Abstract
Background
Delayed graft function (DGF) is a manifestation of acute kidney injury uniquely framed within the transplant process and a predictor of poor long-term graft function1. It is less common in the setting of living donor (LD) kidney transplantation. However, the detrimental impact of DGF on graft survival is more pronounced in LD2.
Purpose
To study the effects of DGF in the setting of LD kidney transplantation.
Methods
We performed a retrospective analysis of LD kidney transplantations performed between 2010 and 2018 in the UNOS/OPTN database for DGF and its effect on graft survival.
Results
A total of 42,736 LD recipients were identified, of whom 1115 (2.6%) developed DGF. Recipient dialysis status, male gender, diabetes, end-stage renal disease, donor age, right donor nephrectomy, panel reactive antibodies, HLA mismatch, and cold ischemia time were independent predictors of DGF. Three-year graft survival in patients with and without DGF was 89% and 95%, respectively. DGF was the greatest predictor of graft failure at three years (hazard ratio = 1.766, 95% CI: 1.514–2.059, P = 0.001) and was associated with higher rates of rejection (9% vs. 6.28%, P = 0.0003). Among patients with DGF, the graft survival rates with and without rejection were not different.
Conclusion
DGF is a major determinant of poor graft functional outcomes, independent of rejection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kidney transplantation is the treatment of choice for the majority of end-stage renal disease (ESRD) patients. It is associated with lower mortality rates and improved quality of life compared to long-term dialysis [1]. However, there exists a shortage of donor kidneys. LD kidney transplantation addresses this public health crisis. LD kidney transplants are associated with superior recipient and graft survival [2], as well as lower incidence of DGF [3, 4]. The incidence of DGF ranges from 27 to 30.4% [4,5,6] in DD and 1.6 to 3.6% in LD kidney transplantation [3, 7].
The most commonly employed definition for DGF is the need for dialysis within 1 week following transplant [8,9,10]. It is a manifestation of acute kidney injury (AKI) uniquely attributed to the transplant process. DGF adversely affects long-term graft function [2, 11, 12] and is associated with an increased incidence of rejection [3, 8, 13, 14] with rates as high as 40–50% [15, 16]. The pathogenesis of DGF is complex, with multiple donor, recipient, and transplant-specific variables contributing to the development of AKI [3, 14]. While risk factors for DGF in the setting of DD kidney transplantation have been well-chronicled, those for LD kidney transplantation are less well understood. Furthermore, identifying the variables which contribute to LD DGF is of considerable importance, as the impact of DGF on graft survival is more significant than in DD transplants [3, 13].
Our aim was to examine known donor, recipient, and surgical risk factors for DGF through a retrospective review of LD transplants that occurred between 2010 and 2018 in the UNOS database. We also sought to elucidate the impact of DGF on long-term graft outcomes by analyzing its effect on 3-year graft survival and rates of rejection.
Methods
Using data from the UNOS/OPTN registry, we conducted a retrospective analysis of all kidney transplantations performed between Jan-01-2010 and Aug-02-2018. This report is in accordance with principles outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism. The recipient population was divided into two groups based on the presence or absence of DGF. This was defined as the requirement for dialysis within 1 week of transplant. Exclusion criteria included multiple organ transplantation, hyperacute rejection, technical failures, and primary nonfunction. Warm ischemic time was not coded in the UNOS data. Demographic factors were compared using t test and chi-squared test. Variables found to be statistically significant in univariate analysis were then included in construction of multivariate models for calculating odds ratio (OR) for DGF using a logistic regression model. A cox proportional hazards model was utilized to calculate the hazard ratio (HR) for the above-mentioned variables on 3-year graft survival. We employed Stata, version 14.0, for statistical analysis.
Results
Recipient and donor characteristics
120,033 kidney transplant recipients were identified between Jan-01-2010 and Aug-02-2018, including 42,736 LD recipients and 77,297 DD recipients. Of the LD recipients, 1115 developed DGF (2.6%). Seven patients (0.02%) suffered primary nonfunction and were excluded from analysis. Baseline characteristics are included in Table 1. Those with DGF had a significantly greater mean age and body mass index (BMI), and were more likely to be African American, diabetic, and hypertensive. The DGF group had a higher mean serum creatinine, were more likely to require pretransplantation dialysis and had a greater mean dialysis duration, and were more likely to have ESRD, defined as estimated GFR (eGFR) < 15 mL/min. Recipients who developed DGF had a greater mean human leukocyte antigen (HLA) mismatch, mean panel reactive antibodies (PRA), and ABO incompatibility. CIT was longer in DGF group with a mean of 2.83 vs. 2.17 h (P < 0.001). In addition, individuals with DGF were less likely to have undergone preemptive transplantation and had a higher median days on the waitlist.
