Abstract
Purpose
The aim of this study was to determine the frequency and the risk factors of acute and chronic nephrotoxicity in patients who received cisplatin due to malignancy.
Materials and methods
Medical records of all patients who received cisplatin-based chemotherapy regimen between January 2013 and July 2019 were retrospectively evaluated. The data of 203 patients who met the study criteria were examined. The patients were evaluated for acute nephrotoxicity at 48 h and late nephrotoxicity at 3rd month after first course of cisplatin. Early and late nephrotoxicity were defined by NCI CTCAE Version 4.0 criteria.
Results
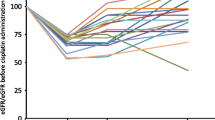
The mean age of the study patients was 56.44 ± 12.69 years, 78.8% were males and 21.2% were females. It is revealed that the incidence of cisplatin-induced acute nephrotoxicity was 9.2% and chronic nephrotoxicity was 37.9%. While the development of acute nephrotoxicity was associated with female gender, history of diabetes mellitus, history of ischemic heart disease and use of antiplatelet drug, the development of chronic nephrotoxicity was associated with older age, female gender and using of diuretics. High serum creatinine, urea and low eGFR value before treatment were found to be associated with both early and late nephrotoxicity (p < 0.05). There was no statistically significant relationship between acute or chronic nephrotoxicity and cumulative dose of cisplatin, hydration or intravenous magnesium supplementation.
Conclusion
High initial serum creatinine value and low initial eGFR are the most important determinants of both early and late nephrotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cisplatin, a platinum-based drug, is widely used in treatment of many cancers, such as lung, testicular, ovarian, genitourinary and gastrointestinal system cancers. Nephrotoxicity associated with cisplatin administration is common and unwanted adverse effect which has significantly restricted its clinical use [1]. Both acute and chronic nephrotoxicities associated with cisplatin tend to occur more frequently at higher doses or especially after repeated administration [2]. The prevalence of cisplatin-induced nephropathy (CIN) has been reported as 7–42% [3, 4]. The aim of this study is to determine the frequency of acute and chronic CIN in patients with any kind of cancer and risk factors related to development of nephropathy.
Materials and methods
Patients and study setting
Medical records of all patients who received cisplatin-based chemotherapy regimen at the medical oncology department between January 2013 and July 2019 were retrospectively evaluated. The patients who had available laboratory and clinical data during the study period and age 18 and over were included in the study. Written informed consent was obtained from all subjects after receiving an explanation of the study. The investigated period included the period from the first application of cisplatin to the 3rd month of cisplatin-based chemotherapy regardless of cisplatin therapy continuation. The study was conducted with the approval of the Clinical Research Ethics Committee of our local hospital, dated 24/07/2019 and numbered 48670771-514.10.
Data collection and assessment
All data were obtained from the medical file of patients and electronic medical records. We collected information about patient gender, age, body mass index (BMI), smoking, past medical history, concurrent radiation therapy, initial and cumulative dose (after the 3th month of chemotherapy) of cisplatin, amount of fluids being infused, use of analgesics, diuretics, antihypertensive and antiplatelet drugs, administration of magnesium and baseline and following laboratory data performed during the investigated period. The baseline laboratory data were obtained from samples drawn on the day before chemotherapy. Laboratory data at 48 h and 3 months were recorded as a following laboratory data. We divided patients into two groups according to amount of fluid infusion where 2000 ml was accepted as the cut-point. Given the treatment strategies, the limit for high-dose cisplatin is accepted as 75 mg/m2 and above, we divided the patients into standard-dose and high-dose cisplatin groups. Renal function was evaluated by the serum creatinine (sCr) and the estimated glomerular filtration rate (eGFR) value calculated using the CKD-EPI formula. Acute and chronic CINs were defined according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTC AE, version 4.0) after first chemotherapy cycle [5].
