cause, BP-lowering medication and lower systolic BP than those without early readmission. There was also a tendency for greater use of intravenous furosemide during the index hospitalization.
Table 2 shows the multivariable analysis adjusting for demographic factors (age, gender, ethnicity), comorbidities (recent hospitalization, cardiovascular disease, atrial fibrillation, diabetes), medications prior to index hospitalization (diuretic, RAS blocker, NSAID), clinical parameters during index hospitalization (systolic BP, eGFR, intravenous furosemide use, LOS) and medications at discharge (diuretic, RAS blocker, statin). Independent predictors for early repeat hospitalization for fluid overload within 30 days were cardiovascular disease (adjusted OR 1.66, 95% CI 1.27–2.17, p < 0.001), prior hospitalization for fluid overload within 3 months preceding index hospitalization (adjusted OR 2.52, 95% CI 1.17–5.44, p = 0.02), prior hospitalization for any cause in the 6 months preceding index hospitalization (adjusted OR 1.33, 95% CI 1.02–1.73, pp = 0.04) and intravenous furosemide during the index hospitalization (adjusted OR 1.58, 95% CI 1.10–2.28, = 0.01). Higher systolic BP on admission (adjusted OR 0.992, 95% 0.986–0.998, p = 0.01) and diuretic at discharge (adjusted OR 0.50, 95% CI 0.26–0.98, p = 0.04) were associated with reduced early hospitalization for fluid overload. There was no multi-collinearity in the model. Sensitivity analysis with Cox regression found that cardiovascular disease (adjusted HR 1.61, 95% CI 1.25–2.08, p < 0.001), prior hospitalization for fluid overload within 3 months (adjusted HR 2.16, 95% CI 1.12–4.16, p = 0.02), prior hospitalization for any cause within 6 months (adjusted HR 1.28, 95% CI 1.001–1.65, p = 0.049) and intravenous furosemide during the index hospitalization (adjusted HR 1.54, 95% CI 1.08–2.18, p = 0.02) were associated with early repeat hospitalization for fluid overload. Higher systolic BP on admission (adjusted HR 0.993, 95% 0.988–0.998, p = 0.01) and diuretic at discharge (adjusted HR 0.50, 95% CI 0.27–0.93, p = 0.03) were associated with reduced early repeat hospitalization for fluid overload.
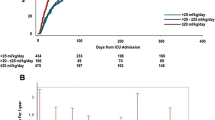
The multivariable logistic regression model with cardiovascular disease, prior hospitalization for fluid overload within 3 months preceding index hospitalization, prior hospitalization for any cause in the 6 months preceding index hospitalization, systolic BP on admission, intravenous furosemide during the index hospitalization and diuretic at discharge had an accuracy of 91.5% (using a cutoff value of 0.5) for the outcome of early repeat hospitalization for fluid overload. The Hosmer and Lemeshow Test Chi-square p was 0.25, suggesting model goodness-of-fit. The C-statistic, also the AUC of the ROC curve in Fig. 2, was 0.639 (95% CI 0.606–0.671), indicating that the model had fair discriminatory ability. After adjusting for age, gender and race, a “high-risk” LACE score was not associated with early repeat hospitalization for fluid overload (adjusted OR 1.26, 95% CI 0.91–1.73, p = 0.16). The model with age, gender, race, and “high-risk” LACE score had a lower C-statistic of 0.537 (95% CI 0.504–0.571), indicating poor discrimination for the outcome.
