Abstract
Purpose
To evaluate the effectiveness and safety of intravesical oxybutynin therapy for patients with neurogenic detrusor overactivity.
Methods
A systematic search in PubMed, MEDLINE, EMBASE, ClinicalTrial.gov, and Cochrane Controlled Trials Register was conducted from 1990 to 2021. Nineteen studies were included for analysis, of which 392 patients including both adults and children were treated with intravesical oxybutynin. The analysis was performed by Cochrane RevMan® software, version 5.3. The primary outcomes were maximum bladder capacity (MBC), detrusor pressure at MBC, and bladder compliance. The secondary outcomes were episodes of urinary incontinence and side effects.
Results
MBC displayed an increase of 77.8 ml (95% CI 56.9 to 98.7) in kids, 110.8 ml (95% CI 58.95 to 162.7) in adults, respectively. Detrusor pressure at MBC demonstrated an improvement of − 18.8 cm H2O (95% CI − 26.2 to − 11.3) in kids, − 23.2 cm H2O (95% CI − 32.6 to − 13.8) in adults, respectively. The bladder compliance increased 5.8 ml/cm H2O (95% CI 3.4 to 8.1) among kids. The mean percentage of patients “dry or improved” after treatment accounted for 76.9% in adults and 74.6% in kids, respectively. Among all patients, 53 (13.5%) reported side effects, 80 (20.4%) discontinued this treatment, 26 (6.6%) withdrew because of side effects, and 35 (8.9%) quit due to inconvenience.
Conclusion
Intravesical oxybutynin treatment could be a feasible treatment for both adults and children with neurogenic detrusor overactivity, because of its good effect and less side effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with different underlying neurologic conditions, such as spinal cord injuries, multiple sclerosis, Parkinson disease, and meningomyelocele, can develop neurogenic overactive bladder, which is the partial or complete loss of urine storage capacity and the ability to empty the bladder under low pressure [1]. Patients often suffer from neurogenic detrusor overactivity, with or without sphincter abnormalities [2]. In these patients, low bladder compliance can lead to urinary incontinence that negatively affects the life quality and it can be dangerous for the kidneys [3]. A report of Woodhouse showed that in English children with neurogenic bladder who had received insufficient treatment, the prevalence of total renal failure before and after puberty was 18% and 30% [4]. Patients with neurogenic detrusor overactivity are usually treated by anticholinergic drugs as the first-line medical therapy, which is combined with clean intermittent self- or third-party catheterization (CIC) when patients cannot adequately empty their bladders [5]. Oxybutynin, which is well studied, cheap, and easily accessible, has been used for decades in patients with detrusor hyperactivity by mainly acting on the parasympathetic nervous system. It has strong smooth muscle spasmolysis, strong analgesic effect, anticholinergic effect, local anesthesia, and calcium-channel blocking activity [6]. However, a number of these patients may become non-responders to the oxybutynin with oral routine or present with a high incidence of systemic side effects, such as dry mouth, constipation, dementia, dizziness, and facial flushing, leading to dose reduction or even discontinuation of treatment [7,8,9]. To minimize the adverse events of oral oxybutynin, another option is the intravascular injection of oxybutynin, which improves effectiveness by increasing the plasma levels while decreases undesired anticholinergic effects by reducing the metabolite [10]. The efficiency and safety of intravesical oxybutynin have been proved by previous report [11]. But few studies have illustrated the therapeutic effect of intravesical oxybutynin for both adults and children. This study was to focus on identifying the outcome of intravesical oxybutynin for adults and children who were refractory to oral drugs or suffered severe side effects.
Methods
Publication searches
This review comprehensively searched all major literature databases, which carried out on September 2021, for studies published since September 1990 in the PubMed, EMBASE, MEDLINE, ClinicalTrial.gov, and Cochrane Controlled Trials Register. Only reports published in English were searched. The following search strategy was used to include all relevant reports: (Neurogenic detrusor overactivity OR Neurogenic bladder OR Bladder overactivity) AND (Intravesical) AND (Oxybutynin OR Anticholinergics OR Antimuscarinics) AND (Efficacy OR Effect OR Safety). The study designs included randomized controlled trial (RCT), clinical trial, controlled clinical trial, prospective cohort study and observational study. A reference list of all systematic reviews and potential reports in this area was also verified for additional recommendations. There were no exclusion criteria according to publication status. It should note that IRB approval was not required for this study because the data came directly from open-source researches rather than proprietary areas.