We first evaluated patients for acute nephrotoxicity at 48 h and chronic nephrotoxicity at 3rd month after first course of cisplatin. Then, we defined the severity of acute kidney injury (early nephrotoxicity) and the severity of chronic kidney injury (late nephrotoxicity) according to NCI CTC AE, version 4.0 criteria. According to acute kidney injury severity criteria, stage 1 was defined as a > 0.3 mg/dl rise in sCr or increase of 1.5- to 2-fold from baseline, stage 2 was defined as a sCr increase > 2.0- to 3.0-fold from baseline, stage 3 was defined as a SCr increase > threefold from baseline or a sCr ≥ 4 mg/dl, stage 4 was defined as a life-threatening conditions or need for renal replacement therapy, stage 5 was defined as death in the patients by acute CIN. According to chronic kidney injury severity criteria/staging, stage 1 was defined as eGFR (estimated Glomerular Filtration Rate) or CrCl (creatinine clearance) > 60 ml/min/1.73 m2 or proteinuria 2 + present; urine protein/creatinine > 0.5, stage 2 was defined as eGFR or CrCl 59—30 ml/min/1.73 m2, stage 3 was defined as eGFR or CrCl 29–15 ml/min/1.73 m2, stage 4 was defined as eGFR or CrCl < 15 ml/min/1.73 m2 or need for renal replacement therapy and transplantation, stage 5 was defined as death in the patients by chronic CIN. Finally, we tried to explore the clinical and laboratory factors in relation with development of nephrotoxicity.
Statistical analysis
Continuous variables are presented as mean (standard deviation) or median (interquartile range [IQR]), whereas categorical variables are described by frequency. The suitability of quantitative data to normal distribution is evaluated with the Shapiro–Wilk test and graphical analysis. Student’s t test was used to compare quantitative variables between two groups that were normally distributed. If the quantitative variables did not showed normal distribution, we used Mann–Whitney U test. Pearson’s Chi-square test, Fisher’s exact test and Fisher–Freeman–Halton tests were used in the comparison of qualitative data. A value of p < 0.05 was considered statistically significant. Analysis was performed using NCSS (Number Cruncher Statistical System) 2007 (Kaysville, Utah, USA).
Results
Medical records and files of 1008 patients with malignancy who were followed up at the medical oncology department of our hospital between January 2013 and July 2019 were reviewed to determine those who received a cisplatin-based chemotherapy regimen. Among them, 228 were found to be treated with cisplatin.
Twenty-five of cisplatin treated patients were excluded from the study because of lack of adequate laboratory or clinical data or refusal to participate in the study. Of the remaining 203 patients, 184 patients had all data necessary to evaluate acute cisplatin nephrotoxicity and 167 was eligible for evaluation of chronic nephrotoxicity.
The mean age of the study patients was 56.44 ± 12.69 years, 78.8% were males and 21.2% were females. The distribution of the patients by malignancy types, their initial and total cisplatin doses (3 months dose), and other therapies simultaneously received with cisplatin, and the treatments administered to prevent nephrotoxicity are given in Table 1.
A total of 184 patients with complete data were analyzed to evaluate cisplatin-induced acute nephrotoxicity. Of these 184 patients, 17 (9.2%) developed cisplatin-induced acute kidney injury. Among patients with cisplatin-induced acute kidney injury, 13 (76.5%) patients had stage 1 patient injury, 1 (5.9%) patient had stage 3 injury and 3 (17.6%) patients had stage 5 injury according to NCI CTC AE. There was no patient who developed stage 2 injury. Actually all patients from stage 5 was defined as stage 4 at beginning of the treatment with cisplatin but since these patients died, the severity of the injury was accepted as stage 5 injury.
Of the 161 patients whose post-treatment 3-month follow-up records could be obtained to evaluate cisplatin-induced chronic nephrotoxicity showed that 61 (37.9%) developed develop chronic nephrotoxicity. Of the patients who developed nephrotoxicity, 46 (75.4%) patients had grade 1 injury, 11 (18.1%) patients had grade 2 injury, 1 (1.6%) patient had grade 3 injury, 1 (1.6%) patient had grade 4 injury, and 2 (3.3%) had grade 5 injury (Table 2).
