Abstract
The overlap syndromes of anti-neutrophil cytoplasmic antibodies (ANCA)-associated crescentic glomerulonephritis (AACGN) and variants of immune complex medicated glomerulopathy (ICMGN) have been reported. But very few have compared AACGN alone with the overlap syndromes (AACGN plus ICMGN). The aim of this retrospective study was to make that comparison, following serum creatinine (sCr) to determine whether the two groups (AACGN-only group versus overlap group) would behave differently over time. We identified 14 cases with dual diagnoses of AACGN and various ICMGN in the overlap group. Data were collected and compared with 15 randomly selected AACGN-only cases over the similar period of time. The overlap syndrome represented 0.35% of our overall biopsies (14/4049). All 14 patients were ANCA positive and had crescentic formation. The percentage of crescents in the biopsies ranged from 10 to 78%. ICMGN included the following: membranoproliferative glomerulonephritis, post-infectious glomerulonephritis, membranous glomerulopathies, idiopathic mesangial proliferative glomerulonephritis, lupus nephritis, and IgA nephropathy. With the exception one biopsy revealing lupus nephritis class III, most of the ICMGN were mild. When compared to the AACGN-only group, there were no significant differences in clinical and histologic indices including age, percent of crescents, and sCr (on biopsy days, and over the follow-up periods), although the numbers of follow-up cases were limited over time. Our findings suggest that AACGN was the dominant disease process in the majority of overlap syndromes between AACGN and ICMGN, similar to the clinical processes of AACGN-only disease, therefore, the AACGN in overlap syndrome cases should be the main target for clinical management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are three types of crescentic glomerulonephritis (CGN)—anti-glomerular basement membrane antibody-mediated CGN (conventionally called type 1 CGN), secondary crescent formation in immune complex-mediated diseases (so-called type 2 CGN), and pauci-immune CGN. The etiology of the pauci-immune CGN was not clear until the 1980s when it was found to be related to ANCA-associated vasculitis [1,2,3]. Then, the ANCA-associated CGN (AACGN) is conventionally regarded as type 3 CGN. The ANCA-associated vasculitis in the kidney is most often present in the glomerular capillary beds causing necrosis and its associated inflammatory cells, leading to the rupture of glomerular basement membranes and stimulates the proliferation of parietal epithelial cells for crescent formation [4]. A small percentage of ANCA-associated CGN is present in small-to-medium-sized arteries. P-ANCA in serology test can be more specifically detected by myeloperioxidase, while C-ANCA is more closely associated with positive proteinase 3. P-ANCA-associated CGN appears to be usually confined to the kidneys, while C-ANCA related CGN can have more systematic involvements such as the lungs [5].
AACGN is usually the sole pathologic etiology for its associated rapidly progressive renal diseases. However, AACGN is considered an autoimmune disorder, thus it is can be seen with other immune complex-mediated glomerulopathies (ICMGN) (so-called overlap syndrome), making the pathologic differential diagnosis difficult, and obscures clinical treatment. The concurrent ICMGN of overlap syndromes have been reported to be either IgA nephropathy [6,7,8,9], lupus nephritis [10,11,12,13,14], membranous glomerulopathy [15,16,17,18,19], or post-infectious glomerulonephritis [20,21,22,23,24]. In this study, we found that there was a high percentage (43%) of membranoproliferative glomerulonephritis (MPGN) (6 out of 14 cases) among variants of immune complex diseases. In addition, our follow-up data for the overlap syndrome indicated that there were generally no significant differences in serum creatinine (sCr) levels over time between the overlap syndrome and AACGN-only cases, implying that AACGN is the dominant disease process in this type of overlap syndrome.
