Abstract
Lithium intoxication is still an undefined and underestimated disease, especially those cases requiring extracorporeal treatment. Lithium is a monovalent cation with small molecular mass of 7 Da that has been regularly and successfully used since 1950 in the treatment of mania and bipolar disorders. However, its careless assumption can lead to a wide spectrum of cardiovascular, central nervous system and kidney diseases in case of acute, acute on chronic and chronic intoxications. In fact, lithium serum range is strict between 0.6 and 1.3 mmol/L, with a mild lithium toxicity observed at the steady-state of 1.5–2.5 mEq/L, moderate toxicity when lithium reaches 2.5–3.5 mEq/L, and severe intoxication with observed serum levels > 3.5 mEq/L. Its favorable biochemical profile allows the complete filtration and partial reabsorption in the kidney due to the similarity to sodium and also the complete removal by renal replacement therapy, that should be considered in specific poisoning conditions. In this narrative and updated review we discussed a clinical case of lithium intoxication, the different pattern of diseases attributable to excessive lithium load and the current indications for extracorporeal treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lithium therapy still stands as one of the most effective therapy for the acute and long-term treatment of bipolar mood diseases. However, due to the narrow and safe therapeutical range, its careless administration may cause lithium intoxication with a wide spectrum of clinical presentations due to the different target organs.
In this context, kidney involvement is frequent and should be always ruled out through a recurring monitoring of renal function on stable maintenance therapy and especially when raised pharmacological doses are required. Further, acute concomitant conditions that may compromise water and sodium balance as hypohydration, acute inflammation statuses, sepsis could promote lithium intoxication and should be promptly assessed.
Herein we reported the clinical case of a patient and the current treatment recommendations.
Case presentation
A 53-year-old Caucasian female patient was admitted to our First-Aid Station due to a clinical condition defined by the presence of confusion, lethargy, nausea, diarrhea and oliguria. Her blood pressure was 100/50 mmHg, heart rate 100 pulse/min, oxygen saturation 98% on air and blood examinations are reported in Table 1. She was affected by bipolar disorder on chronic lithium therapy since several years and she referred the voluntary ingestion of more than 30 tablets of 300 mg lithium carbonate. The admission blood examination evidenced an acute lithium intoxication with [Li+] 2.8 mEq/L and the immediate therapeutic procedures were performed.
After hydration therapy with 2 L saline solution 0.9% and gastric lavage without a clinical improvement, a central venous catheter was placed in the right femoral vein to perform hemodialysis treatment (HD) due to the lack of benefits of the previous therapies.
A 3-h HD session with high flux polysulfone filter was started with low blood flow (QB 150 mL/min) and without weight loss. The HD treatment was well tolerated without complications and the patient was then admitted to Psychiatric Unit for the clinical management. After a single HD session, the patient recovered from the intoxication, the diuresis improved and no following HD therapy was required. Her 12-h following blood examinations revealed [Li+] 1.2 mEq/L and no other significative biochemical alteration were highlighted.
Intermittent HD treatment proved its clinical efficacy on lithium poisoning and herein we discussed the pathophysiology of lithium intoxication and the current standard of care therapy.
Discussion
Lithium salts have been successfully used for the treatment of mania and bipolar disorders since 1949 in Australia with a following growing evidence between 1950s and 1970s [1].
According to the National Ambulatory Medical Care Survey, 17.6% of patients with bipolar disorder in the 2013–2016 period were treated with lithium [2].
Its administration can be either liquid as lithium citrate and more frequently solid as lithium carbonate [3, 4].
Lithium (Li+) is a monovalent cation with a small molecular mass of 7 Da that can lead to acute kidney injury (AKI) through different patterns of poisoning: acute, acute on chronic and chronic intoxication [3].
Acute intoxication occurs in lithium naive patients that exhibit lithium overdose. Acute on chronic lithium intoxication is frequent in those patients who have already an existing burden of lithium maintenance therapy and are exposed to an acute large lithium load, usually for suicidal tendencies, or they are exposed to AKI from other causes such as dehydration that impact with its renal clearance. Instead, chronic poisoning is defined when maintenance lithium therapy patients show a recent increased lithium dose and/or a decline in renal function or a drug-drug interaction that may interfere with lithium withdrawal [4, 5].
