Abstract
Purpose
There have been conflicting data on the relative frequency of common forms of primary nephrotic syndrome (PNS). We undertook this study to look at the causes of PNS in the latest decade from our biopsy population, with a special attention to breakdown by race.
Methods
Retrospective chart review of all cases of adult PNS extracted from a database of 1388 cases for the last 10 years. We were careful to exclude patients with secondary disease and without the full nephrotic syndrome.
Results
There were 115 cases of PNS. Overall, MN was the most common lesion (40.0%), followed by minimal change disease (MCD) (34.0%), focal segmental glomerulosclerosis (FSGS) (13.0%), and IgA nephropathy (IgAN) (11.3%). Among whites, MN was the most common cause of NS (41.7%), followed by MCD (33.3%), IgAN (16.7%), and FSGS (6.3%). Among blacks, FSGS was the most common lesion (33.3%) followed closely by MN (29.6%), and MCD (26.0%). IgAN was present in 7.4%. Among multiracial patients (MR), MGN was the most common (50%) followed by MCD (45.5%) and FSGS (4.5%). In Asians, MCD (50.1%) and MGN (33.3%) were the most common, followed by FSGS and IgAN with 8.3% each.
Conclusions
MN and MCD were the most common causes of PNS in our population, with FSGS much less common overall. This is especially the case among whites and MR. Among blacks, MN and FSGS were almost codominant causes. The apparent decreased prevalence of FSGS may be related to more effective exclusion of secondary and maladaptive causes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been recognized that the incidence of various glomerular diseases varies based on race. Membranous nephropathy (MN) classically has been the most common form of primary nephrotic syndrome (NS) in adults but data from the 1990s showed that focal segmental glomerulosclerosis (FSGS) was increasingly common [1,2,3,4,5]. This was especially true in black [3, 4] and Hispanic populations [5], in whom FSGS far surpassed MN as a NS associated diagnosis in a number of studies. The increase appeared to be true of whites as well, and some studies found that indeed FSGS had become a more common diagnosis than MN in white patients [5, 6]. However, more recent studies have suggested a reversal of this trend, at least in some populations [7, 8] with a resurgence of MN relative to FSGS. We undertook this study to look at the causes of primary NS in the past decade from our biopsy population, with an emphasis on the association of diagnoses with patient race. We were careful to exclude patients with evidence of secondary disease and those who did not meet the full criteria for NS who were not necessarily excluded in prior reports. For FSGS, we only included cases that had diffuse foot process effacement on biopsy, to exclude maladaptive causes of FSGS. We postulate that a factor in the changing frequency of FSGS may be related to a lack of clear definition of FSGS, which could potentially include both a primary podocytopathy as well as maladaptive forms.
Methods
Lenox Hill Hospital kept an independent database of biopsies until the database was joined with the system-wide Northwell database in August of 2017. We reviewed all renal biopsy cases from Lenox Hill Hospital from January 2010 to June 2017 (n = 178) and from the system wide Northwell database system from its inception July 2017 until December 2019 (n = 1210). First, we extracted all biopsy cases with a pathological diagnosis that could be consistent with primary NS: MN, FSGS, minimal change disease (MCD), membranoproliferative glomerulonephritis (MPGN) and IgA nephropathy (IgAN). We excluded patients who had kidney allograft biopsies, those with a history of stem cell transplants, and also children less than 18 years of age. We used only cases meeting full criteria of nephrotic syndrome: edema, serum albumin less than 3.5 gm/dl, and proteinuria exceeding or equal to 3.5 g/day by 24 h urine or by urine protein/creatinine ratio. This data was gathered by reviewing biopsy reports, inpatient and outpatient labs as available and physician consults and progress notes. We then reviewed all biopsy reports with the purpose to exclude cases with evidence of secondary disease. MN cases were considered secondary and excluded if there were mesangial or subendothelial deposits on electron microscopy (EM), or if immunofluorescence (IF) showed positivity for other than IgG and C3 (aside from nonspecific IgM staining). Phospholipase 2 receptor antibody (PLA2-R) positivity in blood and on biopsy (available since 2017) was collected but was not used to determine primary vs secondary MN. FSGS cases were only included if there was diffuse foot process effacement on EM (defined by more than 90% effacement) suggesting a primary podocytopathy. Cases were excluded if there was a diagnosis of concomitant HIV, CMV, EBV, parvovirus B19 infection or pamidronate use. Those with suspected genetic causes of NS based on family history were excluded as well. Racial background was collected from medical records and patient were divided into black, white, Hispanic, Asian, and multiracial races. The racial breakdown was then correlated with NS diagnosis. Hispanic and Native American patients were not included in the breakdown of diagnoses as there were too few patients in each group.