Inclusion criteria and exclusion criteria
Patients (men and women) who were unresponsive to oral oxybutynin therapy (became intolerable to oral drugs or withdrew because of severe side effects) were included. Intervention included intravesical administration of oxybutynin into the bladder. Exclusion criteria included: (a) intravesical therapy using anticholinergic drugs other than oxybutynin; (b) studies reported insufficient data; (c) meeting, case report, review, or abstract; (d) animal studies; (e) language other than English.
Objectives and outcomes
The purpose of this study was to assess the efficiency and safety of intravesical oxybutynin in adults and children with neurogenic detrusor overactivity. The primary outcomes were maximum bladder capacity (MBC), detrusor pressure at MBC, and bladder compliance. The secondary outcomes were episodes of urinary incontinence and the main side effects, such as urinary tract infection (UTI), dry mouth, constipation, dry eye, blurred vision, decreased perspiration, seizures, cognitive effects, hallucination, disturbance in attention, drowsiness, and facial flushing [12,13,14].
Study selection and data collection
Two researchers (S-H.S. and L.P.) independently selected and reviewed the abstracts according to inclusion and exclusion criteria. Since drug metabolism between adults and kids has various behavior and characteristics, they were assessed separately [12]. Studies were conducted in the children group (kids only), adult group (adults only), and mixed group (both adults and children). Data were collected by one author (S-H.S.) and independently doubled examined by another (L.P.). Disagreements were resolved after discussion between reviewers and the corresponding author.
Quality assessment
Reports quality was evaluated by two independent investigators (S-H.S. and L.P.). According to the Cochrane handbook, the risk of bias across RCTs was assessed by RevMan® software using the risk of bias (ROB) graph, which included unclear, high, and low risks [15]. Observational reports were assessed by the Newcastle–Ottawa Scale (NOS) [15, 16]. The semi-quantitative principles of the star system were applied for quality assessment in the NOS, and the maximum achievable score was up to 9 stars. When there were any differences between the authors, a third expert intervened to solve them.
Data analysis
A weighted treatment effect was conducted across trials using Cochrane RevMan® software, version 5.3 (Cochrane Collaboration, Oxford, UK). Weighted mean differences and 95% confidence intervals (CI) (random effects model) were used to express the results. Heterogeneity was calculated statistically using the I2 test. The fixed‐effect model was needed when I2 values were less than 50%, and the random‐effect method was needed when I2 values greater than 50% were produced. Subgroup analyses were performed based on age.
Results
Literature search and study characteristics
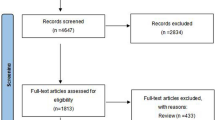
As displayed in Fig. 1, we identified 183 studies via the systematic database search, of which 6 duplicated references were removed. The exclusion of 128 records was that they were not relevant or not met the inclusion criteria. After the removal of studies, 22 individual tests may be appropriate, of which 3 trials were eliminated as we determined they were the same study with long-term follow-up after obtaining full text. However, three studies were not qualified to be analyzed due to insufficient data. Only three RCT studies were included [9, 13, 14]. Subgroup analyses of adults and children were performed for the purpose of theoretically eliminating or reducing the heterogeneity attributed to different age groups. However, the population consisted of both adults and children in five studies, two of them could be analyzed separately [11, 17], and the other three could only be analyzed using the mixed data [18,19,20]. Quality assessments of the included studies were relatively high (Table 1, Fig. 2).
Population and intervention characteristics
Table 2 shows the characteristics of included studies. A total of 392 patients were treated with intravesical oxybutynin, with or without oral oxybutynin. An average dose of 15 mg oxybutynin was instilled into the bladder of the participant through urethral catheter every day. Duration of treatment ranged from 21 days to 36 months across the studies. The oxybutynin can be provided by crushing pills into solution or use a pharmacy-prepared oxybutynin solution [12, 21].