After the evaluation of the baseline demographic and clinical characteristics of the patients with cisplatin-induced acute nephrotoxicity, we found that female gender, presence of comorbidities, such as diabetes and ischemic heart disease, and using anti-aggregant drugs were significantly increasing the incidence of acute nephrotoxicity (p < 0.05). Although the incidence of cisplatin-induced acute nephrotoxicity was increased in advanced age, the difference was not statistically significant. Considering the baseline laboratory values of the patients, the cisplatin-induced acute nephropathy group had statistically significantly lower albumin and eGFR values and significantly higher urea, creatinine and uric acid levels than the group without acute nephropathy (p < 0.05) (Table 3).
After the evaluation of the demographic and clinical characteristics of the patients with cisplatin-induced chronic nephrotoxicity, we found that advanced age and female gender were significantly increasing the incidence of chronic nephrotoxicity (p < 0.001 and p < 0.048, respectively). There was no correlation between comorbid diseases and chronic nephrotoxicity. Apart from diuretics, there was no correlation between other drugs and the development of chronic nephrotoxicity (p = 0.048; p < 0.05). Those using diuretic drugs had 2.476 times higher risk compared to those who did not use diuretics (OR: 2.476; 95% CI 0.976–6.285). In terms of baseline laboratory values, high baseline urea and creatinine value, and low eGFR value were the main predictors of chronic nephrotoxicity (p < 0.001) (Table 4).
While there was no difference in terms of the risk of developing cisplatin-induced acute nephrotoxicity by cancer types, the risk of developing chronic nephrotoxicity showed a statistically significant difference (p = 0.017; p < 0.05). The difference was due to the high incidence of chronic nephrotoxicity in the group with genitourinary cancer (12 of 16 patients, 75%). There was no correlation between initial cisplatin dose, high-dose administration (> 75 mg/m2), simultaneous radio-therapy, other chemotherapeutics administration, amount of fluid infusion, intravenous magnesium replacement and cisplatin-induced acute and chronic nephrotoxicity. Interestingly, the total dose of cisplatin was significantly lower in the chronic nephrotoxicity group (p = 0.06, p < 0.5).
Discussion
In our study, the retrospective evaluation of patients who received cisplatin-based chemotherapy regimens revealed that the incidence of cisplatin-induced acute nephrotoxicity was 9.2% and chronic nephrotoxicity was 37.9%. The most important predictor of cisplatin-induced both acute and chronic nephrotoxicity was baseline kidney functions. The presence of diabetes mellitus and ischemic heart disease was a risk factor for acute nephrotoxicity, but was not correlated with chronic nephrotoxicity. Cancer type was not a predictor of cisplatin-induced acute or chronic nephrotoxicity, while the incidence of cisplatin-induced chronic nephrotoxicity was observed to increase only in genitourinary malignancies. There was no correlation between administration dose of cisplatin and the development of acute and chronic nephrotoxicity. It was found that cisplatin-induced nephrotoxicity could not be reduced with intravenous administrations of hydration and magnesium.
Reviewing the literature, the incidence of cisplatin-induced acute nephrotoxicity has been reported between 6 and 31.5% [6,7,8,9]. Our study is in line with the literature in terms of the incidence of acute nephrotoxicity. In the literature, there are limited data about cisplatin-induced chronic nephrotoxicity. In our study, we found that 37.9% of the patients developed late nephrotoxicity. The majority of these patients were found to have stages 1 and 2 severity of nephrotoxicity. Large retrospective study in USA was evaluated 821 patients according to acute and chronic nephrotoxicity after cisplatin treatment. The incidence of acute nephrotoxicity was 31.5% and the chronic nephrotoxicity was less than < 3% [9]. In their study, the low incidence of chronic nephrotoxicity was associated with the aggressive hydration protocol and the method of eGFR measurement.
In our study, female gender was correlated with an increase in both acute and chronic nephrotoxicity frequencies. Jong et al. [10] reported that women had twofold increased risk of cisplatin-induced nephrotoxicity. The study by Faig et al. [11] that was including only head and neck tumors showed that the incidence of cisplatin-induced nephrotoxicity was significantly increased in female gender. In an another study, the authors showed that renal clearance of unbound form of cisplatin being 15% lower in women than in men [12]. Female gender is associated with a reduced nephron number and therefore might bring about increase in cisplatin nephrotoxicity [13]. The reason for difference in cisplatin nephrotoxicity between genders may be explained with this pathophysiological mechanism.