Descriptive statistics were reported as means ± standard deviation (SD), or counts and proportions (%). ANOVA and logistic regression were used to compare continuous variables across groups. Chi-squared test and logistic regression were used to compare categorical variables across groups. All tests were two-tailed with a significance level of alpha = 0.05. All analyses were performed with Stata 13.0 for Windows, StataCorp LP (College Station, TX). The Northwell Health Institutional review board approved this case series as minimal risk research using date collected as part of standard clinical practice.
Results
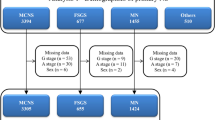
Of the 1388 renal biopsies performed between January 2010 and December 2019, 115 cases of adult PNS were identified. Of the 115 cases of PNS, 48 were white, 27 black, 22 described as multiracial (MR), 12 Asians, 4 Hispanics, 1 Native American and 1 who declined racial identification. Overall, males represented 55.0% and the average age was 49.6 years. In the overall PNS population, MN was the most common lesion (40.0%), followed by MCD (34.0%), FSGS (13.0%), IgAN (11.3%), and MPGN (1.7%).
Among white patients with PNS, MN was the most common diagnosis with 41.7%, followed by MCD with 33.3%, and IgAN with 16.7%. Only 6.3% had FSGS and 2.0% with MPGN. In this racial group, the mean age at biopsy was 50.3 ± 17.9 years. Mean serum creatinine (Cr) was 1.3 ± 0.8 mg/dL, the mean serum albumin was 2.3 ± 0.6 g/dL and mean proteinuria was 7.9 ± 3.9 g. See Table 1 for a summary of clinical data for each racial group.
Among black patients, FSGS was the most common lesion with 33.3%, followed by MN with 29.6%, MCD with 26.0%, IgAN 7.4% and MPGN with 3.7%. In this group, the mean age was 49.4 ± 18.5 years. Mean serum Cr was 2.3 ± 1.9 mg/dL, mean serum albumin 2.1 ± 0.6 g/dL and mean proteinuria 8.8 ± 4.3 g. Black patients had a higher prevalence of FSGS (p < 0.001) and a higher serum creatinine (p = 0.003) when compared with the rest of the population.
Among MR patients, MN was the most common lesion with 50.0%, followed by MCD with 45.5% and FSGS with only 4.5%. There were no IgAN or MPGN cases. In this group, the mean age was 52 ± 16.2 years. Mean serum Cr was 1.1 ± 0.8 mg/dL, mean serum albumin 2.2 ± 0.5 g/dL, and mean proteinuria 9.0 ± 3.2 g.
For Asian patients, MCD and MN represented 50.1% and 33.3% of patients, respectively, followed by FSGS and IgAN with 8.3%. There were only 12 patients overall in this subgroup. The mean age was 49.1 ± 18.2 years. Mean serum Cr was 1.2 ± 0.7 mg/dL, mean serum albumin 2.2 ± 0.7 g/dL, and mean proteinuria 9.3 ± 4.6 g. See Fig. 1 for a summary of the racial percentage breakdown for each diagnosis.
Of the cases of FSGS, one third had the collapsing variant FSGS, one third had tip variant, and one third had FSGS not otherwise specified. Among the 49 cases of MGN for which PLA2-R staining was available, 79.5% had biopsy tissue staining for PLA2- R hyperexpression. The MPGN patients all had immune complex GN; there were no cases of C3 GN.
Discussion
Data from the 1970s and early 1980s established that the most common cause of primary NS in adults was MN followed by MCD. FSGS comprised only a small fraction of all kidney biopsies (2.5%) [1, 9] and as a percentage of nephrotic patients (10–15%) [3]. In the 1990s, a number of reports from various centers found a remarkable increase in the incidence of FSGS. This was most dramatic in the black population, where FSGS became as high as 56% [3] and 64% [4] in 2 series. Most reports found an increase in whites and Hispanics as well [2, 3, 5], to the point, where FSGS was the most common NS associated diagnosis overall in many studies published in the 1990s and early 2000s. Some even found that even among white patients, FSGS became more common than MN [2, 5, 6], though this finding was not universal [3, 4]. The cause of this FSGS “epidemic” has not been clear but it has been speculated there might be some environmental trigger, and a genetic predisposition in blacks might make them especially susceptible.
In 2013, a report from Kraus et al. in Chicago [7], based on biopsies collected from 2001 to 2011, found that the incidence of MN had rebounded relative to FSGS to become again the most common diagnosis in a series of 204 patients with primary glomerular disease. This was especially remarkable, because it occurred in a largely black and Hispanic population; there were only 6 white patients in the series. Another study, by Zhu et al. from China [8] found that MN made up 55% of primary NS and was by far the most common cause if primary NS in that population from 2008 to 2012.Our present study, with biopsies of patients with NS from the most recent decade, extends the above observations to a population containing a plurality of whites. We confirmed the increase of MN relative to FSGS as the primary cause of primary NS. This was especially true among whites, where it represented almost 40% of the NS biopsies. Indeed, among whites the second most common diagnosis was MCD (32%). FSGS was vanishingly rare, representing only 6% of the primary NS patients.