Outcomes
Figure 3 shows the improvement in MBC and detrusor pressure at MBC (Fig. 3a, b). There have been moderate inconsistencies in studies of MBC in the adult group (I2 = 76%) and mixed group (I2 = 59%). The mean MBC showed an increase of 77.8 ml (95% CI 56.9 to 98.7; p < 0.00001) in kids, 110.8 ml (95% CI 58.95 to 162.7; p < 0.00001) in adults and 117.0 ml (95% CI 78.2 to 155.7; p < 0.00001) in the mixed group separately. The mean detrusor pressure at MBC showed an increase of − 18.8 cm H2O (95% CI − 26.2 to − 11.3; p < 0.00001; I2 = 57%) in kids, − 23.2 cm H2O (95% CI − 32.6 to − 13.8; p < 0.00001; I2 = 0) in adults and − 9.4 cm H2O (95% CI − 13.3 to − 5.5; p < 0.00001; I2 = 0) in the mixed group separately. After treatment with intravesical oxybutynin, the bladder compliance increased 5.8 ml/cm H2O (95% CI 3.4 to 8.1; p < 0.00001; I2 = 18%) among kids, and 5.0 ml/cmH2O (95% CI 0.9 to 9.1; p = 0.02; I2 = 59%) among mixed age group, respectively (Fig. 3c).
The changes in urinary incontinence (dry and improvement) are exhibited in Table 3. The mean portion of patients “dry or improved” after treatment accounted for 76.9% in adults and 74.6% in children.
It was demonstrated that detrusor overactivity showed a significant decrease in 33% to 75% of the patients across reports [22,23,24,25]. Detrusor leak point pressure (DLPP) was observed in several studies, and all groups found statistically significant improvements [9, 14, 26]. Buyse et al. reported detrusor-sphincter dyssynergia in 11 kids; however, the follow-up of change was not recorded [21]. Prasad et al. recorded the number of times the patients performed CIC, reduced from 16 ± 7 to 8 ± 3.5 after intravesical therapy (P < 0.05) [17], and Annette et al. also showed a slight decrease in the frequency of CIC (− 0.11 ± 0.88, P < 0.6734) [14].
Three hundred and ninety-two patients initially received intravesical oxybutynin, of whom 53 reported side effects (13.5%) (displayed in Table 4). Dry mouth (6.1%) and constipation (8.2%) were the most frequently mentioned adverse effects of this anticholinergic drug. UTI (5.6%) was mainly attributed to the use of catheters or the drug. Other side effects were rarely reported, including facial flushing (2.8%), drowsiness (0.8%), dizziness (0.8%), abdominal discomfort (0.8%), cognitive effects (0.8%), headaches (0.5%), hallucinations (0.3%), dry eye (0.3%), fatigue (0.3%), somnolence (0.3%), urinary hesitation (0.3%), instillation site pain (0.3%), urge to urinate (0.3%), hypohidrosis (0.3%), hypotension (0.3%), and ileus (0.3%). Among the 80 patients who withdrew from the treatment (20.4%), side effects and inconvenience were responsible for 6.6% (26 patients) and 8.9% (35 patients) of the discontinuations, respectively.
Discussion
Intravesical oxybutynin was known to reduce detrusor activity, increase MBC and decrease detrusor pressure in patients with neurogenic detrusor overactivity [27]. In addition, this review also suggested that intravesical oxybutynin was an efficient therapy option, for both adults and children.
Compared to the baseline values, a significant increase was detected concerning bladder compliance. Since the pressure at MBC and DLPP are clinically significant indicators of renal safety [28], they were selected as the efficacy criteria for the assessment of effectiveness, and the mean detrusor pressure at MBC showed a statistically significant decrease of − 18.8 cm H2O in kids and − 23.2 cm H2O in adults, respectively. However, only a few studies reported DLPP and sphincter abnormalities, with inadequate information to draw a conclusion [9, 12, 14, 21, 25].
It seemed that the increase of MBC in adults (110.8 ml) was greater than that among kids (77.8 ml) (P < 0.05). However, this may wrongly overestimate the efficiency of oxybutynin in adults, because MBC increases with age [29]. Thus, age-related parameters should be used to evaluate the differences in the therapeutic effect between adults and children.
