Abstract
Purpose
Catheter-associated urinary tract infections are of significant medical burden in cost, morbidity, and mortality. Experimental selenium-coated medical devices have demonstrated non-toxic in vitro and in vivo antimicrobial activity. While antimicrobial-coated catheters have shown efficacy in preventing CAUTIs, selenium has not been tested in this context. The purpose of this in vitro study is to evaluate selenium-incorporated urinary catheters for inhibition of uropathogenic bacterial growth and biofilm formation.
Methods
Urinary catheters incorporated with 1% organo-selenium and standard (uncoated) catheters were incubated in vitro with E. coli, K. pneumoniae, P. aeruginosa, H. influenzae, and combinations of these bacteria. Growth was evaluated by colony-forming unit count and visualized with confocal laser and scanning electron microscopy. Organo-selenium catheter material integrity was also tested by soaking the tubing in phosphate-buffered saline for 12 weeks at 37 °C.
Results
Organo-selenium-incorporated catheters demonstrated total reduction (100%) of in vitro bacterial growth and biofilm formation for E. coli, K. pneumoniae, H. influenzae, and a combination of these species when compared to control. P. aeruginosa growth was inhibited by approximately 4 logs (99.99%). Complete inhibition of E. coli growth was maintained after long-term phosphate-buffered saline soaking.
Conclusion
The results demonstrate that organo-selenium was stably incorporated into catheter tubing and inhibited bacterial attachment, growth, and biofilm formation for multiple uropathogenic organisms. Furthermore, long-term soaking of organo-selenium tubing in phosphate-buffered saline did not show any decline in bacterial growth inhibition or biofilm formation. These findings suggest that organo-selenium-incorporated catheters may be advantageous in preventing catheter-associated urinary tract infections and warrant further in vivo and clinical evaluation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary catheter use is common, with an estimated 15–25% of hospitalized patients in the United States undergoing urinary catheter placement during their stay [1]. Indications for urinary catheters include perioperative use, acute urinary retention, urinary obstruction, and treatment of immobilized or hospice patients [2]. Catheter-associated urinary tract infections (CAUTIs) are among the most common preventable and healthcare-acquired infections. Some 70–80% of nosocomial urinary tract infections (UTIs) are associated with urinary catheters, making catheter placement a significant risk factor for infection [3]. CAUTIs are associated with significantly increased length of hospitalization, patient morbidity, and healthcare costs [4]. Additionally, 2–4% of CAUTI cases progress to bacteremia and sepsis, causing a threefold increase in mortality compared to non-bacteremic patients [5]. Escherichia coli, Pseudomonas aeruginosa, and Klebsiella are the most common bacterial culprits for reported nosocomial CAUTIs [6]. Collectively, these organisms contribute to 86% of CAUTI-attributed Gram-negative bacteremia in American and European studies [3].
Different types of antimicrobial catheter coatings have been studied for their potential to prevent CAUTIs by inhibiting bacterial colonization and biofilm formation. Biofilms form when bacteria adhere to host cells or catheter surfaces and then produce an extracellular polymeric substance within which microorganisms can divide and grow, protected from antimicrobial agents [5]. Therefore, biofilm formation is one of the dominant factors in the development of antibiotic resistance and persistent bacteriuria for uropathogenic organisms. Antimicrobial-coated catheters reduce CAUTI risk through material-specific biocidal properties that interrupt colonization and biofilm formation [1]. Several antimicrobial catheter coatings have demonstrated statistically significant decreases in CAUTI incidence compared to uncoated catheters [7,8,9]. A recent meta-analysis of clinical trials on catheters coated with antimicrobial materials including silver alloy, noble metal alloy, and nitrofurazone found that coated catheters significantly reduce CAUTIs in patients when compared to uncoated catheters [10]. However, heterogeneity in methodology, coating materials, study power, and CAUTI criteria among studies has limited the adoption of antimicrobial catheters in current medical practice.
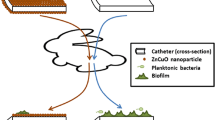
A promising element that has been under investigation for its antimicrobial properties is selenium, which has the unique ability to catalyze the formation of superoxide radicals. As outlined in Fig. 1, selenium takes an electron from sulfur-containing molecules such as glutathione, which is universally found in all biological tissues, and gives it to oxygen, resulting in the formation of superoxide. This radical causes oxidative stress within bacterial cells with secondary cell death [11]. These radicals have a short half-life due to dismutation, and thus have no adverse effect on the surrounding human tissue. Bacterial growth and biofilm inhibition as well as lack of in vivo toxicity are well documented in studies on organo-selenium-coated contact lenses and hemodialysis catheters [12, 13].