Discussion
Among 3423 patients with cardiovascular disease and/or risks who were hospitalized for fluid overload, 8.5% had early repeat hospitalizations for fluid overload within 30 days. Cardiovascular disease, prior hospitalization for fluid overload within 3 months, prior hospitalization for any cause within 6 months and intravenous furosemide use were associated with early repeat hospitalization for fluid overload, while higher systolic BP on admission and diuretic at discharge reduced early hospitalization for fluid overload. The presence of cardiovascular disease and prior hospitalizations for any cause and fluid overload likely indicated the complexity of the patient’s health condition, while the need for intravenous furosemide during the hospitalization and diuretic at discharge may be proxies for the severity of the patient’s fluid overload condition. Although few studies have evaluated repeat hospitalizations for fluid overload among patients with cardiovascular risks, prior studies on all-cause hospital readmissions similarly found that the strongest predictors for readmissions were related to healthcare encounter history and clinical data related to the severity of the health condition [16]. In meta-analyses of heart failure studies [11], current heart failure and previous heart failure were the strongest predictors for 30-day readmissions, while age, gender, arrhythmia, atherosclerosis, cerebrovascular disease and anemia were not significantly associated with readmissions. Although diabetes mellitus and kidney disease were associated with readmissions in some studies [11, 21], diabetes and lower eGFR did not significantly increase early readmissions for fluid overload in this study. However, patients with these conditions appeared to have more severe fluid overload that needed high-dose intravenous or continuous infusion furosemide (Supplementary Table S4). This finding may reflect the diuretic resistance that can develop in patients with cardiorenal disease [22]. Intriguingly, a higher systolic BP was associated with reduced readmissions for fluid overload in this study. By calculating Youden’s index, we determined the optimal systolic BP cutoff was 131 mmHg. The unadjusted and adjusted OR for SBP ≤ 130 mmHg for the primary outcome were 1.50 (95% CI 1.18–1.91) and 1.41 (95% CI 1.10–1.81), respectively. The effect of lower blood pressure on the risk of early repeat readmission for fluid overload was independent of known cardiovascular disease and the use of blood pressure-lowering medications since these were accounted for in the multivariate analysis. There is conflicting evidence for a J-curve relationship between systolic BP and cardiovascular events in observational studies [23], with the threshold approximated at 120 mmHg depending on age and comorbid conditions [23, 24]. However, there may also be confounders such as severe infections or autonomic dysfunction that influenced the outcome. Kidney failure requiring dialysis was a predictor for hospital readmissions in many studies [17], but patients with kidney failure were excluded in our study. These patients with recurrent fluid overload due to kidney failure likely need dialysis for adequate volume control, while we aimed to identify patients amenable to strategies such as self-management or intensified surveillance and diuretic titration to reduce recurrent fluid overload.
The lack of diuretic at discharge was associated with increased early readmission for fluid overload. This observation suggests a quality improvement opportunity to ensure diuretics are prescribed upon discharge. Further, a robust transition care program can empower patients to self-manage their fluid volume and diuretic dose, provide close supervision with home visits or telemonitoring on their medical and fluid volume status, adjust diuretics when diuretic resistance develops [22], and identify and address psychosocial factors that can lead to hospitalizations and, therefore, reduce readmissions [25]. Community-based intensified surveillance and early “rescue” treatment with home- or clinic-administered intravenous diuretics reduced the need for hospitalizations and improved cardiorenal outcomes and health-related quality of life in heart failure and kidney disease [12, 13, 26]. However, these strategies are manpower- and resource-intensive. In the interests of cost-effective health service delivery, a risk prediction tool will be useful to identify patients with considerable risk of early recurrent fluid overload. Our risk prediction model that incorporated the independent risk factors for early repeat hospitalization had fair discrimination for 30-day readmission for fluid overload. In contrast, a recent comprehensive systematic review of 41 validated predictive models for hospital readmissions published between 2105 and 2019 found 17 studies of unselected patients, while 13 models were specific to patients with heart failure or myocardial infarct, and 4 included patients with diabetes [16]. The LACE index had modest discrimination for predicting 30-day readmission in patients with heart failure with C-statistics that ranged from 0.55 to 0.59 [27, 28], while the pooled C-statistic was 0.64 among 45 studies of readmission risk in heart failure [11]. Although the discrimination in the latter studies was comparable with our derived model, many of the studies evaluated all-cause readmissions instead of focusing on preventable readmissions [11]. This distinction is important since not all readmissions were potentially preventable [18, 29, 30], yet reducing these preventable readmissions should be prioritized where healthcare resources are limited. In the absence of validated risk prediction tools for hospital readmissions for fluid overload, further refinement of our prediction model, possibly with the addition of socioeconomic determinants of health or functional status [16], may improve its discriminatory ability and enable its clinical utility to identify high-risk patients.