Overall, most studies had significant improvements in urinary incontinence, with high “dry and improved” rates were reported. The quality of life in patients with neurogenic detrusor overactivity also improved significantly [30].
The effect of intravesical oxybutynin can hypothetically be attributed to the anticholinergic effects and the topical analgesia on the sensitive C fiber afferents in the bladder detrusor, increasing their threshold for activation [31]. Nowadays, Botulinum-A toxin (BTX-A) is widely used to paralyze the bladder detrusor muscle and block the signals to the spinal cord as one second-line treatment [32]. This therapy is not durable and a complicated injection process under anesthesia is needed. Antimuscarinics, such as oxybutynin, are cheaper than BTX-A; however, there is no cost-effectiveness analysis of intravesical oxybutynin vs. BTX-A administration in the treatment of neurogenic detrusor overactivity [33].
The relationship between plasma concentration and its clinical efficacy is still not clear and there is no unified dose for intravesical treatment [27, 34]. In most studies, intravesical oxybutynin was used in dosages between 0.2 and 0.4 mg/kg daily (average 15 mg daily). However, Haferkamp found that increase dosage of intravesical oxybutynin could improve the effectiveness from 66% (0.3 mg/kg daily) to 87% (0.9 mg/kg daily), without significant increase of side effects [35]. Previous studies demonstrated that the oxybutynin solution warmed to 37 °C with a PH of 5.85 before intravesical therapy could reduce irritating reactions in the bladder mucosa [18]. Prasad et al. reported leaving the drug in the bladder for as long as 3 to 4 h or until the next catheterization did not produce any systemic side effects without influencing the effectiveness [17].
N-Desethyl oxybutynin is the first transhepatic metabolite of oxybutynin, which is related to anticholinergic effect. Avoiding oral administration can reduce this risk. Researchers indicated that, among the patients, 20.4% dropped out of the treatment, with side effects accounted for 6.6% (26 patients). According to Weese et al., the incidence of significant side effects of oral treatment ranged from 57 to 94% [36]. In this case, the incidence of adverse reactions of intravesical administration was lower than that of the oral route, which was consistent with the rationality of its use. However, because the drug is absorbed through the bladder mucosa, there is still a chance side effects occur after intravesical installation [10]. Massad and Mohler et al. found significantly higher plasma levels and tissue levels of intravesical oxybutynin compared to oral oxybutynin [10, 18]. On the other hand, this can cause higher cognitive dysfunction owing to that oxybutynin can penetrate the blood–brain barrier because of the lipid-soluble anticholinergic effect and bind to typical muscarinic receptors in the brain, in particular, the postsynaptic subtypes M1 and M2 [37]. Since oxybutynin has been proved to have central anticholinergic adverse effects, such as short-term cognitive impairment [38]. In older patients and those at risk of cognitive impairment or dementia, oxybutynin should be avoided, while β3 agonists, BTX-A administration, and neuro-modulation are available [39, 40]. However, two reports demonstrated that oxybutynin had no harmful effect on children’s attention and memory [41, 42]. Generally, the potential therapeutic efficacy of neurogenic bladder therapy should be weighed against the potential side effects of anticholinergic treatment in all patients, and close monitoring of cognitive and functional performance should be conducted [40].
It was worth noting that a substantial proportion (8.9%) of the patients in these studies dropped out of treatment due to inconvenience of crushing and administering the medication. In some previous studies, sugar-free, sterile, stable, and pharmacy-produced solutions were used to eliminate the complicated crushing procedure by patients, which markedly improved treatment compliance and acceptance of this therapy [12, 21]. Hayashi and Saito et al. suggested that the retention time of modification with hydroxypropyl cellulose (HPC) in bladder was longer than that of intravesical oxybutynin without HPC. Thus, there was a lower concentration of plasma oxybutynin due to the reduction of the absorption of oxybutynin from bladder mucosa, and might reduce the anticholinergic side effects [22, 25]. Oxybutynin 0.1% Grachtenhaus (further ingredients: 0.9% sodium hydrochloride, 0.1% hydrogen chloride) has been used in the treatment of patients suffering from neurogenic detrusor overactivity in a Multi-Center Trial, showing good efficacy and safety [14]. Hence, there is an urgent need to promote the supply of pharmacy-prepared intravesical oxybutynin to improve the compliance of patients.
