Abstract
Background
IgA nephropathy (IgAN), the most common glomerulonephritis in the world, is an important cause of end-stage renal disease (ESRD). It is necessary to explore new prognostic markers for predicting the activity and progress of IgAN. There are few studies on new prognostic markers in IgAN patients with high proportion of glomerulosclerosis. This study aims to explore the value of urine IgG in predicting the prognosis of IgAN patients.
Methods
The primary end point of this retrospective study was a composite event with a reduction in estimated glomerular filtration rate (eGFR) of ≥ 50% or ESRD or death. This study assessed the association between urinary IgG and clinicopathological parameters, as well as the prognosis of a high proportion of patients with global glomerulosclerotic IgAN.
Results
This study included 105 IgAN patients with high proportion of global glomerulosclerotic. The level of urinary protein IgG was significantly correlated with clinical prognostic factors. The level of urinary protein IgG was positively correlated with urinary protein excretion (rs = 0.834, P < 0.001), CRP (rs = 0.375, P < 0.001), and C4 (rs = 0.228, P = 0.019), and negatively correlated with eGFR (rs = – 0.307, P = 0.001). In addition, the level of urinary IgG increased with the increase of tubulointerstitial injury rate, which was positively correlated with endothelial cell proliferation and crescent (all P < 0.05). Prognostic analysis using the Cox proportional hazard regression model and Kaplan–Meier survival curve further determined that urine IgG is an independent risk factor for the prognosis of IgAN with high proportion of global glomerulosclerosis.
Conclusions
This study determined that urine IgG can be used as a useful predictor of the prognosis of IgAN patients with high proportion global glomerulosclerosis. The mechanism of urine IgG trends in IgAN with high proportion of glomerulosclerosis needs further study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
IgA nephropathy (IgAN) is characterized by granular IgA deposition in mesangial area or with IgG, IgM, or both, often accompanied by C3 deposition [1], which is now recognized as the most common primary glomerulonephritis in the world [2]. Currently, about 20–30% of the known patients have disease progression and end-stage renal disease (ESRD) within 20 years after diagnosis [3], and people of Asian Pacific descent have higher risk development to ESRD [4]. Many studies have focused on finding predictors of early IgAN or mild IgAN progression [5, 6], but there are few studies that predict the progress of IgAN with high proportion of global glomerulosclerosis. Although glomerulosclerosis is considered to be a predictor of poor prognosis [7], after years of follow-up of patients with a proportion of not less than 25% of the patients with glomerular sclerosis, a considerable number of patients have stable renal function [8]. For IgA nephropathy with high proportion of global glomerulosclerosis, the speed of disease progression and the response to treatment are different. In addition to non-immune factors, it may be related to different degrees of proliferative lesions, but repeated renal biopsy cannot be carried out at any time, so it is very necessary to explore the related clinical indicators.
Proteinuria is one of the most powerful indicators of IgAN progress [9, 10]. Urine IgG has been widely measured in clinical practice for many years. Studies have shown that urine IgG is a predictive indicator of various kidney diseases [11, 12], but its clinical significance is still uncertain for predicting the development of IgAN with high proportion of global sclerosis. To determine the value of urine IgG in predicting the prognosis of IgAN patients with high proportion of global sclerosis, we conducted a study on 105 high-scale global sclerosis IgAN patients and retrospectively analyzed the relationship between urine IgG and clinical pathological parameters and prognosis of IgAN patients with high proportion of glomerulosclerosis.
Materials and methods
Patients
The present study included adult patients with primary IgAN consecutively diagnosed by renal biopsy in the Guangdong Provincial Hospital of Traditional Chinese Medicine from September 1, 2005 to December 31, 2017. Primary IgAN is defined as the main mesangial IgA, which contains immune complexes detected by immunofluorescence and light microscopy [11]. The inclusion criteria are as follows: (1) the age was between 18 and 65 years; (2) there were more than 8 glomeruli in biopsy; (3) not less than 25% of the glomeruli show global glomerulosclerosis. Exclusion criteria include: (1) ESRD or major coexisting diseases that may affect survival at the time of admission; (2) suffering from other glomerular diseases or systemic diseases (including but not limited to patients with systemic lupus erythematosus, rheumatism, liver disease, and diabetes); (3) patients with insufficient clinicopathological data; (4) patients with a follow-up time of less than 6 months and no end point. (5) Interstitial fibrosis and tubular atrophy are caused by drug. The renal end point was defined as the composite end point event used in this study, reaching eGFR reduction ≥ 50%, blood creatinine doubling, progression to ESRD (eGFR < 15 ml/min/1.73 m2, permanent hemodialysis, peritoneal dialysis, or kidney transplantation), and death. This study was approved by the Ethics Committee of Guangdong Hospital of Traditional Chinese Medicine. The Committee abandoned the need for personal consent.