Given the significant burden of CAUTIs for patients in health care costs, morbidity, and mortality, there is a real need for new preventative measures. To our knowledge, organo-selenium-incorporated urinary catheters have not been experimentally studied or evaluated for this purpose. In this pilot study, we evaluate the effect of organo-selenium-incorporated catheters on the in vitro growth and biofilm formation of E. coli, H. influenzae, P. aeruginosa, K. pneumoniae, and combination incubation of these species.
Materials and methods
Selenium polyurethane tubing production
Diselenide-dimethacrylate was reactively grafted with thermoplastic polyurethane resin pellets at 235 °C. The seleno-methacrylate used was added to the pellet to produce a mixture that contained 1% weight by weight elemental selenium. The coated pellets were extruded with a bench-top injection mold. This process actually polymerizes the selenium methacrylate into the polyurethane of the tubing, forming a co-polymer. The tubing was then tested for chemical and microbiological properties. After this material was validated, the mixture was scaled-up by adding the selenium compound by auger or injection to the heating zones. This procedure was used to prepare master batches at a kilogram scale. The method can be transferred to a commercial injection molding facility to produce the material at scale and volume. The selenium tubing produced by this method was clear, and the physical properties were the same as standard non-selenium tubing. It was also found that both aged and non-aged tubing demonstrated the same physical properties.
Growth conditions, bacterial strains, and media
Escherichia coli GFP strain MM294, which constitutively expresses green fluorescent protein (GFP) from plasmids pCM11 and pMRP9-1, respectively [14], was used along with Pseudomonas aeruginosa PAO1 GFP. Haemophilus influenzae and Klebsiella pneumoniae clinical isolates at our institution were also used. The strains were routinely grown in Luria–Bertani broth (LB) at 37 °C with shaking (250 rpm). The pCM11 was maintained in AH133 using both LB and trypticase soy broth (TSB) supplemented with 1 μg/mL erythromycin. To maintain pMRP9-1 in MM294, both LB and TSB were supplemented with 300 µg/ml carbenicillin. Biofilm formation was examined using TSB (MP Biomedicals, Solon, OH) as the growth medium.
Scanning electron microscopy
After 48 h of incubation at 37 °C, the samples were freeze-dried overnight. Any attached media was gently wiped and shaken off from the samples. After mounting the samples, a thin layer of platinum was coated onto the samples to improve conductivity. The images were taken by Hitachi S/N-4300 under environmental mode with 20 kV accelerating voltage and 300 Pa air pressure.
In vitro biofilm testing
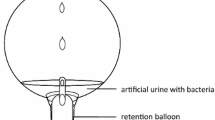
(I) Biofilm: The biofilm assays were performed as previously described [13]. Pieces (1 cm in size) of control and selenium catheter tubing were placed in 24-well plates and incubated in media with inoculums of either E. coli GFP MM294, H. influenzae, K. pneumoniae, or P. aeruginosa for 24 h. The biofilms formed on the tubing were quantified by determining the colony-forming units (CFU) per tubing segment and examined by confocal laser scanning microscopy (CLSM) using an Olympus Fluoview FV300 (Olympus America, Center Valley, PA). Each segment was removed carefully from the well, rinsed gently with sterile distilled water to remove loosely attached bacteria, and placed into a microcentrifuge tube containing 1 ml of phosphate-buffered saline (PBS). The tubes were sonicated for 10 min to loosen the cells within the biofilm and then vigorously vortexed three times for 1 min to detach the cells. The cells were then serially diluted in PBS, and 10 μl aliquots of each dilution were spotted onto LB agar plates. The plates were incubated at 37 °C for 24 h, and the CFU were determined. Using the following formula, the CFU per segment was determined: CFU x dilution factor × 100. Each sample was done in triplicate.
(ii) Biofilm image quantification: The biofilms were visualized using CLSM [14]. The biofilms were developed as described above. After 24 h of incubation at 37 °C, the pieces were gently rinsed to remove loosely attached bacteria. Visualization of the E coli MM294 GFP biofilms was accomplished using a Nikon A1 + /AIR + Confocal Microscope (Nikon Inc., Melville, NY, USA) with images acquired at 2 µm intervals through the biofilms. Two dimensional images were acquired using the Nis Elements Imaging software v. 4.20 (Nikon Inc., Melville, NY, USA). Experiments were done in triplicate.