CIC treatment has been successfully used even in newborns and infants with neurogenic bladder who are not able to effectively empty the bladder [43]. Proper education, guidance, training for both patients and caregivers are needed to assist the child catheterize properly, and the detailed procedure of CIC in kids were parented in this study [44]. CIC can be successfully taught to boys and girls who are motivated and who have developed the required dexterity, mostly around the age of 6 years [1]. Catheter lubrication and avoidance of forceful manipulation during CIC are necessary to avoid urethral strictures and false passage in boys [45].
Duration of treatment ranged from 21 days to 36 months across the reports. Even though Kato suggested pharmacological effect of it was time-dependent, while Painter et al. revealed only a 53% durable long-term effect [46, 47]. Kaplinsky et al. also believed the therapeutic efficiency of intravesical oxybutynin was durable in patients, with MBC markedly increased during extended follow-up [23]. Meanwhile, after a 15-year follow-up, Humblet illustrated that intravesical oxybutynin remained valid, which could still inhibit the detrusor overactivity without adverse effects [29].
The first limitation was that not all studies included revolving urodynamic study (UDS) or with overall UDS parameters to evaluate the function of bladder in patients with neurogenic detrusor overactivity [48], and some data were incomplete, thus we were not able to collect valid data from these studies [14]. Second, the symptoms of neurogenic bladder are related to the dysfunction of detrusor and sphincter; however, included articles only investigated the therapeutic effects of oxybutynin for the dysfunction of detrusor overactivity, we could only draw a conclusion based on included studies, and future study should aim to assess the effects of single or combined treatments for different subtypes of neurogenic bladder, especially with combined sphincter disorder. In addition, only a few studies illustrated the long-term outcomes of this therapy. Furthermore, only three RCTs were included; more well-designed RCT trials should be conducted to confirm our findings.
Conclusions
Meta-analysis revealed that intravesical oxybutynin was a potential treatment for both adult and pediatric patients with neurogenic detrusor overactivity who were intolerable to oral drugs or withdrew because of serious side effects. Generally, this treatment improves mean detrusor compliance, decreases MBC and pressure at MBC, and improves incontinence. So far there is no sufficient evidence that intravesical oxybutynin treatment may have advantages over other treatments including BTX-A injection, other anticholinergic drugs or surgery for patients with neurogenic detrusor overactivity. However, it is undeniably that intravesical oxybutynin therapy is well studied, cheap, and easily accessible, and it could be a feasible treatment for both adults and children. In the future, high-quality trials that include validity index and overall UDS parameters should be conducted to confirm our findings. Meanwhile, age-related parameters should be used to evaluate the therapeutic effect of intravesical oxybutynin between adults and children.
Data availability
The data supporting the finding of this article are available from the corresponding author.
Code availability
Not applicable.