Donors of grafts that developed DGF had a higher mean age and BMI. They were also more likely to be African American (AA).
DGF risk factors
37,058 LD recipients had data available for logistic regression analysis. The risk factors for DGF are shown in Table 2. Five of these variables were recipient characteristics, namely pretransplantation dialysis, ESRD, diabetes, male gender, and polycystic kidney disease (PKD). Pretransplantation dialysis was the greatest risk factor for DGF (OR 2.75, 95% CI 2.24–3.37, P < 0.001), which corresponded to the increased odds seen in recipients with ESRD. These were followed by diabetes and male gender. Diagnosis of PKD was associated with a 30% decreased risk of DGF (P = 0.014).
Among surgical factors, right kidney donor nephrectomy increased the risk of DGF by 66% (P < 0.001). Operative technique was not included for final analysis due to insufficient data in the UNOS database.
Recipient BMI and Transplant recipient registration (TRR) serum creatinine minimally increased the odds of DGF on multivariate analysis. Recipient age was no longer significant. Although ESRD increased risk of DGF, preemptive transplantation, defined as transplantation performed before initiation of maintanence dialysis [17], did not decrease the risk of DGF.
Among donor factors, age and BMI marginally increased the risk of DGF. Greater PRA, higher HLA mismatch, and longer CIT also slightly increased the risk of DGF. We compared the difference between donor and recipient BMI, and the average donor BMI was 26.90; (SD = 4.11; 95% CI is 26.86–26.94) and average recipient BMI was 27.73; (SD = 5.47; 95% CI is 27.67–27.78). After doing a paired t test analysis we obsereved that average donor BMI was significantly less than average recipient BMI; (P < 0.0001). We also analyzed if donor BMI had an effect on the incidence of DGF by dividing arbitrarily into BMI of < 18, between 18 and 27, above 27, and observed that higher donor BMI increased the odds of DGF; BMI > 27 increased the odds of DGF by 15% compared to donor BMI category of 18–27 (P value = 0.03, 95% CI 1.01–1.31).
Among the other variables investigated, recipient history of hypertension, recipient ethnicity, and previous transplants did not substantially impact the odds of developing DGF. Live donor ethnicity was not predictive of DGF. Recipient glomerulonephritis (GN) and interstitial nephritis (IN) were commonly coded diagnoses in the UNOS database which did not influence the odds of DGF on multivariate analysis.
Graft survival
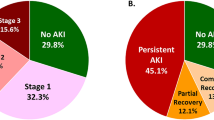
We performed a Cox regression analysis to identify factors which predict risk of graft failure at 3 years posttransplantation (Table 3). 37,318 patients (87%) had 3-year graft function data available in the UNOS database. Three-year graft survival in patients with and without DGF was 89% and 95%, respectively (Fig. 1). DGF was the greatest predictor of graft failure at 3 years, with an increased risk of 77% (HR 1.77, 95% CI 1.51–2.06, P < 0.001). Recipient dialysis before transplantation increased the risk of graft failure by 62% (P < 0.001). Notably, recipient African American race increased the risk of graft failure (HR 1.312, 95% CI 1.21–1.43, P < 0.001), but did not affect the odds of DGF. Greater PRA and HLA mismatch marginally increased the risk of graft failure, similar to their effect on DGF, as did recipient age. Right-sided donor nephrectomy, duration on the transplant waitlist, and recipient gender did not affect the risk of graft failure (see Table 4).
Rejection
Finally, we assessed the correlation between DGF and rejection, and also sought to determine whether rejection mediates the detrimental impact of DGF on graft survival rates. There were too many missing data points for analysis of rejection with regard to acute versus chronic. The rate of graft rejection at 3 years was significantly higher in patients with DGF compared to those without (9% vs. 6.28%, P = 0.0003). However, we did not observe a significant increase in graft loss secondary to rejection in patients with DGF. In patients with DGF who developed rejection, the 3-year graft survival rate was 78.5%, while it was 80.2% with DGF alone (P = 0.6).