The 24-h biofilms were also studied by scanning electron microscopy (SEM). The tubing was fixed in 2% glutaraldehyde in 0.1 mol/L phosphate buffer pH 7.2. This was followed by a 1% OsO4 postfix. The SEM studies were then carried out according to the method of Hazlett [15].
(iii) Stability testing of the selenium in the tubing: This was carried out by soaking the tubing in PBS for 12 weeks at 37 °C. The tubing was then tested for its ability to inhibit biofilm formation as described above.
Statistical analyses
Results of the CFU assays were analyzed and drawn with GraphPad Prism® version 7 (GraphPad Software, San Diego, CA, USA) with 95% confidence intervals (CIs) of the difference. Comparisons of the in vitro biofilms formed on Se-free and Se-polyurethane tubings were analyzed, using GraphPad InStat 3 (GraphPad Software, San Diego, CA) with 95% confidence intervals (CIs) of the difference, by a two-tailed unpaired t test to determine significant differences. In minimum, all experiments were done in triplicate. Differences were considered significant when the p value was ≤ 0.05. Results were statistically analyzed using GraphPad InStat 3 (GraphPad Software, San Diego, CA) with 95% confidence intervals (CIs) of the difference.
Results
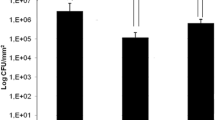
The antibacterial study results are outlined in Fig. 2. For both E. coli and K. pneumoniae, the bacteria grew over 7 logs in 24 h on the control tubing, while none grew on the selenium tubing. H. influenzae grew over 6 logs in 24 h on the control tubing, but none grew on the selenium tubing. P. aeruginosa was not inhibited completely, as seen for the other three bacteria; however, it was inhibited by approximately 4 logs (99.99%). Thus, the incorporation of selenium into the tubing material was very effective in blocking the bacterial attachment and colonization for each bacterial species.
As described above, the stability of the selenium-incorporated tubing material was tested by soaking in PBS at 37 °C for 12 weeks. The tubing was then exposed to E. coli for 24 h. As shown in Fig. 3, control tubing showed over 6 logs of bacterial growth, while the selenium tubing had no growth. Thus, the antimicrobial activity of the selenium-incorporated catheter was stably maintained.
This same soaking technique was applied to tubing treated with the E. coli GFP MM294. CLSM was used to visualize bacterial growth with marker green fluorescence protein expression. As seen in Fig. 4, the E. coli grew on both the inside and on the outer surface of the control tubing, but no fluorescence was seen on the selenium tubing.
The 24-h biofilms were also studied by SEM to visualize internal and external biofilm formation in control versus experimental catheters exposed to a combination of all three pathogens (E. coli, K. pneumoniae, P. aeruginosa) to test whether the combination could be resistant to the selenium. The results are summarized in Fig. 5.
Discussion
CAUTIs are the most common nosocomial infection, and infection risk increases with duration of catheter placement [16]. Although CAUTIs are considered preventable, there are approximately 450,000 cases in the United States annually, with Medicare and Medicaid-ineligible treatment costs exceeding $350 million each year [17]. Uropathogenic E. coli (UPEC) represents the most commonly isolated bacteria in nosocomial UTIs [18], while Klebsiella spp. and P. aeruginosa cause 10.1 and 10.3% of CAUTIs, respectively [19]. Thus, the substantial reduction of bacterial colonization of these organisms should help prevent CAUTIs.
Emerging antibiotic resistance among bacterial species is considered a global health crisis that threatens clinicians’ ability to treat serious bacterial infections [20]. CAUTIs caused by E. coli, K. pneumoniae, and P. aeruginosa often show antibiotic resistance mediated by important virulence factors. UPEC strains use adhesins, toxins, and fimbriae that facilitate catheter colonization, host cell invasion, and biofilm formation [18]. Klebsiella species have capsule lipoproteins and external membrane proteins that enable to resistance of some strains against even carbapenems [21]. P. aeruginosa is recognized for its ability to cause severe biofilm-mediated infections often highly refractory to antibiotic therapy [22]. A recent intensive care unit study found most CAUTIs were caused by Gram-negative bacteria already resistant to common antibiotics [23]. Primary preventative efforts that interrupt colonization and biofilm formation of these species would reduce incidence of clinically challenging infections and slow antibiotic resistance development.