References
Verpoorten C, Buyse GM (2008) The neurogenic bladder: medical treatment. Pediatr Nephrol 23(5):717–725. https://doi.org/10.1007/s00467-007-0691-z
Tijnagel MJ, Scheepe JR, Blok BF (2017) Real life persistence rate with antimuscarinic treatment in patients with idiopathic or neurogenic overactive bladder: a prospective cohort study with solifenacin. BMC Urol 17(1):30. https://doi.org/10.1186/s12894-017-0216-4
MacDiarmid SA (2003) Overactive bladder: improving the efficacy of anticholinergics by dose escalation. Curr Urol Rep 4(6):446–451. https://doi.org/10.1007/s11934-003-0025-z
Woodhouse CRJ (2005) Myelomeningocele in young adults. BJU Int 95(2):223–230. https://doi.org/10.1111/j.1464-410X.2005.05374.x
Groen J, Pannek J, Castro Diaz D, Del Popolo G, Gross T, Hamid R, Karsenty G, Kessler TM, Schneider M, Hoen L, Blok B (2016) Summary of European association of urology (EAU) guidelines on neuro-urology. Eur Urol 69(2):324–333. https://doi.org/10.1016/j.eururo.2015.07.071
Wein AJ (1998) Pharmacologic options for the overactive bladder. Urology 51(2A Suppl):43–47. https://doi.org/10.1016/s0090-4295(98)90009-7
Bishara D, Perera G, Harwood D, Taylor D, Sauer J, Funnell N, Stewart R, Mueller C (2021) Centrally acting anticholinergic drugs used for urinary conditions associated with worse outcomes in dementia. J Am Med Dir Assoc. https://doi.org/10.1016/j.jamda.2021.08.011
Coupland CAC, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J (2019) Anticholinergic drug exposure and the risk of dementia: a nested case-control study. JAMA Intern Med 179(8):1084–1093. https://doi.org/10.1001/jamainternmed.2019.0677
Ferrara P, D’Aleo CM, Tarquini E, Salvatore S, Salvaggio E (2001) Side-effects of oral or intravesical oxybutynin chloride in children with spina bifida. BJU Int 87(7):674–678. https://doi.org/10.1046/j.1464-410x.2001.02152.x
Massad CA, Kogan BA, Trigo-Rocha FE (1992) The pharmacokinetics of intravesical and oral oxybutynin chloride. J Urol 148(2 Pt 2):595–597. https://doi.org/10.1016/s0022-5347(17)36663-6
Kasabian NG, Vlachiotis JD, Lais A, Klumpp B, Kelly MD, Siroky MB, Bauer SB (1994) The use of intravesical oxybutynin chloride in patients with detrusor hypertonicity and detrusor hyperreflexia. J Urol 151(4):944–945. https://doi.org/10.1016/s0022-5347(17)35130-3
Guerra LA, Moher D, Sampson M, Barrowman N, Pike J, Leonard M (2008) Intravesical oxybutynin for children with poorly compliant neurogenic bladder: a systematic review. J Urol 180(3):1091–1097. https://doi.org/10.1016/j.juro.2008.05.056
Lehtoranta K, Tainio H, Lukkari-Lax E, Hakonen T, Tammela TL (2002) Pharmacokinetics, efficacy, and safety of intravesical formulation of oxybutynin in patients with detrusor overactivity. Scand J Urol Nephrol 36(1):18–24. https://doi.org/10.1080/003655902317259319
Schröder A, Albrecht U, Schnitker J, Reitz A, Stein R (2016) Efficacy, safety, and tolerability of intravesically administered 0.1% oxybutynin hydrochloride solution in adult patients with neurogenic bladder: a randomized, prospective, controlled multi-center trial. Neurourol Urodynam 35(5):582–588. https://doi.org/10.1002/nau.22755
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA (2011) The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605. https://doi.org/10.1007/s10654-010-9491-z
Prasad KV, Vaidyanathan S (1993) Intravesical oxybutynin chloride and clean intermittent catheterisation in patients with neurogenic vesical dysfunction and decreased bladder capacity. Br J Urol 72(5 Pt 2):719–722. https://doi.org/10.1111/j.1464-410x.1993.tb16255.x
Brendler CB, Radebaugh LC, Mohler JL (1989) Topical oxybutynin chloride for relaxation of dysfunctional bladders. J Urol 141(6):1350–1352. https://doi.org/10.1016/s0022-5347(17)41304-8