[Li+] therapeutic serum range is strict between 0.6 and 1.3 mmol/L of [Li+], with a mild lithium toxicity observed at the steady-state [Li+] of 1.5–2.5 mEq/L, moderate toxicity when [Li+] reach 2.5–3.5 mEq/L, and severe intoxication with observed serum levels > 3.5 mEq/L [3, 5].
After therapeutical assumption, Li+ distributes widely and without protein binding in total body water, with initial volume distribution of 0.5 L/kg that can increase to 0.9 L/kg [5, 6].
Serum [Li+] accumulates rapidly in the kidney, bone and thyroid, while the diffusion into cerebral fluid and the brain is delayed by approximately 24 h compared with its appearance with plasma.
In the kidney, Li+ is totally filtered in the glomerulus, with 80% reabsorbed (60% by the proximal tubule and 20% by the tick ascending limb of the Henle Loop and collecting duct) and then excreted through urine output [4, 6].
Clinical conditions that impair glomerular filtration rate such as volume depletion may promote an increase in Li+ proximal tubule reabsorption due to its biochemical similarity to sodium leading to raised serum [Li+] levels [7].
Usually the half-life of [Li+] is 12–27 h but it is affected by the age, kidney function and duration of Li therapy and it can be longer up to 58 h in patient on chronic lithium therapy [8].
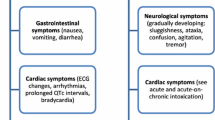
Clinical manifestations of lithium poisoning have a wide spectrum, involving the gastrointestinal tract, the central nervous system, the heart and the kidney.
Nausea, vomit and diarrhea are mostly prevalent in acute and acute on chronic clinical patterns [5]. Electrocardiographic alterations may involve QT, ST and T waves modifications and also bradycardic syndrome, sinus node dysfunction, atrioventricular blockade, ventricular fibrillation and sudden cardiac death [4, 8]. Indeed, these pathological findings are not always related to serum [Li+] levels and can be attributable to individual susceptibility [5]. Lithium neurotoxicity can range from confusion, ataxia, convulsions, lithium encephalopathy to the most problematic condition also known as syndrome of irreversible lithium-effectuated neurotoxicity (SILENT) [9,10,11].
SILENT causes chronic, largely cerebellar sequelae, tremor, extrapyramidal symptoms, gait alterations, nystagmus, dysarthria and cognitive impairment. Actually, the prevalence of SILENT is not known as well as the definitive management [9,10,11].
Also the kidney is frequently involved and may be injured by high [Li+] serum levels with different histological and clinical presentations, see Table 2.
The most common kidney involvement in lithium intoxication is the nephrogenic diabetes insipidus (NDI) [12]. NDI is due to the lithium induced resistance to antidiuretic hormone in the collecting duct with polyuria and polydipsia even after the first weeks or months of lithium treatment. Usually, it can develop in almost 40% of the patients under lithium therapy and it is reversible after lithium discontinuation.
Li+ inhibits the antidiuretic hormone action in the renal collection duct by entering in the principle cells through the epithelial sodium channel (ENaC) [12]. [Li+] inhibits glycogen synthase kinase-3 (GSK3) activity and the generation of cyclic adenosine monophosphatase (cAMP) [13]. As a consequence, the Aquaporin-2 (AQP2) water channel cannot be phosphorylated and cannot be redistributed from storage vesicles to the apical membranes leading to a reduced water reabsorption. Further, Li+ might also reduce AQP2 gene transcription and/or mRNA stability [12].
A less frequent lithium induced renal disease is the chronic tubule-interstitial nephritis with classical tubular atrophy, interstitial fibrosis and cystic dilation of distal tubules/collecting in patients on chronic lithium treatment. It can lead to end stage renal disease after almost 20 years of lithium exposition and the subnephrotic range proteinuria (< 3 g/day) occasionally described in this condition could be expression of secondary glomerulosclerosis due to the chronic tubule-interstitial disease [14].