It is well documented that antimicrobial catheter coatings reduce the risk for CAUTIs through material-specific biocidal properties, and thus interrupting biofilm formation [1]. A recent meta-analysis of 15 clinical studies comparing coated catheters to standard catheters demonstrated a statistically significant decrease in CAUTIs with antimicrobial catheters [10]. However, heterogeneity between studies in coating material, sample size, and study criteria has hindered the adoption of antimicrobial catheter technology in current clinical practice. Silver in alloy, polymer, and nanoparticle forms is one of the most studied elements in this area [1]. Despite its FDA-approval for this purpose, silver has not been widely adopted due to lingering safety concerns. For example, silver-nanoparticle coatings have demonstrated cytotoxicity and genotoxicity in human cell lines, raising concern for patient safety [24]. Although other antimicrobial catheter materials have been studied, none have been widely adopted despite meta-analytical findings that support reduction of CAUTI incidence. Therefore, there is a need to study other bactericidal materials that may help prevent CAUTI development with less risk of host tissue toxicity. Additionally, coating technologies covering only the surface of the catheter material have brought up concerns such as substance stability and leaching over time [24]. Complete incorporation by polymerization of the antimicrobial substance with the catheter material is considered superior to more superficial, less stable modifications.
Prior non-urological studies demonstrate the effectiveness of covalent selenium in preventing bacterial colonization and biofilm formation against P. aeruginosa and S. aureus [12, 13]. Furthermore, animal studies demonstrate that organo-selenium coatings are non-toxic to surrounding host tissue [13], supporting the potential for implementing organo-selenium-incorporated catheters in future clinical trials. The antibacterial effectiveness of organo-selenium has been credited to its superoxide radicals that inhibit bacterial attachment on coated surfaces for a wide spectrum of pathogens. In a recently published in vitro study, standard urinary catheters were inoculated with UPEC and then treated with inorganic selenium (sodium selenite), which demonstrated antimicrobial activity in preventing UPEC biofilm formation when compared to catheters not exposed to selenium [25]. Additionally, in vivo animal studies on covalently incorporated organo-selenium contact lenses and hemodialysis catheters have demonstrated significant inhibition of bacterial colonization and biofilm formation on surfaces without any evidence of tissue toxicity for the host, in part due to its covalent attachment to the catheter [12, 13]. These prior findings support the promise for selenium in a urinary antimicrobial setting as well as the potential and safety of organo-selenium incorporating technology, respectively, but our study applies these principles in a novel way.
The results of our study demonstrate that organo-selenium-incorporated catheters significantly inhibit the bacterial in vitro growth and biofilm formation of common uropathogenic Gram-negative bacteria. E. coli, K. pneumoniae, and H. influenzae demonstrated complete growth inhibition, while P. aeruginosa was inhibited by approximately 4 logs (99.99%). SEM revealed qualitative growth inhibition of all individual isolates as well as multi-bacterial inoculation. Based on these findings, organo-selenium-incorporated catheters may reduce the risks for CAUTIs, catheter-associated bacteremia, and further development of pathogenic antibiotic resistance. The addition of H. influenzae further demonstrates the ability of organo-selenium to inhibit unusual, less common CAUTI-causative pathogens [26], suggesting potential for broad-spectrum antimicrobial action. Organo-selenium-incorporated catheters are also not expected to cause any local bladder toxicity when used in patients given results of prior animal studies [12, 13]. These results should encourage investigators to further evaluate its clinical potential in preventing CAUTIs.
While the results are promising, we are well aware of the limitations in our study. This pilot study provides in vitro data only requiring further verification by in vivo and clinical studies. While our results suggest broad antimicrobial action, efficacy of organo-selenium in growth inhibition of other CAUTI-causing organisms cannot be extrapolated from this study. Although in vitro growth and biofilm inhibition suggest a reduction in CAUTIs, clinical studies are needed to prove this. Additionally, while previous in vivo studies have demonstrated non-toxicity of selenium in other, non-urological medical devices, in vivo studies on organo-selenium-incorporated catheters have to confirm this prior to clinical trials in patients. While, in our study, stability testing demonstrated efficacy following incubation in PBS at 37 °C for 12 weeks, additional stability evaluation after longer-term storage is necessary.
Conclusion
CAUTIs are common nosocomial infections contributing extensively to patient morbidity, mortality, and health care costs. More effective prevention of CAUTIs would contribute to improved patient outcomes and reduced antibiotic resistance among causative organisms. In the past, antimicrobial-coated catheters have proven effective in lowering the risks of CAUTIs. However, their clinical implementation has been limited by factors such as heterogeneity in antimicrobial substances, study design, and limited statistical power, all factors which have prevented the wide acceptance of antimicrobial catheters in current clinical practice.