Ersoz M, Yildiz N, Akyuz M, Koseoglu F (2010) Efficacy of combined oral-intravesical oxybutynin hydrochloride treatment for patients with overactive detrusors and indwelling urethral catheters. Rehabil Nurs 35(2):80–86. https://doi.org/10.1002/j.2048-7940.2010.tb00036.x
Mizunaga M, Miyata M, Kaneko S, Yachiku S, Chiba K (1994) Intravesical instillation of oxybutynin hydrochloride therapy for patients with a neuropathic bladder. Paraplegia 32(1):25–29. https://doi.org/10.1038/sc.1994.5
Buyse G, Verpoorten C, Vereecken R, Casaer P (1998) Intravesical application of a stable oxybutynin solution improves therapeutic compliance and acceptance in children with neurogenic bladder dysfunction. J Urol 160(3 Pt 2):1084–1087. https://doi.org/10.1097/00005392-199809020-00031
Hayashi A, Saito M, Okada S, Hanada T, Watanabe T, Satoh K, Kanzaki S (2007) Treatment with modified intravesical oxybutynin chloride for neurogenic bladder in children. J Pediatr Urol 3(6):438–442. https://doi.org/10.1016/j.jpurol.2007.05.007
Kaplinsky R, Greenfield S, Wan J, Fera M (1996) Expanded followup of intravesical oxybutynin chloride use in children with neurogenic bladder. J Urol 156(2 Pt 2):753–756. https://doi.org/10.1097/00005392-199608001-00053
Pannek J, Sommerfeld HJ, Bötel U, Senge T (2000) Combined intravesical and oral oxybutynin chloride in adult patients with spinal cord injury. Urology 55(3):358–362. https://doi.org/10.1016/s0090-4295(99)00540-3
Saito M, Watanabe T, Tabuchi F, Otsubo K, Satoh K, Miyagawa I (2004) Urodynamic effects and safety of modified intravesical oxybutynin chloride in patients with neurogenic detrusor overactivity: 3 years experience. Int J Urol 11(8):592–596. https://doi.org/10.1111/j.1442-2042.2004.00871.x
Szollar SM, Lee SM (1996) Intravesical oxybutynin for spinal cord injury patients. Spinal Cord 34(5):284–287. https://doi.org/10.1038/sc.1996.51
Madersbacher H, Knoll M (1995) Intravesical application of oxybutynine: mode of action in controlling detrusor hyperreflexia. Preliminary results. Eur Urol 28(4):340–344. https://doi.org/10.1159/000475078
Madersbacher H, Mürtz G, Alloussi S, Domurath B, Henne T, Körner I, Niedeggen A, Nounla J, Pannek J, Schulte-Baukloh H, Schultz-Lampel D, Bock P, Strugala G (2009) Propiverine vs oxybutynin for treating neurogenic detrusor overactivity in children and adolescents: results of a multicentre observational cohort study. BJU Int 103(6):776–781. https://doi.org/10.1111/j.1464-410X.2008.08093.x
Humblet M, Verpoorten C, Christiaens MH, Hirche H, Jansen K, Buyse G, van Gool JD (2015) Long-term outcome of intravesical oxybutynin in children with detrusor-sphincter dyssynergia: with special reference to age-dependent parameters. Neurourol Urodyn 34(4):336–342. https://doi.org/10.1002/nau.22560
Vaidyananthan S, Soni BM, Brown E, Sett P, Krishnan KR, Bingley J, Markey S (1998) Effect of intermittent urethral catheterization and oxybutynin bladder instillation on urinary continence status and quality of life in a selected group of spinal cord injury patients with neuropathic bladder dysfunction. Spinal Cord 36(6):409–414. https://doi.org/10.1038/sj.sc.3100573
De Wachter S, Wyndaele JJ (2003) Intravesical oxybutynin: a local anesthetic effect on bladder C afferents. J Urol 169(5):1892–1895. https://doi.org/10.1097/01.ju.0000049903.60057.4b
Softness KA, Thaker H, Theva D, Rajender A, Cilento BG Jr, Bauer SB (2021) Onabotulinumtoxin A (Botox): a reasonable alternative for refractory neurogenic bladder dysfunction in children and young adults. Neurourol Urodyn 40(8):1981–1988. https://doi.org/10.1002/nau.24778
Shabir H, Hashemi S, Al-Rufayie M, Adelowo T, Riaz U, Ullah U, Alam B, Anwar M, de Preux L (2021) Cost-utility analysis of oxybutynin vs onabotulinumtoxin A (Botox) in the treatment of overactive bladder syndrome. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph18168743
Amark P, Eksborg S, Juneskans O, Bussman G, Palm C (1998) Pharmacokinetics and effects of intravesical oxybutynin on the paediatric neurogenic bladder. Br J Urol 82(6):859–864. https://doi.org/10.1046/j.1464-410x.1998.00888.x