This study had some limitations. There may be a misclassification bias for the outcome since we did not include readmissions to other hospitals. However, by including only those who were on active follow-up (for laboratory investigations, medication prescription and subsequent hospitalizations), the patients were likely to have been re-admitted to our institution if there had been a need for repeat hospitalization for fluid overload. While the use of discharge codes may under-estimate the true prevalence of the condition of interest [31], we have attempted to ensure a comprehensive capture of fluid overload cases by including codes for conditions such as congestive heart failure, pulmonary edema and generalized edema that suggest a fluid overload state. ICD codes related to other conditions that may contribute to fluid overload, such as nephrotic syndrome and liver cirrhosis, were not excluded, but no cases were not identified. We did not differentiate the underlying causes for fluid overload since heart failure with preserved ejection fraction is an under-recognized condition; and fluid overload, regardless of cause, indicates overall sodium and fluid retention that will benefit from similar strategies (early recognition of symptoms, dietary and fluid intake modification, and judicious diuretic use) to prevent progression to severe symptomatic fluid overload that will necessitate hospitalization. Despite including a wide range of potential confounders in the logistic regression, there is a possibility of residual uncontrolled confounders including psychosocial determinants that may contribute to early repeat hospitalization [17]. These data were not readily available in our medical records for the study period. Other studies that used electronic medical records faced similar issues [16, 30]. Hence, among 41 studies included in a systematic review, only 7 studies (17.0%) included functional status and 16 studies (39.0%) used various proxies such as education level or residential address in a low-income neighborhood for socioeconomic status. In addition, health ecosystem-level factors such as quality of medication reconciliation, care coordination and medical social welfare services may also impact readmission [15, 29], but were not captured in this study. Factors such as left ventricular ejection fraction and N-terminal (NT) pro b-type natriuretic (BNP) were not included since the former does not take into account heart failure with preserved ejection fraction, and not all patients with left ventricular dysfunction have symptomatic heart failure [32], while BNP or NT-proBNP can be elevated in comorbidities such as older age, diabetes and kidney disease that formed our study population [32]. We did not include sodium/glucose cotransporter-2 inhibitors (SGLT2i) as a variable since few patients used SGLT2i during the study period, possibly because there were no government subsidies for these medications at our public healthcare institution then. However, there is growing evidence of their benefits in heart failure and chronic kidney disease [33]. Increased SGLT2i initiation among patients with these conditions can potentially impact the risk of repeat hospitalizations for fluid overload and thus the study findings may not be generalizable to populations where the use of SGLT2i is prevalent. The generalizability of our results may also be limited where the patient characteristics or healthcare systems, policies and organization differ significantly [34].
In conclusion, this study identified independent predictors for early repeat hospitalizations for fluid overload that can be used to develop a pragmatic tool for quality improvement to reduce readmissions.
Data availability
Data available upon reasonable request and subject to institutional approval.
References
Mayne KJ, Shemilt R, Keane DF, Lees JS, Mark PB, Herrington WG (2022) Bioimpedance indices of fluid overload and cardiorenal outcomes in heart failure and chronic kidney disease: a systematic review. J Card Fail 28(11):1628–1641
Zoccali C, Mallamaci F (2018) Mapping progress in reducing cardiovascular risk with kidney disease: managing volume overload. Clin J Am Soc Nephrol 13(9):1432
Koratala A, Ronco C, Kazory A (2022) Diagnosis of fluid overload: from conventional to contemporary concepts. Cardiorenal Med 12(4):141–154
Blecker S, Ladapo JA, Doran KM, Goldfeld KS, Katz S (2014) Emergency department visits for heart failure and subsequent hospitalization or observation unit admission. Am Heart J 168(6):901-908.e901
Ronksley PE, Tonelli M, Manns BJ et al (2017) Emergency department use among patients with CKD: a population-based analysis. Clin J Am Soc Nephrol 12(2):304
Ambrosy AP, Fonarow GC, Butler J et al (2014) The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 63(12):1123–1133
Costanzo MR, Fonarow GC, Rizzo JA (2019) Ultrafiltration versus diuretics for the treatment of fluid overload in patients with heart failure: a hospital cost analysis. J Med Econ 22(6):577–583
Mark PB, Mangion K, Rankin AJ et al (2022) Left ventricular dysfunction with preserved ejection fraction: the most common left ventricular disorder in chronic kidney disease patients. Clin Kidney J 15(12):2186–2199
McHugh K, DeVore AD, Wu J et al (2019) Heart failure with preserved ejection fraction and diabetes: JACC state-of-the-art review. J Am Coll Cardiol 73(5):602–611
Kasiakogias A, Rosei EA, Camafort M et al (2021) Hypertension and heart failure with preserved ejection fraction: position paper by the European society of hypertension. J Hypertens 39(8):1522–1545