The picture was somewhat different among blacks, where FSGS remained the most common diagnosis, almost certainly reflecting the genetic predisposition to this form of glomerular damage. We did not have information on APOL1 mutations in our population, but suspect it was prevalent. However, as in Krauss’study, we also found a dramatic increase in the relative percentage of MN, which made up almost 30% of the cases. MCD also made up a quarter of cases, and there was thus a much more coequal distribution of these three causes of NS than has been seen in earlier studies of this population.
How do we explain the changing trends over the past half century? If the prior spike in FSGS was due to some environmental factor, perhaps this factor is less prevalent. Aside from HIV, a number of infections such as parvovirus B19, EBV and CMV, have been putatively linked to FSGS [10] and perhaps these are indeed less common. More likely, we may have become better at identifying and excluding secondary and maladaptive forms of FSGS when analyzing trends of primary glomerular disease or primary nephrotic syndrome.
We have gained insight into the many genetic and familial causes of FSGS that may have been included in prior series. We have also discovered a number of drug induced causes of FSGS, including from lithium [11], pamidronate [12], and anabolic steroids [13], among others that would only be excluded after the associations were identified only in the past 20 years. Most importantly, we are likely more precise about distinguishing primary FSGS, understood as a primary podocytopathy presenting with diffuse podocyte injury and full nephrotic syndrome, from hemodynamic or maladaptive causes.
It is important to emphasize that the light microscopy findings can be virtually identical in primary and maladaptive FSGS, because both segmental and global glomerulosclerosis can occur with both. However, these lesions are a nonspecific pattern of injury that can have very different causes. In primary FSGS this is due to a primary podocytopathy, most likely due to some circulating factor that causes a generalized injury to podocytes. It is perhaps better described as “primary diffuse podocytopathy with FSGS lesions”. On the other hand, maladaptive FSGS is due to nephron loss and ensuing endocapillary hypertension, hyperfiltration, and hypertrophy, which causes a secondary injury to podocytes [10, 14]. The nephron loss can be accumulated over a lifetime in any individual patient due to multiple causes including obesity, previous glomerular or interstitial disease, obstructive sleep apnea, congenital heart disease, obstetric or urologic insults [14]. It could also be related to a low initial nephron endowment due to premature birth or low birth weight [14]. Maladaptive FSGS due to glomerular hyperfiltration and glomerular hypertension is a more patchy or segmental process which typically does not produce the nephrotic syndrome [10]. The presence or absence of the full nephrotic syndrome is likely a more effective way to distinguish primary FSGS from maladaptive, as opposed to any light microscopic pathologic finding including glomerulomegaly [10]. In contrast to previous literature that described primary FSGS as only having the full NS between 54 and 90% of the time [10, 15], a more recent study found that if one required an absence of conditions associated with secondary disease and the presence of diffuse foot process effacement on EM, the prevalence of NS was 100% in primary disease [10, 16]. So it is notable that only one of the previous studies documenting a high prevalence of FSGS (with a racial breakdown) required the full NS for inclusion, and none required the presence of diffuse foot process effacement on EM [3]. See Table 2 for a summary of studies assessing the racial breakdown of nephrotic syndrome associated diagnoses.
For this study, we were careful to exclude all patients without the full NS to avoid the pitfall of including patients with secondary FSGS. The report from China also only included NS patients and found a very low incidence of FSGS, though this could be attributed to the Asian population being studied [8]. The study from Chicago is all the more remarkable in its finding that FSGS has receded and MN increased, because this study, besides having a predominantly black and Hispanic population also did not require the NS for inclusion [7]. As the mean serum albumin was as high as 3.1 ± 1.0 in the FSGS group and only 57.9% of the FSGS patients had edema, it is likely that many of the FSGS patients would not have been included if the full NS were required for inclusion.
A strength of this study, besides the requirement to have the full NS to be included as discussed, and the fact that it includes a decade of biopsies, is that it is the first to our knowledge to include a multiracial category. We found that almost 20% of the patients in our PNS patients were described this way. We did not have further information on the racial background of these patients, though the pattern of NS causes quite closely resembled that of the white or Asian patients in our study.
Limitations of this study include that racial identification was based either on patient self-report or provider assessment as documented in patient charts, and could be subjective. There is obviously no gold standard for assessing race, but the fact that identified races differed in their incidences of NS supports there was a true difference between the groups. Another limitation is the relatively small number of patients, especially in certain populations such as Asian and Hispanic.