Discussion
In this retrospective analysis of UNOS LD kidney transplantation data between 2010 and 2018, we identified several recipient, donor, and technical attributes with notable impact on the development of DGF. We also demonstrated that DGF is the most important correlate with decreased 3-year graft survival. Severity of pre-existing kidney disease, as measured by both need for dialysis prior to transplant and ESRD, male gender, diabetes, PKD, and right-sided donor nephrectomy were significant predictors of DGF. While most of our results are in accordance with other reports, some were in contrast.
Maintenance dialysis prior to transplant increased the odds of DGF by 174%, similar to earlier reports [3, 18, 19]. Need for pretransplant dialysis correlates with severity of renal disease. Chronic kidney disease triggers chronic inflammation, which becomes maladaptive and contributes to the uremic phenotype, namely cardiovascular disease, protein energy wasting, depression, osteoporosis, and frailty and is a predictor of both cardiovascular and total mortality [20]. We did not observe decreased incidence of DGF with preemptive renal transplant, defined as transplantation done before starting long term dialysis. However, Asderakis et al. observed lower incidence of DGF and improved graft function at 1, 5, and 10 years in preemptively transplanted patients compared to those who received pretransplantation dialysis [21]. In a large retrospective analysis by Bertoni et al., preemptive transplant was associated with less DGF, better graft, and patient survival rate [22]. Thus, our observation is in contrast to previous reports, probably due to low numbers of preemptive LD transplants in the UNOS database. It should be noted that DGF is more difficult to diagnose in preemptive transplants, and residual renal function may allow dialysis avoidance in those patients who would otherwise have been diagnosed with DGF. Improvements in detection of DGF in these patients is needed in addition to a more comprehensive definition of DGF to include them.
We also observed that diabetes imparts a 50% greater likelihood of developing DGF. Parekh et al. analyzed the role of diabetes in the development of DGF in DD kidney transplantation and demonstrated that it was associated with increased risk [23]. While they did not identify a specific reason to explain why diabetes may lead to DGF, they proposed several potential mechanisms. Diabetes may pose an increased technical challenge surgically, as it is often associated with obesity and atherosclerotic vascular disease, which could lengthen warm ischemia time. Unfortunately, warm ischemia time could not be included in our analysis. Likewise, diabetics have a higher incidence of cardiovascular events after kidney transplant [24] and may be predisposed to hemodynamic instability, which can contribute to development of DGF. Furthermore, diabetic recipients may suffer more severe ischemia–reperfusion injury, a known contributor to DGF [14], as various reports have shown diabetes to be associated with chronic inflammation and increased oxidative stress. Schachtner et al. investigated the contribution of obesity-related comorbidities to adverse outcomes in deceased kidney transplantation. They found that among obese recipients, only those with diabetes were observed to have inferior patient and allograft survival, worse allograft function, and delayed graft function [25]. No differences were observed for normal weight diabetics or obese non-diabetics. In addition, obese diabetic recipients had significantly higher frequencies of donor-reactive T-cell posttransplantation [25]. Their results indicate that the combination of obesity-related inflammation and hyperglycemia may trigger increased alloreactivity. Another analysis reviewed the relationship between pretransplant blood glucose levels and the occurrence of DGF among kidney transplant recipients without a prior diagnosis of diabetes mellitus. A significant correlation between hyperglycemia and risk of DGF was found [26]. Taken together, we suggest that LD kidney transplant recipients with diabetes should be monitored for appropriate immunosuppression, strict blood glucose control, and hemodynamic parameters.
Obesity marginally increased the risk of DGF in our analysis. In a meta-analyis of 138,081 patients Hill et al. observed an increased risk of DGF and graft loss in obese kidney transplant recipients. There was a 68% increased incidence of DGF in their report. Molnar et al. hypothesized that vasospasm due to sympathetic overacticity and longer operative time due to obesity are causal reasons for DGF [27]. In addition, postsurgical complications were higher in obese patients [28]. Obesity results in elevated levels of proinflammatory cytokines that may mediate glomerular injury, such as tumour necrosis factor alpha, as well as adipokines released from adipose tissue that can cause endothelial dysfunction. Both may contribute to a higher rate of graft loss [29]. Interestingly, survival of obese transplant recipients was not inferior to patients with normal BMI in the analysis by Hill et al. Increasing donor BMI also correlated with higher risk of DGF, as noted in previous studies [19].