Methods
The retrospective study protocol was approved by the Institutional Research Board of Beaumont Health System. In total, 4049 renal biopsies from 2008 to 2019 reviewed, we identified 14 cases with dual diagnoses of AACGN and various ICMGN. The patients were divided into two groups. The first group of 14 cases was composed of AACGN and variants of ICMGN (also called overlap group). The second group included 15 cases with only AACGN in the absence of ICMGN, which were randomly but relatively timely matched to the cases in the overlap group. For each patient, clinical indices including age, gender, ANCA status (by immunofluorescent method for either perinuclear (p) or cytoplasmic (c) staining pattern), serum myeloperoxidase/proteinase status (by ELISA method) and sCr levels, were collected. The sCr levels were followed-up for available patients over five years from each renal biopsy.
Each renal biopsy was processed in a routine protocol. Light microscopy sections using paraffin embedded tissue (2 µm) were stained for hematoxylin and eosin, PAS, Jones’ and Trichrome. Frozen sections were cut at 3 µm for immunofluorescent staining of IgG, IgA, IgM, C3, C1q, fibrinogen, kappa and lambda. The third specimen was fixed in 3% glutaaldehyde and routinely processed for transmission electron microscopy.
Statistics The values were represented as mean ± standard error. An unpaired student test was used to compare the indices between the two groups. P value less than 0.05 was considered significantly different.
Results
The overlap syndrome represented 0.35% of our overall biopsies (14/4049) over 11 years of period. In this study, 6 of 14 cases showed concurrent AACGN with membranoproliferative glomerulonephritis (MPGN). Five were classified as type 1 MPGN as the immune complex deposits were found at subendothelial spaces (Table 1). One MPGN type 3 was also identified, because the immune complex deposits were found at multiple locations of the glomeruli. In addition, we had two cases of post-infectious glomerulonephritis, two cases of membranous glomerulopathies, one cases of IgA nephropathy and one non-specific mesangial proliferative glomerulopathy, coexisting with AACGN. As cellular crescents in the cases appeared rather prominent and ICMGN were relatively mild in patients with positive ANCA, we determined that these cases represented overlap syndromes between the two entities.
We also had two lupus nephritis cases, concurrently present with AACGN. The first case was a mesangial proliferative lupus nephritis, ISN/RPS class II. The accompanied AACGN was easily identified as the lupus nephritis class II with deposits only in mesangial areas, which was impossible to cause the crescent formation in the patient with positive ANCA. The second lupus nephritis case, classified as ISN/RPS class III, was associated with extensive crescent formation involving in up to 70% of glomeruli in a woman with positive ANCA and lupus serology (Fig. 1). Electron microscopy revealed minor subendothelial immune complex deposits, which can argue against their capacity to break through the glomerular basement membranes leading to extensive crescent formation. The factors favoring an ANCA-associated crescentic glomerulonephritis included a high P-ANCA serology with MPO positivity, light microscopy revealing a subtle proliferative pattern and electron microscopy with very few subendothelial and mesangial deposits. Factors that would implicate lupus derived crescents include hypocomplementemia with a positive ANA and double stranded DNA with a full house pattern on immunofluorescence. Of note, there were limited loops for the evaluation of subendothelial deposition on electron microscopy. This patient had a poor response to treatment and her renal failure persisted. In summary, the majority of our cases demonstrated minor ICMGN when they were overlapped with AACGN while a minority of cases behaved aggressively if assocaited with lupus nephritis.
Overlap syndrome between AACGN and lupus nephritis class III. Large cellular crescents, replacing almost entire glomeruli, were seen in 70% of glomeruli such as seen in a (hematoxylin–eosin staining). Focal proliferative glomerulus was present in minority of the glomeruli on hematoxylin and eosin stained section (b) with 3 + positive immunofluorescent staining for C1q in c (full house pattern of positive immunofluorescent stainins for IgG at 3 + , IgM at 1–2 + , IgA at 1 + , C3 at 3 + , kappa at 3 + and lambda at 3 + , not shown). Electron microscopy in d revealed some immune complex deposits in mesangial areas (indiced by white arrow) but relatively small subendothelial immune complex deposits with focal duplication of glomerular basement membrane (blue arrow); the insert shows enlarged subendothelial deposits, indicated by another bigger yellow arrow. (Magnifications ×400 in a, b, × 600 in c, and × 5,200 in d)
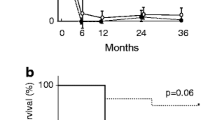
We randomly compared 15 renal biopsies with AACGN without other disease processes (called AACGN-only group) over a similar period of time with the overlap syndrome group (Table 1). Patient’s mean age and percent of crescentic glomeruli in the AACGN-only group were similar to the overlap syndrome group (Table 2). Serum creatinine levels on the day of biopsy and over 5-years of follow-up were also similar between the two groups (no significant difference is statistically found), despite limited numbers of cases available in each group over time.