In our study, advanced age was correlated with both cisplatin-induced acute and chronic nephrotoxicity; however, the difference was only significant for cisplatin-induced chronic nephrotoxicity. There are many studies in the literature showing an association between advanced age and the increased incidence of cisplatin-induced nephrotoxicity [9, 10, 14]. The increase in the incidence of nephrotoxicity in elderly patients has been attributed to age-related changes in renal vascularity, filtration and tubular functions [15, 16]. However, there is also a study indicating that age is not a risk factor for cisplatin-induced nephrotoxicity [8]. The study by Liu et al. [14] found the incidence of cisplatin-induced acute kidney injury as 9.4% in the group over 60 years of age and as 3.3% in the group fewer than 60 years of age. They evaluated the patient’s kidney functions 30 days after the first dose of cisplatin. However, in our study, cisplatin-induced acute kidney injury was evaluated at 48 h after the administration. The different results may be attributed to the difference in the evaluation method.
There are many studies in the literature showing the relationship between hypoalbuminemia and cisplatin-induced acute kidney injury [6, 7, 10]. Similar to the results of these studies, we also observed that hypoalbuminemia significantly increased the incidence of cisplatin-induced acute kidney injury. Hypoalbuminemia may increase acute kidney injury by increasing the level of free (non-protein bound) cisplatin and the renal clearance of the drug or by causing a change in its half-life [7, 17].
In our study, the patients with cisplatin-induced acute and chronic nephrotoxicity were found to have significantly higher baseline creatinine and urea values and a lower basal eGFR value. Similar to our study, Liu et al. [14] showed that, the mean baseline serum creatinine was higher in the cisplatin-induced acute kidney injury group, while it was lower in the group without acute kidney injury. Moreover, in our study, the incidence of chronic kidney failure was increased in the acute kidney injury group. Von der Vorst et al. showed that the cisplatin-induced acute kidney injury group had significantly higher creatinine value at 3 and 12 months compared to those without acute kidney injury. However, this study included only patients with head and neck cancer, who received radio-therapy in addition to cisplatin, and high-dose cisplatin (100 mg/m2) [18].
Given the comorbidities of the patients, the cisplatin-induced acute kidney injury group had significantly higher rates of diabetes and ischemic heart disease. There was no such correlation in the group with chronic kidney disease. In the literature, some of the studies suggested that diabetes and/or coronary artery diseases pose a risk for cisplatin-induced nephrotoxicity [6, 8, 19, 20]. Diabetes mellitus and coronary artery disease may increase the incidence of cisplatin-induced nephrotoxicity by increasing atherosclerosis and associated glomerulosclerosis [21].
A limitation of our study is that it was a retrospective analysis of the patients. In addition, blood pressure, volume status, accompanying nausea-vomiting history, which may particularly contribute to the development of cisplatin-induced acute nephropathy (CIN), were not evaluated.
In conclusion, cisplatin-induced nephrotoxicity is still an important issue today. Before administration of cisplatin treatment, the physician should be alert in patients with advanced age, female gender and comorbidities including diabetes mellitus and ischemic heart disease. However, baseline kidney functions are the most important risk factor for the development of nephrotoxicity. The prevention of acute kidney injury in patients with high baseline kidney functions will also protect the development of chronic kidney disease.