Male recipient gender increased the risk of DGF by 30%, similar to previous reports [3, 18]. However, it is unclear whether male gender truly increases the risk of DGF or whether female gender decreases the risk. Potential explanations include that males may be more susceptible to ischemia–reperfusion injury, due to testosterone [30]. Estrogen also plays a protective role in preventing ischemia reperfusion injury [31]. In addition, size mismatch between opposite gender donor and recipient blood vessels may favor a female recipient [32]. Female to male live donor transplantation in DD is associated with inferior graft outcomes, as the smaller allograft functional mass from a female may not meet the increased metabolic demands of a male [33], although this has not been reported in LD transplantation.
Right donor nephrectomy was associated with a 65% higher chance of DGF. Shorter donor renal vein length relative to the renal artery may create a more technically challenging anastomosis, resulting in longer operative times, as well as a higher chance of conversion to open nephrectomy. Unfortunately, data for open conversion rates were not available. Özdemir-van Brunschot et al. report prolonged warm ischemia time, higher technical failure rate, and elevated serum creatinine at 3 months post transplant with implantation of right renal allografts compared to left, using data from Dutch Organ Transplant Registry. Interestingly, this difference was unique to live donors and was not seen with deceased donor transplantation [34]. The plausible explanation for this difference is the shorter renal vein with live donors makes vascular anastomosis more challenging, while right kidneys from deceased donors usually have a renal vein with a caval patch. Also, the relatively shorter right renal vein compared to renal artery can be easily compressed by hematoma or urinary obstruction [35].
Prior to widespread paired organ donation programs, Simpkins et al. queried the UNOS database for the effect of CIT on long-term graft functional outcomes in LD kidney transplantation. Recipients were grouped based on CIT: 0–2, 2–4, 4–6, and 6–8 h, with 85.1% in the 0- to 2-h reference group. While low for all groups, the adjusted probability of DGF increased with increased CIT duration, but was only significant on comparison of the 4- to 6-h and 0- to 2-h groups. There was no significant difference in graft loss between all CIT groups during 10 years posttransplantation [36]. Similarly, Treat et al. compared the outcomes for shipped (mean CIT of 12.1 h) and non-shipped donor kidneys (mean CIT of 1.0 h), which demonstrated that while DGF incidence was slightly greater in the shipped cohort (1.8% vs. 0%), 1-year graft survival was identical between the two groups [37]. Gill et al. evaluated even longer CITs in 48,498 LD recipients from the SRTR registry. Only CITs of 8.1–16 h had increased odds of DGF and CITs of fewer than 16 h showed no correlation with allograft loss of any cause [38]. Krishnan et al. analyzed 3717 live donor recipients from Australia and New Zealand between 1997 and 2012 and observed that each hour of increase in CIT was associated with an adjusted OR of 1.14 for DGF. However, age was determined to be an effect modifier. In recipients who received kidneys from older donors (> 50 years), the adjusted OR for DGF was 1.28, while no association between CIT and DGF was observed for younger donor kidneys. Prolonged CIT was also associated with worse long-term graft outcomes, as demonstrated by lower 5- and 10-year graft survival rates for CIT of > 4–8 h [11]. In 2019, Nassiri et al. published an analysis evaluating the impact of donor age and CIT on DGF using the National Kidney Registry database. Their findings opposed those of Krishnan et al. in that they observed no significant association between donor age or CIT and DGF, with significant implications for utilization of kidneys from older donors or those with prolonged shipping times [39]. In the present analysis, we did observe a trend of increased DGF with prolonged CIT. However, it was marginal and no correlation was seen between CIT and graft survival at 3 years. It should be noted that due to limited data in the UNOS database, we did not stratify recipients into groups based on increasing CIT. Rather, we dichotomously divided them based on whether CIT was fewer or greater than 2 h.
Ethnic disparities in graft survival outcomes are well documented. AA individuals more commonly harbor gene polymorphisms in apolipoprotein APOL1, which predisposes to chronic kidney disease and end stage renal disease at a younger age [40]. Our results demonstrated African American to be associated with increased incidence of acute rejection, as well as poor graft survival outcomes [41, 42]. Socioeconomic status and transplant center outcome differences are also potential reasons for this finding [42]. We did observe a trend towards increased incidence of DGF in African American individuals. However, it was not significant on multivariate analysis.