Discussion
The exact prevalence of overlap syndrome between AACGN and ICMGN is difficult to determine, since most of the overlap syndrome cases were reported as case reports or a small series between AACGN and one particular type of ICMGN such as IgA nephropathy, lupus nephritis or membranous glomerulopathy (Table 3) [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24]. Our study revealed only 0.35% biopsies showing both AACGN and ICMGN (with positive ANCA serology), which were documented in our pathologic reports. Several overlap syndrome studies between AACGN and lupus nephritis indicates that this type of overlap syndrome cases between AACGN and one particular type of ICMGN are indeed rare (possibly less than 2%) [10,11,12].
There are two major findings in our study. First, we found that MPGN represented 43% of all overlap syndrome cases (6 out of 14). As to our knowledge, no single study has revealed the dominance of MPGN cases in the overlap syndrome. Theoretically, subendothelial deposits can have the potential to break glomerular basement membranes stimulating secondary crescent formation, while primary crescents associated with ANCA result from the erosion of glomerular endothelial cells and glomerular basement membranes by released myeloperoxidase or proteinase-3 from neutrophils and activated complements, leading to the stimulation of parietal epithelial proliferation. Our cases showed a large portion of crescent formation (some with necrosis), but mild features of MPGN, supporting that the crescent formation was most likely linked to the positive ANCA in these overlap syndrome cases. Other overlap syndrome cases in the study were also characterized with prominent crescent formation but minor immume complex depositis. With positive serology for ANCA (and/or positive myeloperoxidase or proteinase-3), we have determined that our 14 cases qualified as an overlap syndrome. Second, we compared the overlap syndrome cases between AACGN and ICMGN with AACGN-only cases for the analysis of histologic and clinical indies. We found that there was no significant difference in the histologic and clinical indices. The percent of crescent formation and sCr was similar between the two groups. This indicates that the majority of overlap syndromes had AACGN as the dominant disease process, similar to AACGN-only group. Certainly, our case numbers appeared relatively small, particularly the number of sCr values dropped during the follow-up check over 5 years. Larger studies are needed to confirm our findings. Another limitation of this study is that our overlapped ICMGN were composed of different variants of ICMGN. Some presented with significant proteinuria such as membranous nephropathy and some were mainly associated with hematuria as IgA nephropathy and post-infectious glomerulonephritis, therefore, proteinuria values cannot be reliably evaluated in our overlap syndrome cases for comparison with AACGN-only cases.
There are several studies approaching the overlap syndromes between AACGN and ICMGN from different angles. Early on, two retrospective studies investigated whether AACGN would be “pure” pauci-immune [25, 26]. They retrospectively reviewed electron microscopy for immune complex deposits. Haas et al. found that up to 54% of so-called pauci-immune crescentic glomerulonephritis actually had some electron dense deposits [25]. As some showed positive immunofluoresccent stains, they regarded the electron dense deposits as immune complex deposits. Furthermore, they demonstrated that cases with mixed AACGN and “immune complex deposits” had significantly higher levels of proteinuria than AACGN only cases. However, we discovered that pathologic diagnoses were critical for patients’ clinical management. A retrospective identification of electron dense deposits would change the initial diagnoses and miss a large range of ICMGN, particularly as the authors did not specify the subtype of ICMGN. The study did not show accompanied kappa and lambda staining data, which would be critical to determine true immune complex deposits versus non-specific electron dense deposits such as fibrin or necrotic material. Because we believe that 54% is too high a percentage and does not support our finding in “the overlap syndrome”, we hesitate to put the two studies into the current Table 3.