References
Barton CD, Pizer B, Jones C, Oni L, Pirmohamed M, Hawcutt DB (2018) Identifying cisplatin-induced kidney damage in paediatric oncology patients. Pediatr Nephrol 33(9):1467–1474
Hanigan MH, Devarajan P (2003) Cisplatin nephrotoxicity: molecular mechanisms. Cancer Ther 1:47–61
Almanric K, Marceau N, Cantin A, Bertin É (2017) Risk factors for nephrotoxicity associated with cisplatin. Can J Hosp Pharm 70(2):99–106
Bodnar L, Wcisle G, Gasowska-Bodnar A, Synowies A, Szarlec-Wcislo K, Szczylik C (2008) Renal protection with magnesium subcarbonate and magnesium sulphate in patients with epithelial ovarian cancer after cisplatin and paclitaxel chemotherapy: a randomised phase II study. Eur J Cancer 44:2608–2614
US Department of Health and Human Services: Common Terminology Criteria for Adverse Events (CTCAE version) 4.0 (2020). https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE-4.03-JUNE
Miyoshi T, Misumi N, Hiraike M, Mihara Y, Nishino T, Tsuruta M et al (2016) Risk factors associated with cisplatin-induced nephrotoxicity in patients with advanced lung cancer. Biol Pharm Bull 39:2009–2014
Motwani SS, McMahon GM, Humphreys BD, Partridge AH, Waikar SS, Curhan GC (2018) Development and validation of a risk prediction model for acute kidney injury after the first course of cisplatin. J Clin Oncol 36:682–688
Lavole A, Daniel S, Baudrin L, Gounant V, Ruppert AM, Epaud C et al (2012) Routine administration of a single dose of cisplatin ≥ 75 mg/m2 after short hydration in an outpatient lung-cancer clinic. Bull Cancer 99:E43-348
Latcha S, Jaimes EA, Patil S, Glezerman IG, Mehta S, Flombaum CD (2016) Long-term renal outcomes after cisplatin treatment. Clin J Am Soc Nephrol 11:1173–1179
De Jongh FE, Van Veen RN, Veltman SJ, De Wit R, Van der Burg ME, Van den Bent MJ et al (2003) Weekly high-dose cisplatin is a feasible treatment option: analysis on prognostic factors for toxicity in 400 patients. Br J Cancer 88:1199–1206
Faig J, Haughton M, Taylor RC, D’Agostino RB Jr, Whelen MJ, Porosnicu Rodriguez KA et al (2018) Retrospective analysis of cisplatin nephrotoxicity in patients with head and neck cancer receiving outpatient treatment with concurrent high-dose cisplatin and radiotherapy. Am J Clin Oncol 41:432–440
De Jongh FE, Verweij J, Loos WJ, De Wit R, De Jonge MJ, Planting AS et al (2001) Body-surface area-based dosing does not increase accuracy of predicting cisplatin exposure. J Clin Oncol 19:3733–3739
Luyckx VA, Shukha K, Brenner BM (2011) Low nephron number and its clinical consequences. Rambam Maimonides Med J 2:e0061
Liu JQ, Cai GY, Wang SY, Song YH, Xia YY, Liang S et al (2018) The characteristics and risk factors for cisplatin-induced acute kidney injury in the elderly. Ther Clin Risk Manag 14:1279–1285
Wen J, Zeng M, Shu Y, Guo D, Sun Y, Guo Z et al (2015) Aging increases the susceptibility of cisplatin-induced nephrotoxicity. Age (Dordr) 37:112
Rosner MH (2013) Acute kidney injury in the elderly. Clin Geriatr Med 29:565–578
Ulldemolins M, Roberts JA, Rello J, Paterson DL, Lipman J (2011) The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin Pharmacokinet 50:99–110
van der Vorst MJDL, Neefjes ECW, Toffoli EC, Oosterling-Jansen JEW, Vergeer MR, Leemans CR et al (2019) Incidence and risk factors for acute kidney injury in head and neck cancer patients treated with concurrent chemoradiation with high-dose cisplatin. BMC Cancer 19:1066
Mizuno T, Ishikawa K, Sato W, Koike T, Kushida M, Miyagawa Y et al (2013) The risk factors of severe acute kidney injury induced by cisplatin. Oncology 85:364–369
Bhat ZY, Cadnapaphornchai P, Ginsburg K, Sivagnanam M, Chopra S, Treadway CK et al (2015) Understanding the risk factors and long-term consequences of cisplatin-associated acute kidney injury: an observational cohort study. PLoS ONE 10:e0142225
Thakar CV, Christianson A, Himmelfarb J, Leonard AC (2011) Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol 6:2567–2572
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kamacı, Ş.Ç., Koçak, G., Yeşilova, A. et al. Evaluation of acute and chronic nephrotoxicity in patients received cisplatin-based chemotherapy: has anything changed over time?. Int Urol Nephrol 54, 1085–1090 (2022). https://doi.org/10.1007/s11255-021-02975-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-021-02975-8