Among immunological factors, elevated PRA and HLA mismatch marginally increased the odds of DGF, which supports the role of recipient immune system in promoting pathologic responses to an ischemic insult. DGF leads to upregulation of HLA class I and II antigens with enhanced expression of adhesion and costimulatory molecules, leading to enhanced immunogenicity of the graft, culminating in increased risk of acute rejection [14].
Three-year graft survival in those with and without DGF was 89% versus 95%, with a hazard ratio of 1.76, (P < 0.001). Generally, patients experiencing DGF have a higher incidence of rejection, and the combination of DGF and acute rejection has an adverse effect on long-term graft survival [43, 44]. Chronic rejection, or chronic allograft injury is the major cause of graft failure other than patient death. It is characterized by diminished renal function with nonspecific pathology of tubular atrophy, interstitial fibrosis, and fibrous intimal thickening of arteries. DGF is a major contributor to chronic allograft injury and results in graft failure through a combination of acute injury, inflammation, and adaptive immune response as proposed by Halloran et al. [45]. The 5-year graft survival in live donor transplantation from an older UNOS data set was 85% and 65% with and without DGF, with a hazard ratio of 2.3, which is comparable to our present analysis [3]. The known association between DGF and graft outcomes necessitates closer follow-up, maximized immunosuppression, and perhaps the liberal use of biopsy.
DGF is associated with increased risk of rejection [15, 16, 19]. In our analysis, we observed a significantly higher rate of rejection in patients with DGF. We also investigated whether the increased incidence of graft failure observed with DGF was due to direct causal effect or mediated by rejection. Martinez-Mier et al. demonstrated rejection within 1 year of live donor transplantation to be the strongest predictor of 5-year graft survival in pediatric live donor transplants [46]. Zhang et al. determined acute rejection to be a significant factor affecting graft survival in their live donor cohort [47]. Similarly, Mustian et al. observed significantly higher rates of acute rejection among ABO incompatible LD transplants and subsequent early graft loss [10]. However, our stratified analysis of DGF patients with and without rejection did not demonstrate a significant difference in graft survival outcomes. This could be due to missing data points for causally associating rejection with graft failure in the UNOS database, despite a history of rejection in the past, limiting meaningful interpretation.
Strengths
To our knowledge, this is the most recent analysis of DGF risk factors and their association with graft and patient outcomes in LD kidney transplantation in the United States. Our large dataset with multi-institutional representation allows for detection of minute differences between groups and permits reasonable generalization to transplants being performed across the country. Determination of significant risk factors for DGF on univariate anaylsis allowed us to adjust for potential confounding variables on multivariate analysis. Most of these variables concurred with the previous UNOS data base analysis from 2000 to 2014 [3]; in addition we report correlation with PRA and PKD. We analyzed preemptive transplant data and correlation of DGF with rejection, which has not been reported prior.
Limitations
Our investigation had several limitations inherent for large data base analyses. Several definitions for DGF exist, accounting for a lack of uniform diagnostic criteria between different transplant centers. However, we do believe that the majority of transplant centers employ the requirement for dialysis within 1 week of transplant as criteria for DGF and it has been reported to be the most frequently cited definition in research analyses [8, 9]. This definition also has inherent disadvantages. First, dialysis may be used in the first week after transplant despite good graft function due to hyperkalemia or volume overload [8]. This definition also underestimates the rate of DGF in preemptive transplants because these patients may not require or may have delayed postoperative dialysis initiation due to residual native kidney function, despite poor allograft function [8, 19].
Several potential confounding variables were unable to be measured including intensity and type of immunosuppression, complexity of donor kidneys, type of perfusion fluid, intraoperative technical complications, anastomosis times, warm ischemia time, and open conversion rates. The number of primary nonfunctioning grafts was insignificant (0.02%) for meaningful statistical correlation. Intraoperative fluid replacement therapy can vary between transplant centers and may impact results. While normal saline is the traditional fluid replacement in most transplant centers, half saline-bicarbonate is also increasingly utilized in this setting [48]. Systematic differences in the management of recipients between transplant centers and clinicians may also impact outcomes and could also not be accounted for in our analysis [4]. We were also unable to analyze rejection in a more detailed manner due to insufficient data. There may be unverifiable immunologic or technical events we cannot assess through a data base study. Finally, a major limitation was insufficient data for laparoscopic and other minimally invasive techniques in the UNOS database, which prevented correlation between surgical techniques and DGF.