In recent years, two other studies focused on serologic findings of both positive ANCA and positive serology for lupus [27, 28]. We applaud their suggestions to check serum ANCA for patients with lupus nephritis, as they found up to 14% dual positive serology for both ANCA and lupus. But they did not mention what was the percent of biopsies showing crescents in the lupus nephritis cases. Therefore, the dual positive serology studies are addressing different issues from the histologically identified overlap syndrome with both primary crescent formation and lupus nephritis reported by others [10,11,12,13,14]. Here, we have to emphasize that “true” overlap syndrome with matched histologic and serologic identification are indeed relatively rare [10,11,12] as we only observed 0.35% in our practice (documented on our pathologic reports). It appears that these overlap syndrome cases between AACGN and lupus nephritis carry a worse prognosis, but our study with only 2 such lupus cases out of 14 overlap syndrome cases cannot either confirm or disapprove their findings.
Endocarditis-associated glomerulonephritis can present with 53% of crescent formation and 28% of positive rate for ANCA, while there is often concurrent focal or diffuse prolifearative glomerulonephritis up to 53% as well [29]. This may represent a unique finding indicating that one etiology may trigger two disease processes. As the authors did not mention their endocarditis-associated glomerulonephritis cases as overlap syndrome [29] and we have limited experience for this entity [30], we leave to future studies to determine if some of endocarditis-associated glomerulonephritis can be counted as overlap syndrome.
In conclusion, most of our overlap syndrome cases with positive ANCA showed profound crescent formation but minor immune complex-mediated renal diseases, which represented a small percent of our renal biopsy cases. The overlap syndrome cases revealed similar percentage of crescent formation in renal biopsies and similar levels of sCr over time with those of only AACGN, implying that this type of overlap syndrome behaves similarly to AACGN only cases, therefore, the AACGN in overlap syndrome cases should be the main target for clinical management.
References
Davies DJ, Moran JE, Niall JF, Ryan GB (1982) Segmental necrotising glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology? Br Med J (Clin Res Ed) 285:606
van der Woude FJ, Rasmussen N, Lobatto S et al (1985) Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet 1:425–429
Falk RJ, Jennette JC (1988) Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med 318:1651–1657
Falk RJ, Jennette JC (2010) ANCA disease: where is this field heading? J Am Soc Nephrol 21:745–752
Hilhorst M, van Paassen P, Tervaert JW (2015) Proteinase 3-ANCA vasculitis versus myeloperoxidase-ANCA vasculitis. J Am Soc Nephrol 26:2314–2327
Allmaras E, Nowack R, Andrassy K, Waldherr R, van der Woude F, Ritz E (1997) Rapidly progressive IgA nephropathy with anti-myeloperoxidase antibodies benefits from immunosuppression. Clin Nephrol 48:269–273
Richer C, Mouthon L, Cohen P et al (1999) IgA glomerulonephritis associated with microscopic polyangiitis or Churg-Strauss syndrome. Clin Nephrol 52:47–50
Rollino C, Mazzucco G, Basolo B et al (1999) cANCA positivity in a case of IgA glomerulonephritis (IgAGN) with necrotizing lesions. Nephrol Dial Transplant 14:797–798
Vrtovsnik F, Queffeulou G, Skhiri H et al (1999) Simultaneous IgA nephropathy and Wegener's granulomatosis–overlap or coincidence (the role of renal biopsy). Nephrol Dial Transplant 14:1266–1267