A rarer complication is the lithium related nephrotic syndrome defined as proteinuria > 3 g/day, edema, hypoalbuminemia, and hyperlipidemia and currently it has been described in less of 30 patients on lithium therapy on the available literature [15, 16]. Most of them were related to Li+ levels and a prompt cessation of renal disease was noticed after lithium discontinuation. The most common lesions on renal biopsies performed during the first year of lithium therapy were minimal change disease and focal segmental glomerulosclerosis [15, 16].
Therapeutical indications
The current recommendations in the management of lithium intoxication include: lithium discontinuation volume replacement with intravenous saline isotonic solution and also considering the extracorporeal therapy according to the Extracorporeal Treatments in Poisoning (EXTRIP) Workgroup indications [17].
Both gastric lavage and/or whole bowel irrigation with polyethylene glycol electrolyte lavage solution have not produced a better result according to the recent literature [18, 19].
Forced diuresis with furosemide increases the Li+ clearance by reducing the reabsorption in the thick ascending limb of the Henle loop; however, it is no longer recommended because of the risk of dehydration in lithium exposed patients and the consequent increased reabsorption of sodium and Li+ in the proximal tubule due to the similar chemical profile of the two cations [20, 21].
The favorable pharmacokinetic pattern of Li+ (low molecular weight, low protein binding, relatively low volume distribution and low endogenous clearance) allows an efficient clearance on intermittent HD [5, 22].
It is still underestimated the application of renal replacement therapy (RRT) in patients affected by lithium poisoning, as it is reported in small case series or case reports. In a Sweden population-based retrospective cohort study the 13.4% of 77 patients with lithium intoxication requires HD treatment with benefit and without adverse effects over a 17-year period of observation [23].
High efficiency intermittent HD can achieve a Li+ clearance of almost 180 mL/min while physiological kidney clearance of Li+ can reach in 30–40 mL/min which may decrease to 10.6 mL/min in patient with chronic kidney disease [24].
Both intermittent HD and sustained low-efficiency HD (SLED) provide excellent Li+ clearance [25], while continuous renal replacement therapy (CRRT) offers a lower efficacy profile with Li+ clearance of 28–62 mL/min, due to the lower effluent and blood flow rates [26]. CRRT is not recommended as first substitutive treatment, while it is indicated if intermittent HD is not available or because of the high risk of patient’s clinical conditions.
If CRRT is selected as extracorporeal treatment, the delivered dialytic dose should be above 25 mL/kg/h which is usually prescribed for patient with Acute Kidney Injury (AKI) [27].
Peritoneal dialysis provides a limited Li+ clearance of 9–4 mL/min and it is not indicated as alternative therapy as extracorporeal treatment [28].
Li+ rebound commonly occurs after extracorporeal treatment cessation and it is due to a redistribution of Li+ from deeper compartments/red blood cells to the plasma or due to the ongoing absorption from the gastrointestinal tract. The increase of [Li+] is maximal after 6–12 h and it can be asymptomatic due to the Li+ shift form the toxic compartment to blood [29, 30]. However, when it is related to altered absorption of gastrointestinal tract, it can progressively accumulate in the central nervous system leading to the onset of neurological symptoms [31].
Li+ rebound is more frequent after intermittent HD, while it is limited during CRRT or SLED due to the longer duration of extracorporeal treatment [32].
A correct [Li+] measurement is necessary to assess the real state of lithium poisoning, the rebound and the need for RRT. Green-top tubes should be avoided since they contain lithium heparin that can raise the [Li+] levels factitiously, so blood samples for lithium measurements should be performed in blood collected in red-top tubes [4].
According to the EXTRIP group, the indications for extracorporeal treatment in lithium poisoning include recommendations and suggestions according to the [Li+], the clinical symptoms and the kidney function [17]. Recommendations are [Li] > 4 mEq/L and impaired kidney function; the presence of decreased level of consciousness, seizures or life-threatening dysrhythmias independently of [Li+] levels. Instead, suggestions are [Li+] > 5 mEq/L, the onset of significant confusion and if the expected time to reduce [Li+] to < 1 mEq/L with optimal management is > 36 h. The indications are summarized in Table 3.