The presented in vitro study results demonstrate that organo-selenium can be stably incorporated into catheter tubing to inhibit bacterial attachment, growth, and biofilm formation of multiple uropathogenic organisms. Polymerization of organo-selenium into catheter tubing material does not show any loss of antimicrobial activity even after soaking in PBS for 12 weeks at 37 °C, thus demonstrating material stability and lasting effectiveness.
Our findings suggest that organo-selenium-incorporated catheters may help in preventing catheter-associated urinary tract infections. This pilot study should encourage other investigators to proceed with in vivo and randomized clinical trials to further verify the prophylactic value of organo-selenium with the long-term clinical goal of reducing catheter-associated complications.
Data availability
The data of this study will be available on request.
Abbreviations
- CAUTI:
-
Catheter-associated urinary tract infection
- CFU:
-
Colony-forming units
- CLSM:
-
Confocal laser scanning microscopy
- GFP:
-
Green fluorescent protein
- LB:
-
Luria–Bertani broth
- PBS:
-
Phosphate-buffered saline
- SEM:
-
Scanning electron microscopy
- TSB:
-
Trypticase soy broth
- UTI:
-
Urinary tract infection
- UPEC:
-
Uropathogenic E. coli
References
Singha P, Locklin J, Handa H (2017) A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater 50:20–40
Toolkit for reducing catheter-associated urinary tract infections in hospital: Appendix D. poster on indications for urinary catheters. Agency for Healthcare Research and Quality, Rockville, MD Web site. https://www.ahrq.gov/hai/cauti-tools/impl-guide/implementation-guide-appendix-d.html. Updated 2015.
Nicolle LE (2014) Catheter associated urinary tract infections. Antimicrob Resist Infect Control 3:23. https://pubmed.ncbi.nlm.nih.gov/25075308/https://doi.org/10.1186/2047-2994-3-23
Chant C, Smith OM, Marshall JC, Friedrich JO (2011) Relationship of catheter-associated urinary tract infection to mortality and length of stay in critically ill patients: A systematic review and meta-analysis of observational studies. Crit Care Med 39(5):1167–1173. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&NEWS=n&CSC=Y&PAGE=fulltext&D=ovft&AN=00003246-201105000-00034. https://doi.org/10.1097/CCM.0b013e31820a8581
Majumder MMI, Ahmed T, Ahmed S, Khan AR (2018) Microbiology of catheter associated urinary tract infection. In: Behzadi P, ed. Microbiology of urinary tract infections: Microbial agents and predisposing factors. IntechOpen. https://www.intechopen.com/chapters/62973. https://doi.org/10.5772/intechopen.80080
Weiner LM, Webb AK, Limbago B et al (2016) Antimicrobial-resistant pathogens associated with healthcare-associated infections: Summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2011–2014. Infect Control Hosp Epidemiol 37(11):1288–1301. https://doi.org/10.1017/ice.2016.174
Karchmer TB, Giannetta ET, Muto CA, Strain BA, Farr BM (2000) A randomized crossover study of silver-coated urinary catheters in hospitalized patients. Arch Int Med 160(21):3294–3298. https://doi.org/10.1001/archinte.160.21.3294.
Stenzelius K, Persson S, Olsson U, Stjärneblad M (2011) Noble metal alloy-coated latex versus silicone foley catheter in short-term catheterization: A randomized controlled study. Scand J Urol Nephrol 45(4):258–264. http://www.tandfonline.com/doi/abs/10.3109/00365599.2011.560007. https://doi.org/10.3109/00365599.2011.560007.