Finally, it should be noted that it may be difficult to carry out a similar biopsy based population study in the future. This is because of the now widely available serum PLA2-R autoantibody assay. It has been suggested recently [17, 18] that a renal biopsy might not be necessary if a nephrotic patient tests positive by this assay and there is no renal dysfunction, nor any apparent secondary diseases. If this suggestion is followed, the proportion of PNS MN cases in a biopsy population would clearly fall relative to other diagnoses considering that PLA2-R antibodies can be positive in serum in up to 70–80% of primary MN cases.
Conclusion
In conclusion, our study has extended the observations of two other groups which found a recent increase in MN relative to FSGS in predominantly black and Hispanic [7] and Asian [8] populations. We found that in a population with a plurality of whites, MN was the most common cause of primary NS followed by MCD. FSGS was rather uncommon in the overall population. Among blacks it was still the most common cause but MGN and MCD were almost as common, in contrast to reports from the 1990s and early 2000s.The reason for this trend remains unclear, though it may be related to an improved exclusion of secondary and maladaptive causes of FSGS, and limiting FSGS cases to those defined more precisely as a primary podocytopathy with FSGS lesions.
References
D’Agati V (1994) The many masks of focal segmental glomerulosclerosis. Kidney Int 46(4):1223–1241
Haas M, Spargo BH, Coventry S (1995) Increasing incidence of focal-segmental glomerulosclerosis among adult nephropathies: a 20-year renal biopsy study. Am J Kidney Dis 26(5):740–750
Haas M, Meehan SM, Karrison TG, Spargo BH (1997) Changing etiologies of unexplained adult nephrotic syndrome: a comparison of renal biopsy findings from 1976–1979 and 1995–1997. Am J Kidney Dis 30(5):621–631
Korbet SM, Genchi RM, Borok RZ, Schwartz MM (1996) The racial prevalence of glomerular lesions in nephrotic adults. Am J Kidney Dis 27(5):647–651
Braden GL, Mulhern JG, O’Shea MH, Nash SV, Ucci AA, Germain MJ (2000) Changing incidence of glomerular diseases in adults. Am J Kidney Dis 35(5):878–883
Dragovic D, Rosenstock JL, Wahl SJ, Panagopoulos G, De Vita MV, Michelis MF (2005) Increasing incidence of focal segmental glomerulosclerosis and an examination of demographic patterns. Clin Nephrol 63:1–7
Kraus MA, Punj S, Cimbaluk D, Hart PD (2013) Resurgence of membranous nephropathy in African Americans in inner city Chicago. Clin Kidney J 6(5):373–378
Zhu P, De ZF, Wang SX, Zhao MH, Wang HY (2015) Increasing frequency of idiopathic membranous nephropathy in primary glomerular disease: a 10-year renal biopsy study from a single Chinese nephrology centre. Nephrology 20(8):560–566
Barisoni L, D’Agati V (1994) The changing epidemiology of focal segmental glomerulosclerosis in New York City. Mod Pathol 7(156A):1
De Vriese AS, Sethi S, Nath KA, Glassock RJ, Fervenza FC (2018) Differentiating primary, genetic, and secondary FSGS in adults: a clinicopathologic approach. J Am Soc Nephrol 29(3):759–774
Markowitz GS, Radhakrishnan J, Kambham N, Valeri AM, Hines WH, D’Agati VD (2000) Lithium nephrotoxicity: a progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol 11(8):1439–1448
Markowitz GS, Appel GB, Fine PL et al (2001) Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J Am Soc Nephrol 12(6):1164–1172
Herlitz LC, Markowitz GS, Farris AB et al (2010) Development of focal segmental glomerulosclerosis after anabolic steroid abuse. J Am Soc Nephrol 21(1):163–172
Fogo AB (2015) Causes and pathogenesis of focal segmental glomerulosclerosis. Nat Rev Nephrol. https://doi.org/10.1038/nrneph.2014.216
Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD (2001) Obesity-related glomerulopathy: an emerging epidemic. Kidney Int 59(4):1498–1509
Hommos MS, De Vriese AS, Alexander MP et al (2017) The incidence of primary vs secondary focal segmental glomerulosclerosis: a clinicopathologic study. Mayo Clin Proc 92:1772–1781
De Vriese AS, Glassock RJ, Nath KA, Sethi S, Fervenza FC (2017) A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol. https://doi.org/10.1681/ASN.2016070776
Bobart SA, De Vriese AS, Pawar AS et al (2019) Noninvasive diagnosis of primary membranous nephropathy using phospholipase A2 receptor antibodies. Kidney Int 95:429–438
Acknowledgements
We thank Kaveh Hajifathalian MD, MPH for assistance with statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mbakop, C., DeVita, M.V., Wahl, S.J. et al. Adult primary nephrotic syndrome trends by race: a diminished frequency of focal segmental glomerulosclerosis in non-black patients. Int Urol Nephrol 53, 719–724 (2021). https://doi.org/10.1007/s11255-020-02658-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-020-02658-w