The EXTRIP workgroup defines impaired kidney function according to KDIGO guidelines the stages 3B, 4 or 5 CKD, stage 2 or 3 AKI, the presence of oligo-anuria, and in the absence of baseline creatinine value, a serum creatinine ≥ 1.5 mg/dL [17].
Intermittent HD is currently the best standard care as initial RRT in lithium poisoning, CRRT should be considered according to the clinical setting and/or if intermittent HD is not practicable. After initial intermittent HD treatment, both CRRT and intermittent HD are equally acceptable for additional lithium removal.
Cessation of RRT should be considered if [Li+] is < 1 mEq/L, or clinical improvement is apparent, and/or after a minimum of 6 h of extracorporeal treatment if [Li+] is not readily measurable. After RRT cessation, [Li+] values should be analyzed in the next 12 h to assess the rebound and the need for following RRT sessions [17].
In conclusion, a single HD treatment proved to be efficient in our case of lithium intoxication. However, more cohort studies with a larger number of patients are needed to define the best dialyzer membrane as well as the best RRT modality: intermittent HD or SLED.
Data availabiltiy
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Baird-Gunning J, Lea-Henry T, Hoegberg LCG et al (2017) Lithium poisoning. J Intensive Care Med 32(4):249263
Rhee T, Olfson M, Nierenberg A, Wilkinson S (2020) 20-Year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry 177(8):706–715. https://doi.org/10.1176/appi.ajp.2020.19091000
Meltzer H (2012) Antipsychotic agents and lithium. In: Katsung BG, Masters SB, Trevor AJ (eds) Basic and clinical pharmacology, 12th edn. McGraw-Hill Medical, New York, pp 501–520
Timmer RT, Sands JM (1999) Lithium intoxication. J Am Soc Nephrol 10:666–674
Khasraw M, Ashley D, Wheeler G, Berk M (2012) Using lithium as a neuroprotective agent in patients with cancer. BMC Med 10:131–132
Gosselin S, Juurlink DN, Kielstein JT, Ghannoum M, Lavergne V, Nolin TD, Hoffman RS (2014) Extracorporeal treatment for acetaminophen poisoning: recommendations from the EXTRIP workgroup. Clin Toxicol (Phila) 52:856–867
Finley PR, Warner MD, Peabody CA (1995) Clinical relevance of drug interactions with lithium. Clin Pharmacokinet 29:172–191
Okusa MD, Crystal LJ (1994) Clinical manifestations and management of acute lithium intoxication. Am J Med 97:383–389
Porto FH, Leite MA, Fontenelle LF, Marrocos RP, Szczerback NF, de Freitas MR (2009) The syndrome of irreversible lithium-effectuated neurotoxicity (SILENT): one-year follow-up of a single case. J Neurol Sci 277:172–173
Adityanjee, Munshi KR, Thampy A (2005) The syndrome of irreversible lithium-effectuated neurotoxicity. Clin Neuropharmacol 28:38–49
ZalloAtxutegi E, Pacheco MT, Izaguirre NB, Ansorena MAP (2008) Syndrome of irreversible lithium-effectuated neurotoxicity. A propos of a case. Psiquiatr Biol 15:56–58
Robben JH, Knoers NV, Deen PM (2006) Cell biological aspects of the vasopressin type-2 receptor and aquaporin 2 water channel in nephrogenic diabetes insipidus. Am J Physiol Ren Physiol 291(2):F257–F270
Christensen BM, Zuber AM, Loffing J, Stehle JC, Deen PM, Rossier BC, Hummler E (2011) alphaENaC-mediated lithium absorption promotes nephrogenic diabetes insipidus. J Am Soc Nephrol 22(2):253–261
Grunfeld J, Rossier B (2009) Lithium nephrotoxicity revisited. Nat Rev Nephrol 5(5):270–276. https://doi.org/10.1038/nrneph.2009.43
Tandon P, Wong N, Zaltzman J (2015) Lithium-induced minimal change disease and acute kidney injury. N Am J Med Sci 7(7):328. https://doi.org/10.4103/1947-2714.161252