Clinical data
All clinical data were obtained from the patient’s medical history, sociodemographic characteristics, medical history, and current medications were recorded, and blood and urine samples were collected to measure study variables. Variables included gender, age, hospital stay, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), serum creatinine (SCR), eGFR, uric acid (UA), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL), electrolyte, C-reactive protein (CRP), albumin (ALB), serum immunoglobulin A (IgA), serum immunoglobulin G (IgG), serum complement 3 (C3), serum complement 4 (C4), serum total complement active complement (CH5O), urine red blood cell count (uRBC), 24-h proteinuria quantification (24hupro), and urine immunoglobulin G (IgGU).
Renal pathology data
Renal biopsies were reviewed by two pathologists who were blinded to patients’ outcome and were scored according to the updated Oxford Classification (MEST-C). The histologic classification was defined as follows: mesangial hypercellularity, mesangial score of ≤ 0.5 (M0) or > 0.5 (M1); endocapillary hypercellularity absent (E0) or present (E1); segmental glomerulosclerosis/adhesion absent (S0) or present (S1); tubular atrophy/interstitial fibrosis ≤ 25% (T0), 26–50% (T1), > 50% (T2); and cellular or fibrocellular crescents absent (C0), present in less than one-fourth of glomeruli (C1), crescents in at least 25% of glomeruli (C2) [13, 14], due to the small number of C2 cases, C1 and C2 were combined into a group for analysis. Focal glomerulosclerosis is defined as hyaluronan deposition or scarring in not less than 25% of the glomeruli.
Treatment
According to the treatment plan, we divide the patients into immunosuppressive therapy [glucocorticoid, immunosuppressant (cyclophosphamide, mycophenolate mofetil, tacrolimus, etc.), Chinese patent medicine with immunosuppressive effect (Tripterygium wilfordii, Kunxian capsule, etc.)] and non-immunosuppressive therapy, and no patients choose bias. The prescription of the drug is based on each the doctor’s own decision.
Statistical analysis
SPSS 23.0 software (SPSS Company, Chicago, Illinois, USA) and MedCalc15 software were used for statistical analysis. Skewed distributed continuous variables were expressed as median and interquartile range and compared with the non-parametric test. Normally distributed continuous variables were expressed as the means ± SD and compared with the T test, and categorical variables were expressed as absolute frequencies and percentages and compared with the Chi-square test. Spearman correlation was applied to analyze the relationship between IgGU and clinical indicators and pathology. To compare IgGU levels between different grades of pathological parameters, non-parametric tests were performed. To determine the independent prognostic value of IgGU levels, the receiver-operating characteristic (ROC) curves calculated the area under the curve (AUC) with 95% confidence intervals (95% CI) were used to compare 24hupro with UIgG risk score, the Cox proportional hazard regression model was used for univariate and multivariate analyses using the "input" method, and the results were expressed as hazard ratio (HR) and 95% confidence interval (CI). In addition, we divided the IgGU levels into two categories according to the median: low (≤ 85.79 mg / L) and high (> 85.79 mg / L). Kaplan–Meier survival analysis was performed to evaluate the ability of IgGU to predict the prognosis of IgAN patients. If both sides p < 0.05, it is considered statistically significant.
Results
Characteristics of the patients
During the period from September 1, 2005 to December 31, 2017, a total of 180 patients with global sclerosis rate of no less than 25% were collected in Guangdong Hospital of Traditional Chinese Medicine, and finally, 105 patients met the inclusion and exclusion criteria and entered the study. Patients were followed up with renal puncture day as the starting point, composite terminal event or January 31, 2020 or lost visit day as the end point and the median follow-up time was 37 (27.5, 59) months, a total of 35 patients (33.3%) had a composite end point event. The mean age was 36 (28, 43) years at baseline, 51.4% of patients were females, the SBP was 132.00 (125.00, 142.50) mmHg, the DBP was 86.10 ± 14.52 mmHg, MAP was 101.67 (92.17, 109.00) mmHg, eGFR was 51.96 (29.95, 77.25) ml/min/1.73 m2, and 24hupro was 1.31 (0.61, 2.46) g/d. 61 patients were treated with immunosuppressive therapy.