Conclusions
Despite the lower incidence of DGF with LD kidney transplantation, the negative impact of DGF on LD graft survival is more pronounced. Our report of a recent UNOS dataset provides an updated analysis of DGF risk factors. Improved understanding of modifiable risk factors is vital for mitigating their effect, reducing associated healthcare costs, and optimizating long-term graft survival. We determined a marginal increase in risk of DGF with prolonged CIT, without an effect on graft survival. Our results demonstrate that DGF is the most significant predictor of graft failure at 3 years, suggesting that recipients who develop DGF require closer follow-up and potentially immunosuppression adjustments to prevent graft loss.
Availability of data and material
Available in UNOS/OPTN registry.
References
Wolfe RA et al (1999) Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341(23):1725–1730
Nemati E et al (2014) Does kidney transplantation with deceased or living donor affect graft survival? Nephrourol Mon 6(4):e12182
Redfield RR et al (2016) Predictors and outcomes of delayed graft function after living-donor kidney transplantation. Transpl Int 29(1):81–87
Orandi BJ et al (2015) Center-level variation in the development of delayed graft function after deceased donor kidney transplantation. Transplantation 99(5):997–1002
Butala NM et al (2013) Is delayed graft function causally associated with long-term outcomes after kidney transplantation? Instrumental variable analysis. Transplantation 95(8):1008–1014
Aceto P et al (2019) Perioperative-, recipient-, and donor-related factors affecting delayed graft function in kidney transplantation. Exp Clin Transplant 17(5):575–579
Park HS et al (2012) Delayed graft function in living-donor renal transplantation: 10-year experience. Transplant Proc 44(1):43–46
Yarlagadda SG et al (2008) Marked variation in the definition and diagnosis of delayed graft function: a systematic review. Nephrol Dial Transplant 23(9):2995–3003
Mallon DH et al (2013) Defining delayed graft function after renal transplantation: simplest is best. Transplantation 96(10):885–889
Mustian MN et al (2018) Landscape of ABO-incompatible live donor kidney transplantation in the US. J Am Coll Surg 226(4):615–621
Krishnan AR et al (2016) Prolonged ischemic time, delayed graft function, and graft and patient outcomes in live donor kidney transplant recipients. Am J Transplant 16(9):2714–2723
Ojo AO et al (1997) Delayed graft function: risk factors and implications for renal allograft survival. Transplantation 63(7):968–974
Weber S et al (2018) Delayed graft function is associated with an increased rate of renal allograft rejection: a retrospective single center analysis. PLoS ONE 13(6):e0199445
Siedlecki A, Irish W, Brennan DC (2011) Delayed graft function in the kidney transplant. Am J Transplant 11(11):2279–2296
Brennan DC et al (2006) Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med 355(19):1967–1977
Hanaway MJ et al (2011) Alemtuzumab induction in renal transplantation. N Engl J Med 364(20):1909–1919
Kasiske BL et al (2002) Preemptive kidney transplantation: the advantage and the advantaged. J Am Soc Nephrol 13(5):1358–1364
Doshi MD et al (2011) Recipient risk factors associated with delayed graft function: a paired kidney analysis. Transplantation 91(6):666–671
Mogulla MR, Bhattacharjya S, Clayton PA (2019) Risk factors for and outcomes of delayed graft function in live donor kidney transplantation - a retrospective study. Transpl Int 32(11):1151–1160
Cobo G, Lindholm B, Stenvinkel P (2018) Chronic inflammation in end-stage renal disease and dialysis. Nephrol Dial Transplant 33(suppl_3):ii35-iii40
Asderakis A et al (1998) Pre-emptive kidney transplantation: the attractive alternative. Nephrol Dial Transplant 13(7):1799–1803
Bertoni E, Salvadori M (2009) Preemptive living donor kidney transplant. G Ital Nefrol 26(4):478–487
Parekh J, Bostrom A, Feng S (2010) Diabetes mellitus: a risk factor for delayed graft function after deceased donor kidney transplantation. Am J Transplant 10(2):298–303
Cosio FG et al (2008) Patient survival and cardiovascular risk after kidney transplantation: the challenge of diabetes. Am J Transplant 8(3):593–599
Schachtner T, Stein M, Reinke P (2017) Increased alloreactivity and adverse outcomes in obese kidney transplant recipients are limited to those with diabetes mellitus. Transpl Immunol 40:8–16
Hekmat R, Eshraghi H (2010) Correlation of pretransplant hyperglycemia and delayed graft function in kidney transplantation. Iran J Kidney Dis 4(2):147–152