Stensballe J, Tvede M, Looms D, et al (2007) Infection risk with nitrofurazone-impregnated urinary catheters in trauma patients: A randomized trial. Ann Intern Med 147(5):285–293. https://www.ncbi.nlm.nih.gov/pubmed/17785483. https://doi.org/10.7326/0003-4819-147-5-200709040-00002
Vopni R, Voice A, de Riese CS, Garza J, de Riese WT (2021) Use of antimicrobial-coated catheters in preventing catheter-associated urinary tract infections and bacteriuria: A meta-analysis for clinicians. Urol Prac 8(6):705–712. https://doi.org/10.1097/UPJ.0000000000000254
Vatansever F, de Melo WC, Avci P, et al (2013) Antimicrobial strategies centered around reactive oxygen species–bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol Rev 37(6):955–989. https://doi.org/10.1111/1574-6976.12026
Mathews S, Spallholz J, Grimson M, Dubielzig R, Gray T, Reid T (2006) Prevention of bacterial colonization of contact lenses with covalently attached selenium and effects on the rabbit cornea. Cornea 25(7):806–814. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&NEWS=n&CSC=Y&PAGE=fulltext&D=ovft&AN=00003226-200608000-00009. https://doi.org/10.1097/01.ico.0000224636.57062.90
Tran PL, Lowry N, Hamood AN, et al (2012) An organoselenium compound inhibits Staphylococcus aureus biofilms on hemodialysis catheters in vivo. Antimicrob Agents Chemoth 56(2):972–978. http://aac.asm.org/content/56/2/972.abstract. https://doi.org/10.1128/AAC.05680-11
Malone CL, Boles BR, Lauderdale KJ, Thoendel M, Kavanaugh JS, Horswill AR (2009) Fluorescent reporters for Staphylococcus aureus. J Microbiol Methods 77(3):251–260. https://doi.org/10.1016/j.mimet.2009.02.011
Hazlett LD (1993) Corneal and ocular surface histochemistry. Prog Histochem Cytochem 25(3):1–60. https://doi.org/10.1016/s0079-6336(11)80031-8
Foxman B (2003) Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Dis Mon 49(2):53–70. https://doi.org/10.1067/mda.2003.7
Lo J, Lange D, Chew BH (2014) Ureteral stents and foley catheters-associated urinary tract infections: The role of coatings and materials in infection prevention. Antibiotics (Basel) 3(1):87–97. https://www.ncbi.nlm.nih.gov/pubmed/27025736. https://doi.org/10.3390/antibiotics3010087
Jcobsen SM, Stickler DJ, Mobley HLT, Shirtlife ME (2008) Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin Microb Rev 21(1):26–59. http://cmr.asm.org/content/21/1/26.abstract. https://doi.org/10.1128/CMR.00019-07
Flores-Mireles A, Hreha TN, Hunstad DA (2019) Pathophysiology, treatment, and prevention of catheter-associated urinary tract infection. Top Spinal Cord Inj Rehabil 25(3):228–240. https://doi.org/10.1310/sci2503-228
Alós JI (2015) Resistencia bacteriana a los antibióticos: Una crisis global [Antibiotic resistance: A global crisis]. Enferm Infecc Microbiol Clin 33(10):692–699. https://doi.org/10.1016/j.eimc.2014.10.004
Candan ED, Aksöz N (2015) Klebsiella pneumoniae: Characteristics of carbapenem resistance and virulence factors. Acta Biochim Pol 62(4):867–874. https://doi.org/10.18388/abp.2015_1148
Cole SJ, Records AR, Orr MW, Linden SB, Lee VT (2014) Catheter-associated urinary tract infection by Pseudomonas aeruginosa is mediated by exopolysaccharide-independent biofilms. Infec Immun 82(5):2048–2058. https://www.ncbi.nlm.nih.gov/pubmed/24595142. https://doi.org/10.1128/IAI.01652-14
Peng D, Li X, Liu P, et al (2018) Epidemiology of pathogens and antimicrobial resistance of catheter-associated urinary tract infections in intensive care units: A systematic review and meta-analysis. Am J Infect Control 46(12):e81–e90
Knetsch MLW, Koole LH (2011) New strategies in the development of antimicrobial coatings: The example of increasing usage of silver and silver nanoparticles. Polymers 3(1):340–366. https://doi.org/10.3390/polym3010340
Narayanan A, Nair MS, Muyyarikkandy MS, Amalaradjou MA (2018) Inhibition and inactivation of uropathogenic Escherichia coli biofilms on urinary catheters by sodium selenite. Int J Mol Sci 19(6):1703. https://www.ncbi.nlm.nih.gov/pubmed/29880781. https://doi.org/10.3390/ijms19061703
Diedrich LK, Manby CL (2017) Haemophilus species as a urinary tract pathogen. Lab Med 48(1):e1–e3. https://www.ncbi.nlm.nih.gov/pubmed/28039378. https://doi.org/10.1093/labmed/lmw063
Funding
The authors have no sources of funding, financial or non-financial interests to declare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jacobo, U., Vopni, R., Tran, P. et al. Efficacy of organo-selenium-incorporated urinary catheter tubing for in vitro growth inhibition of E. coli, K. pneumoniae, P. aeruginosa, and H. influenzae. Int Urol Nephrol 55, 503–510 (2023). https://doi.org/10.1007/s11255-022-03422-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03422-y