Correlations between urine protein IgG and clinical parameters
In terms of correlation between IgGU and clinical indicators, in terms of renal function, IgGU was negatively correlated with eGFR (rs = – 0.307, P = 0.001) and positively correlated with Cr (rs = 0.291, P = 0.003); in terms of urine test, IgGU was positively correlated with 24hupro (rs = 0.834, P < 0.001) and uRBC (rs = 0.321, P = 0.001); IgGU was negatively correlated with ALB (rs = – 0.604, P < 0.001) and TP (rs = – 0.510, P < 0.001); in the aspect of blood lipid, IgGU was positively correlated with TC (rs = 0.427, P < 0.001), TG (rs = 0.247, P = 0.001), and LDL (rs = 0.384, P < 0.001); in the aspect of immunity, IgGU was negatively correlated with IgG (rs = − 0.442, P < 0.001), while IgGU was positively correlated with CRP (rs = 0.375, P < 0.001) and C4 (rs = 0.228, P < 0.001) (Table 1).
Correlations between urine protein IgG and histopathological parameters
For endothelial cell proliferation, IgGU was positively correlated with E score (rs = 0.340, P = 0.001), and the level of IgGU in E1 patients (170.00, IQR 95.26–593.75) was significantly higher than that in no E0 patients (70.30, IQR 22.31–157.62) (P = 0.001). The level of IgGU was not related to segmental glomerulosclerosis. There was a positive correlation between IgGU and T score (rs = 0.394, P < 0.001), and the increase of IgGU was parallel to the severity of tubulointerstitial lesions (T0, 22.50, IQR 12.30–74.20; T1, 68.15, IQR 29.95–164.53; t2130.00, IQR 59.65–283.50; P 0,1 = 0.013, P 1,2 = 0.026, P 0,2 < 0.001). There was a positive correlation between IgGU and C score (rs = 0.231, P = 0.018), and the level of IgGU in crescent was higher than that in non-crescent (C0, 70.68, IQR 21.20–142.00; C1&2, 100.22, IQR 39.65–262.25; P = 0.019) (Table 1; Fig. 1).
Comparison between 24hupro and UIgG
The area under the ROC curve of UIgG is 0.724 (95%CI 0.629–0.807) and the area under the ROC curve of 24upro is 0.736 (95%CI 0.641–0.817); the difference of the area under the ROC curve between the two tests is 0.0114, the Z statistic is 0.376, P = 0.7070, and there is no statistically significant difference between the two tests in prognostic prediction Fig. 2.
Correlation between urine protein IgG levels and prognosis
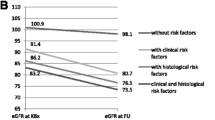
Univariate analysis of the Cox proportional hazard regression model determined that SBP, MAP, LDL, 24hupro, IgGU, SCr, UA, immunosuppressive therapy, renal tubular atrophy/interstitial fibrosis, crescent, and global sclerosis rates were risk factors for the occurrence of the composite endpoint; IgG, ALB, eGFR, and RASIs’ treatment are protective factors for the occurrence of the composite endpoint. Multivariate analysis of Cox proportional hazard regression model (Model 1) further validates IgGU as an independent risk factor for the composite endpoint of IgAN [hazard ratio (HR) 1.004, 95% confidence interval (CI), 1.000–1.008, P = 0.038]; we added traditional factors (age, immunosuppressive therapy) to another multivariate model (model 2), and the results showed that IgGU was an independent risk factor for the IgAN composite endpoint [HR 1.004, 95% CI, 1.000–1.009, P = 0.042)]. Kaplan and Meier survival analysis showed that two groups of patients with low and high urine protein IgG statistical differences in renal cumulative survival rate (P < 0.001) (Fig. 1), the group of patients with lower urinary protein IgG, median renal cumulative survival time was 113 month higher than group a high urinary protein IgG renal 61 month cumulative survival time, and Kaplan–Meier survival curve shows that the urine protein IgG group has a good degree of differentiation, predictable high proportion of global to the prognosis of patients with IgAN glomerular sclerosis Table 2; Fig. 3.
Discussion
IgAN is a chronic progressive glomerular disease, but the rate of progression is very heterogeneous. Because high proportion of global glomerulosclerosis is accompanied by different degrees of proliferative lesions, the prognosis is different. At present, there is still a lack of research on the prognostic indicators of patients with IgAN with a global sclerosis rate greater than 25%. Proteinuria is a risk factor for the progression of IgAN [9, 10]. Due to the difference of protein group size and charge, the clinical significance of different urinary microproteins is different. Urinary IgG is a macromolecular protein with molecular weight of 160kd, which is generally discharged in large quantities when the glomerulus is seriously damaged, such as endothelial cell proliferation and crescent [11], forming the so-called non selective proteinuria in clinic. Urine IgG is a predictive indicator of various kidney diseases [11, 12], according to previous studies [15, 16], UIgG indicators are included in the risk stratification of membranous nephropathy in the 2020 KDIGO guidelines (UIgG < 250 mg/d is medium risk; UIgG > 250 mg/d is high risk), but its clinical significance is still uncertain for the progress of predicting a high proportion of global sclerosis IgAN.