Molnar MZ et al (2011) Higher recipient body mass index is associated with post-transplant delayed kidney graft function. Kidney Int 80(2):218–224
Lynch RJ et al (2009) Obesity, surgical site infection, and outcome following renal transplantation. Ann Surg 250(6):1014–1020
Navaneethan SD et al (2009) Weight loss interventions in chronic kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol 4(10):1565–1574
Park KM et al (2004) Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J Biol Chem 279(50):52282–52292
Aufhauser DD Jr et al (2016) Improved renal ischemia tolerance in females influences kidney transplantation outcomes. J Clin Invest 126(5):1968–1977
McGee J et al (2010) Donor-recipient gender and size mismatch affects graft success after kidney transplantation. J Am Coll Surg 210(5):718-725e1 ((725-6))
Oh CK et al (2006) Gender-related differences of renal mass supply and metabolic demand after living donor kidney transplantation. Clin Transplant 20(2):163–170
Ozdemir-van Brunschot DM et al (2016) Is the reluctance for the implantation of right donor kidneys justified? World J Surg 40(2):471–478
Amezquita Y et al (2008) Risk factors for early renal graft thrombosis: a case-controlled study in grafts from the same donor. Transplant Proc 40(9):2891–2893
Simpkins CE et al (2007) Cold ischemia time and allograft outcomes in live donor renal transplantation: is live donor organ transport feasible? Am J Transplant 7(1):99–107
Treat EG et al (2014) Outcomes of shipped live donor kidney transplants compared with traditional living donor kidney transplants. Transpl Int 27(11):1175–1182
Gill J et al (2017) Cold ischemia time up to 16 hours has little impact on living donor kidney transplant outcomes in the era of kidney paired donation. Kidney Int 92(2):490–496
Nassiri N et al (2020) The “oldest and coldest” shipped living donor kidneys transplanted through kidney paired donation. Am J Transplant 20(1):137–144
Foster MC et al (2013) APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol 24(9):1484–1491
Woodle ES et al (2005) Multivariate analysis of risk factors for acute rejection in early corticosteroid cessation regimens under modern immunosuppression. Am J Transplant 5(11):2740–2744
Kasiske BL et al (1991) The effect of race on access and outcome in transplantation. N Engl J Med 324(5):302–307
Humar A et al (2002) Risk factors for slow graft function after kidney transplants: a multivariate analysis. Clin Transplant 16(6):425–429
Troppmann C et al (1995) Delayed graft function, acute rejection, and outcome after cadaver renal transplantation. The multivariate analysis. Transplantation 59(7):962–968
Halloran PF, Melk A, Barth C (1999) Rethinking chronic allograft nephropathy: the concept of accelerated senescence. J Am Soc Nephrol 10(1):167–181
Martinez-Mier G et al (2013) Rejection is a strong graft survival predictor in live donor pediatric renal transplantation using cyclosporine, mycophenolate mofetil, and steroids: 5-year outcomes in a single Mexican center. Transplant Proc 45(4):1442–1444
Chen GD et al (2013) Donor factors predictive for poor outcomes of living donor kidney transplantation. Transplant Proc 45(4):1445–1448
Pourfakhr P et al (2020) Half saline-bicarbonate solution as intraoperative fluid replacement therapy leads to less acidosis and better early renal function during deceased-donor transplant. Exp Clin Transpl 18(1):34–38
Funding
None.
Author information
Authors and Affiliations
Contributions
SD: concept/design, Data analysis/interpretation, Drafting article, Critical revision of article. BB: drafting article, critical revision of article. MN: data analysis/interpretation, Statistics, Data collection. NK: data analysis/interpretation, Statistics, Data collection. OE—Critical revision of article, Approval of article. PS: critical revision of article, Approval of article. JO: concept/design, Data analysis/interpretation, Critical revision of article, Approval of article.
Corresponding author
Ethics declarations
Conflict of interest
None.
Consent to participate
Deidentified data from UNOS/OPTN registry, not applicable.
Consent for publication
Deidentified data from UNOS/OPTN registry, not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Damodaran, S., Bullock, B., Ekwenna, O. et al. Risk factors for delayed graft function and their impact on graft outcomes in live donor kidney transplantation. Int Urol Nephrol 53, 439–446 (2021). https://doi.org/10.1007/s11255-020-02687-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-020-02687-5