Haferkamp A, Staehler G, Gerner HJ, Dörsam J (2000) Dosage escalation of intravesical oxybutynin in the treatment of neurogenic bladder patients. Spinal Cord 38(4):250–254. https://doi.org/10.1038/sj.sc.3100995
Weese DL, Roskamp DA, Leach GE, Zimmern PE (1993) Intravesical oxybVnin chloride: experience with 42 patients. Urology 41(6):527–530. https://doi.org/10.1016/0090-4295(93)90098-u
Donnellan CA, Fook L, McDonald P, Playfer JR (1997) Oxybutynin and cognitive dysfunction. BMJ 315(7119):1363–1364. https://doi.org/10.1136/bmj.315.7119.1363
Gish P, Mosholder AD, Truffa M, Johann-Liang R (2009) Spectrum of central anticholinergic adverse effects associated with oxybutynin: comparison of pediatric and adult cases. J Pediatr 155(3):432–434. https://doi.org/10.1016/j.jpeds.2009.01.074
Nambiar AK, Bosch R, Cruz F, Lemack GE, Thiruchelvam N, Tubaro A, Bedretdinova DA, Ambühl D, Farag F, Lombardo R, Schneider MP, Burkhard FC (2018) EAU guidelines on assessment and nonsurgical management of urinary incontinence. Eur Urol 73(4):596–609. https://doi.org/10.1016/j.eururo.2017.12.031
Welk B, Richardson K, Panicker JN (2021) The cognitive effect of anticholinergics for patients with overactive bladder. Nat Rev Urol 18(11):686–700. https://doi.org/10.1038/s41585-021-00504-x
Giramonti KM, Kogan BA, Halpern LF (2008) The effects of anticholinergic drugs on attention span and short-term memory skills in children. Neurourol Urodyn 27(4):315–318. https://doi.org/10.1002/nau.20507
Veenboer PW, Huisman J, Chrzan RJ, Kuijper CF, Dik P, de Kort LM, de Jong TP (2013) Behavioral effects of long-term antimuscarinic use in patients with spinal dysraphism: a case control study. J Urol 190(6):2228–2232. https://doi.org/10.1016/j.juro.2013.06.036
van Gool JD, Dik P, de Jong TP (2001) Bladder-sphincter dysfunction in myelomeningocele. Eur J Pediatr 160(7):414–420. https://doi.org/10.1007/s004310100741
Joseph DB, Bauer SB, Colodny AH, Mandell J, Retik AB (1989) Clean, intermittent catheterization of infants with neurogenic bladder. Pediatrics 84(1):78–82
Sutherland RS, Kogan BA, Baskin LS, Mevorach RA (1996) Clean intermittent catheterization in boys using the LoFric catheter. J Urol 156(6):2041–2043
Kato K, Kondo A, Saito M, Miyake K, Wein AJ, Levin RM (1991) In vitro intravesical instillation of anticholinergic, antispasmodic and calcium blocking agents to decrease bladder contractility. Urol Int 47(Suppl 1):36–38. https://doi.org/10.1159/000282246
Painter KA, Vates TS, Bukowski TP, Fleming P, Freedman AL, Smith CA, Gonzalez R, Perlmutter AD (1996) Long-term intravesical oxybutynin chloride therapy in children with myelodysplasia. J Urol 156(4):1459–1462
Schäfer W, Abrams P, Liao L, Mattiasson A, Pesce F, Spangberg A, Sterling AM, Zinner NR, van Kerrebroeck P (2002) Good urodynamic practices: uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn 21(3):261–274. https://doi.org/10.1002/nau.10066
Funding
This study was funded by the National Natural Science Fund of China (Grant Nos.81770673), 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (Grant Nos.ZY2017310).
Author information
Authors and Affiliations
Contributions
SS and XJ: data collection, data analysis, manuscript writing, and revision. LP, XZ and HS: patient follow-up. De-yi Luo: protocol/project development and revision.
Corresponding author
Ethics declarations
Conflict of interest
There are no competing economic interests to declare.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shen, Sh., Jia, X., Peng, L. et al. Intravesical oxybutynin therapy for patients with neurogenic detrusor overactivity: a systematic review and meta-analysis. Int Urol Nephrol 54, 737–747 (2022). https://doi.org/10.1007/s11255-022-03129-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03129-0