In this study, urine IgG was positively correlated with E score and C score. Claudio Bazzi et al. [11] compared 37 patients with cell crescent with 111 patients without any type of crescent, and found that the urine IgG of the patients with cell crescent was six times higher than that of the patients without crescent, and there was a positive correlation between the urine IgG and cell crescent (r = 0.414, P = 0.01). It is suggested that when the global sclerosis ratio is not less than 25%, pathological changes of endothelial cell proliferation and crescent formation damage the basement membrane, which causes large amounts of large protein IgG to be excreted. In addition, urinary IgG is positively correlated with C4, and complement component is a well-known acute phase protein, including C3 and C4 [17]. Studies have shown that C4 level may damage kidney through mannose-binding lectin pathway, and C4 is related to severe renal pathological changes and independent risk factor for prognosis [18]. There was a positive correlation between urinary IgG and the clinical indexes of 24hupro, uRBC indicating active pathological changes. It is suggested that urine IgG can reflect the degree of active renal inflammation damage.
Urine IgG was positively correlated with the T score, but not with the global proportion of sclerosis in our study. Previous studies have found that high-molecular-weight proteinuria is also associated with proximal tubular cell apoptosis [19]. When the primary and HK-2 proximal renal tubular cells were exposed to human plasma-derived high-molecular-weight fractions (100–440 kDa), they showed characteristics consistent with the pro-apoptotic phenotype, namely Fas and FasL expression. This is invisible when cells are exposed to low-molecular-weight proteins [20]. Therefore, it is reasonable to conclude that long-term exposure of proximal tubular cells in the body to high-molecular-weight proteins will produce toxicity and promote tubular atrophy and interstitial fibrosis. It is suggested that in patients with IgA nephropathy with a global hard rate greater than 25%, urinary IgG will aggravate the damage of tubule interstitial, and can reflect the degree of tubule atrophy and interstitial fibrosis. Urine IgG is positively correlated with Cr and negatively correlated with eGFR, suggesting that urine IgG is related to abnormal renal function, which is consistent with the previous studies [21, 22]. It is suggested that urinary IgG can reflect the degree of chronic renal pathological damage.
After ROC curve analysis, there was no significant difference in the prognosis between the two tests. 24hupro was a powerful predictor of the progression of IgA nephropathy, and UIgG was a component of urinary protein, suggesting that UIgG has a certain predictive value for the progression of IgA nephropathy with a high proportion of global glomerulosclerosis. 24hupro test needs to collect 24 h of urine, the operation is more troublesome, and diet will have a certain impact on the results, but the test of UIgG does not have the above inconvenience, in clinical operation is more convenient, higher accuracy.
Previous studies on other kidney diseases have suggested that urine IgG is a predictor of prognosis. Wetzels and colleagues show that total excretion of urine IgG in a 24-h urine sample can predict decreased renal function and preserve renal function in patients with idiopathic membranous nephropathy [16]. Bazzi et al. [11] studied the partial excretion of IgG and α1 microglobulin (Mol Wt = 26–33 kDa) in 37 patients with crescent-type IgA nephropathy as indicators for predicting end-stage renal failure or doubling of serum creatinine. Survival analysis in this study suggests that in patients with IgA nephropathy with a global sclerosis ratio > 25%, the cumulative renal survival rate of high urine IgG is lower than that of urine IgG; independent risk factors. It suggests that urine IgG has predictive value for the prognosis of different kidney diseases.
However, this study has some limitations. First, the included sample size is small, the follow-up time is relatively short, the number of cases with composite endpoint events is small, and the statistical power is limited. Second, as a single-center study, our study cannot exclude the limitations of race and region, so its external validity may be limited. Finally, we have not studied the mechanism of urinary IgG increase in the process of global sclerosis > 25% IgAN, which should be discussed in future studies.
Conclusions
In conclusion, urine IgG levels in patients with global sclerosis not less than 25% IgAN are significantly correlated with recognized IgAN clinical pathological prognostic factors, and urine IgG is associated with active lesions of high proportion of global glomerulosclerosis and indicates a worse prognosis, which is a very good non-invasive index. However, the mechanism of elevated urine IgG must be further studied.