Bosquet S, Descombes E, Gauthier T, Fellay G, Regamey C (1997) Nephrotic syndrome during lithium therapy. Nephrol Dial Transplant 12(12):2728–2731. https://doi.org/10.1093/ndt/12.12.2728
Lavergne V, Nolin TD, Hoffman RS, Roberts D, Gosselin S, Goldfarb DS, Kielstein JT, Mactier R, Maclaren R, Mowry JB, Bunchman TE, Juurlink D, Megarbane B, Anseeuw K, Winchester JF, Dargan PI, Liu KD, Hoegberg LC, Li Y, Calello DP, Burdmann EA, Yates C, Laliberté M, Decker BS, Mello-DaSilva CA, Lavonas E, Ghannoum M (2012) The EXTRIP (EXtracorporeal TReatments In Poisoning) workgroup: guideline methodology. Clin Toxicol (Phila) 50:403–413
Anonymous (2004) Position paper: whole bowel irrigation. J Toxicol Clin Toxicol 42(6):843–854
BretaudeauDeguigne M, Hamel JF, Boels D, Harry P (2013) Lithium poisoning: the value of early digestive tract decontamination. Clin Toxicol (Phila) 51:243–248
Scharman EJ (1997) Methods used to decrease lithium absorption or enhance elimination. J Toxicol Clin Toxicol 35:601–608
Zimmerman JL (2003) Poisonings and overdoses in the intensive care unit: general and specific management issues. Crit Care Med 31:2794–2801
Waring WS (2006) Management of lithium toxicity. Toxicol Rev 25:221–230
Ott M, Stegmayr B, Renberg ES, Werneke U (2016) Lithium intoxication: incidence, clinical course and renal function- a population based retrospective cohort study. J Psychopharmacol 30(10):1008–1019. https://doi.org/10.1177/0269881116652577
Von Hartitzsch B, Hoenich NA, Leigh RJ, Wilkinson R, Frost TH, Weddel A, Posen GA (1972) Permanent neurological sequelae despite haemodialysis for lithium intoxication. BMJ 4:757–759
Bailey AR, Sathianathan VJ, Chiew AL, Paterson AD, Chan BS, Arora S (2011) Comparison of intermittent haemodialysis, prolonged intermittent renal replacement therapy and continuous renal replacement haemofiltration for lithium toxicity: a case report. Crit Care Resusc 13:120–122
Goodman JW, Goldfarb DS (2006) The role of continuous renal replacement therapy in the treatment of poisoning. Semin Dial 19:402–407
Hazouard E, Ferrandière M, Rateau H, Doucet O, Perrotin D, Legras A (1999) Continuous veno-venous haemofiltration versus continuous veno-venous haemodialysis in severe lithium self-poisoning: a toxicokinetics study in an intensive care unit. Nephrol Dial Transplant 14:1605–1606
Wilson JH, Donker AJ, van der Hem GK, Wientjes J (1971) Peritoneal dialysis for lithium poisoning. BMJ 2:749–750
Jaeger A, Sauder P, Kopferschmitt J, Tritsch L, Flesch F (1993) When should dialysis be performed in lithium poisoning? A kinetic study in 14 cases of lithium poisoning. J Toxicol Clin Toxicol 31:429–447
Bellomo R, Boyce N (1992) Current approaches to the treatment of severe lithium intoxication. Lithium 3:245–248
Borrás-Blasco J, Sirvent AE, Navarro-Ruiz A, Murcia-López A, Romero-Crespo I, Enriquez R (2007) Unrecognized delayed toxic lithium peak concentration in an acute poisoning with sustained release lithium product. South Med J 100:321–323
Eyer F, Pfab R, Felgenhauer N, Lutz J, Heemann U, Steimer W, Zondler S, Fichtl B, Zilker T (2006) Lithium poisoning: pharmacokinetics and clearance during different therapeutic measures. J Clin Psychopharmacol 26:325–330
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Spatola, L., Maringhini, S., Canale, C. et al. Lithium poisoning and renal replacement therapy: pathophysiology and current clinical recommendations. Int Urol Nephrol 55, 2501–2505 (2023). https://doi.org/10.1007/s11255-023-03558-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03558-5