Van Grootven B, Jepma P, Rijpkema C et al (2021) Prediction models for hospital readmissions in patients with heart disease: a systematic review and meta-analysis. BMJ Open 11(8):e047576
Inglis SC, Clark RA, Dierckx R, Prieto–Merino D, Cleland JGF (2015) Structured telephone support or non‐invasive telemonitoring for patients with heart failure. Cochrane Database Syst Rev 2015(10):CD007228. https://doi.org/10.1002/14651858.CD007228.pub3
Bahar J, Rahman A, Wong G et al (2022) 126 Safety and effectiveness of acute heart failure care as outpatient (safe): a meta-analysis of studies comparing outpatient based management with standard inpatient care. BMJ Publishing Group Ltd and British Cardiovascular Society
Lim DYZ, Chia SY, Abdul Kadir H, Mohamed Salim NN, Bee YM (2021) Establishment of the SingHealth diabetes registry. Clin Epidemiol 13:215–223
Kansagara D, Englander H, Salanitro A et al (2011) Risk prediction models for hospital readmission: a systematic review. JAMA 306(15):1688–1698
Mahmoudi E, Kamdar N, Kim N, Gonzales G, Singh K, Waljee AK (2020) Use of electronic medical records in development and validation of risk prediction models of hospital readmission: systematic review. BMJ 2020(369):m958
Low LL, Liu N, Wang S, Thumboo J, Ong MEH, Lee KH (2016) Predicting 30-day readmissions in an Asian population: building a predictive model by incorporating markers of hospitalization severity. PLoS One 11(12):e0167413
van Walraven C, Dhalla IA, Bell C et al (2010) Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. Can Med Assoc J 182(6):551
Zhou H, Della PR, Roberts P, Goh L, Dhaliwal SS (2016) Utility of models to predict 28-day or 30-day unplanned hospital readmissions: an updated systematic review. BMJ Open 6(6):e011060
Department of Statistics MoTaI, Republic of Singapore. Singapore Census of Population 2020, Statistical Release 1: Demographic Characteristics, Education, Language and Religion. https://www.singstat.gov.sg/publications/reference/cop2020/cop2020-sr1/census20_stat_release1. Updated 16th June 2021. Accessed 15 July 2021
Arora S, Patel P, Lahewala S et al (2017) Etiologies, trends, and predictors of 30-day readmission in patients with heart failure. Am J Cardiol 119(5):760–769
Masella C, Viggiano D, Molfino I et al (2019) Diuretic resistance in cardio-nephrology: role of pharmacokinetics, hypochloremia, and kidney remodeling. Kidney Blood Press Res 44(5):915–927
Rahman F, McEvoy JW (2017) The J-shaped curve for blood pressure and cardiovascular disease risk: historical context and recent updates. Curr Atheroscler Rep 19(8):34
Jiang C, Wu S, Wang M, Zhao X, Li H (2021) J-curve relationship between admission SBP and 2-year cardiovascular mortality in older patients admitted for acute coronary syndrome. J Hypertens 39(5):926
Low LL, Vasanwala FF, Ng LB, Chen C, Lee KH, Tan SY (2015) Effectiveness of a transitional home care program in reducing acute hospital utilization: a quasi-experimental study. BMC Health Serv Res 15(1):1–8
Bamforth RJ, Chhibba R, Ferguson TW et al (2021) Strategies to prevent hospital readmission and death in patients with chronic heart failure, chronic obstructive pulmonary disease, and chronic kidney disease: a systematic review and meta-analysis. PLoS One 16(4):e0249542
Ibrahim AM, Koester C, Al-Akchar M et al (2020) HOSPITAL Score, LACE Index and LACE+ Index as predictors of 30-day readmission in patients with heart failure. BMJ Evid Based Med 25(5):166–167
Yazdan-Ashoori P, Lee SF, Ibrahim Q, Van Spall HGC (2016) Utility of the LACE index at the bedside in predicting 30-day readmission or death in patients hospitalized with heart failure. Am Heart J 179:51–58
Auerbach AD, Kripalani S, Vasilevskis EE et al (2016) Preventability and causes of readmissions in a national cohort of general medicine patients. JAMA Intern Med 176(4):484–493
Meurs EA, Siegert CE, Uitvlugt E et al (2021) Clinical characteristics and risk factors of preventable hospital readmissions within 30 days. Sci Rep 11(1):1–8
McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA (2014) Validity of heart failure diagnoses in administrative databases: a systematic review and meta-analysis. PLoS One 9(8):e104519
Worster A, Balion CM, Hill SA et al (2008) Diagnostic accuracy of BNP and NT-proBNP in patients presenting to acute care settings with dyspnea: a systematic review. Clin Biochem 41(4):250–259
van der Aart-van der Beek AB, de Boer RA, Heerspink HJ (2022) Kidney and heart failure outcomes associated with SGLT2 inhibitor use. Nat Rev Nephrol 18(5):294–306
Carlson MD, Roy B, Groenewoud AS (2020) Assessing quantitative comparisons of health and social care between countries. JAMA 324(5):449–450
Acknowledgements
The authors thank Ms Hanis Bte Abdul Kadir from the Health Services Research Unit, Singapore General Hospital, for her help in data management and processing.
Funding
This study was supported by the SHF-Foundation Research Grant (SHF/HSRHO014/2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare no relevant conflict of interest.
Ethical approval
This study abided by the Declaration of Helsinki and the Centralized Institutional Review Board (2020/3061) determined that the study did not require further ethical deliberation for the use of de-identified data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lim, C.C., Huang, D., Huang, Z. et al. Early repeat hospitalization for fluid overload in individuals with cardiovascular disease and risks: a retrospective cohort study. Int Urol Nephrol 56, 1083–1091 (2024). https://doi.org/10.1007/s11255-023-03747-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03747-2