Data availability statement
The data used to support the findings of this study are restricted by the Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine to protect the patient privacy. Data are available from Guangdong Provincial Hospital of Chinese Medicine for researchers who meet the criteria for access to confidential data.
References
Roos A et al (2006) Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol 17(6):1724–1734
Cheung C, Bashir S, Barratt J (2014) IgA nephropathy. Br J Hosp Med (Lond) 75(Suppl 11):C173–C176
D’Amico G (2000) Natural history of idiopathic IgA nephropathy: role of clinical and histological prognostic factors. Am J Kidney Dis 36(2):227–237
Barbour SJ et al (2013) Individuals of Pacific Asian origin with IgA nephropathy have an increased risk of progression to end-stage renal disease. Kidney Int 84(5):1017–1024
Knoop T et al (2017) Long-term outcome in 145 patients with assumed benign immunoglobulin A nephropathy. Nephrol Dial Transplant 32(11):1841–1850
Walsh M et al (2010) Histopathologic features aid in predicting risk for progression of IgA nephropathy. Clin J Am Soc Nephrol CJASN 5(3):425–430
Coppo R (2017) Clinical and histological risk factors for progression of IgA nephropathy: an update in children, young and adult patients. J Nephrol 30(3):339–346
Wang J et al (2020) The role of hypertriglyceridemia and treatment patterns in the progression of IgA nephropathy with a high proportion of global glomerulosclerosis. Int Urol Nephrol 52(2):325–335
Donadio JV et al (2002) Proteinuria patterns and their association with subsequent end-stage renal disease in IgA nephropathy. Nephrol Dial Transplant 17(7):1197–1203
Reich HN et al (2007) Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol 18(12):3177–3183
Bazzi B et al (2009) In crescentic IgA nephropathy, fractional excretion of IgG in combination with nephron loss is the best predictor of progression and responsiveness to immunosuppression. Clin J Am Soc Nephrol 4(5):929–935
Bazzi C et al (2001) Urinary excretion of IgG and alpha(1)-microglobulin predicts clinical course better than extent of proteinuria in membranous nephropathy. Am J Kidney Dis 38(2):240–248
Cattran DC et al (2009) The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int 76(5):534–545
Trimarchi H et al (2017) Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int 91(5):1014–1021
Branten AJ, du Buf-Vereijken PW, Klasen IS et al (2005) Urinary excretion of beta2-microglobulin and IgG predict prognosis in idiopathic membranous nephropathy: a validation study. J Am Soc Nephrol 16(1):169–174
Reichert LJ, Koene RA, Wetzels JF (1997) Urinary IgG excretion as a prognostic factor in idiopathic membranous nephropathy. Clin Nephrol 48(2):79–84
Gabay C, Kushner I (1999) Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340(6):448–454
Bi T et al (2019) Serum complement C4 is an important prognostic factor for IgA nephropathy: a retrospective study. BMC Nephrol 20(1):244
Abbate M, Zoja C, Remuzzi G (2006) How does proteinuria cause progressive renal damage. J Am Soc Nephrol 17(11):2974–2984
Morais C et al (2005) High molecular weight plasma proteins induce apoptosis and Fas/FasL expression in human proximal tubular cells. Nephrol Dial Transplant 20(1):50–58
Berg UB, Bohman SO, Widstam-Attorps U (1991) Renal histological changes in relation to renal function and urinary protein excretion in IgA nephropathy. Arch Dis Child 66(5):593–597
Tencer J, Bakoush O, Torffvit O (2000) Diagnostic and prognostic significance of proteinuria selectivity index in glomerular diseases. Clinica Chimica Acta Int J Clin Chem 297:73–83
Acknowledgements
The study was funded by Research Project for Practice Development of National TCM Clinical Research Bases (Project no. JDZX2015202) and National Key R&D Program "Research on Modernization of Traditional Chinese Medicine" (Project no. 2019YFC1709903).
Author information
Authors and Affiliations
Contributions
Research idea, study design, article writing, and guidance on revision: CZ; article writing and revision: XX; article revision and submission: XH; data collection, collation, and summary: YC, JL, MS, YH, and XL; provide medical records and renal biopsy specimens: QL, XL, KB, LW, and LL; pathological diagnosis: HY.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest that may influence the results.
Ethical approval
The study was approved by the Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, X., Huang, X., Chen, Y. et al. The role of urine IgG in the progression of IgA nephropathy with a high proportion of global glomerulosclerosis. Int Urol Nephrol 54, 323–330 (2022). https://doi.org/10.1007/s11255-021-02858-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-021-02858-y