Jarrot PA, Chiche L, Hervier B et al (2016) Systemic lupus erythematosus and antineutrophil cytoplasmic antibody-associated vasculitis overlap syndrome in patients with biopsy-proven glomerulonephritis. Medicine (Baltimore) 95:e3748
Marshall S, Dressler R, D'Agati V (1997) Membranous lupus nephritis with antineutrophil cytoplasmic antibody-associated segmental necrotizing and crescentic glomerulonephritis. Am J Kidney Dis 29:119–124
Nasr SH, D'Agati VD, Park HR et al (2008) Necrotizing and crescentic lupus nephritis with antineutrophil cytoplasmic antibody seropositivity. Clin J Am Soc Nephrol 3:682–690
Schwartz MM, Roberts JL, Lewis EJ (1983) Necrotizing glomerulitis of systemic lupus erythematosus. Hum Pathol 14:158–167
Sen D, Isenberg DA (2003) Antineutrophil cytoplasmic autoantibodies in systemic lupus erythematosus. Lupus 12:651–658
Dwyer KM, Agar JW, Hill PA, Murphy BF (2001) Membranous nephropathy and anti-neutrophil cytoplasmic antibody-associated glomerulonephritis: a report of 2 cases. Clin Nephrol 56:394–397
Gaber LW, Wall BM, Cooke CR (1993) Coexistence of anti-neutrophil cytoplasmic antibody-associated glomerulonephritis and membranous glomerulopathy. Am J Clin Pathol 99:211–215
Nasr SH, Said SM, Valeri AM et al (2009) Membranous glomerulonephritis with ANCA-associated necrotizing and crescentic glomerulonephritis. Clin J Am Soc Nephrol 4:299–308
Taniguchi Y, Yorioka N, Kumagai J, Ito T, Yamakido M, Taguchi T (1999) Myeloperoxidase antineutrophil cytoplasmic antibody-positive necrotizing crescentic glomerulonephritis and membranous glomerulonephropathy. Clin Nephrol 52:253–255
Tse WY, Howie AJ, Adu D et al (1997) Association of vasculitic glomerulonephritis with membranous nephropathy: a report of 10 cases. Nephrol Dial Transplant 12:1017–1027
Bauer A, Jabs WJ, Sufke S, Maass M, Kreft B (2004) Vasculitic purpura with antineutrophil cytoplasmic antibody-positive acute renal failure in a patient with Streptococcus bovis case and Neisseria subflava bacteremia and subacute endocarditis. Clin Nephrol 62:144–148
Choi HK, Lamprecht P, Niles JL, Gross WL, Merkel PA (2000) Subacute bacterial endocarditis with positive cytoplasmic antineutrophil cytoplasmic antibodies and anti-proteinase 3 antibodies. Arthritis Rheum 43:226–231
Couzi L, Morel D, Deminiere C, Merville P (2004) An unusual endocarditis-induced crescentic glomerulonephritis treated by plasmapheresis. Clin Nephrol 62:461–464
Majumdar A, Chowdhary S, Ferreira MA et al (2000) Renal pathological findings in infective endocarditis. Nephrol Dial Transplant 15:1782–1787
Subra JF, Michelet C, Laporte J et al (1998) The presence of cytoplasmic antineutrophil cytoplasmic antibodies (C-ANCA) in the course of subacute bacterial endocarditis with glomerular involvement, coincidence or association? Clin Nephrol 49:15–18
Haas M, Eustace JA (2004) Immune complex deposits in ANCA-associated crescentic glomerulonephritis: a study of 126 cases. Kidney Int 65:2145–2152
Neumann I, Regele H, Kain R, Birck R, Meisl FT (2003) Glomerular immune deposits are associated with increased proteinuria in patients with ANCA-associated crescentic nephritis. Nephrol Dial Transplant 18:524–531
Turner-Stokes T, Wilson HR, Morreale M et al (2017) Positive antineutrophil cytoplasmic antibody serology in patients with lupus nephritis is associated with distinct histopathologic features on renal biopsy. Kidney Int 92:1223–1231
Wang Y, Huang X, Cai J et al (2016) Clinicopathologic characteristics and outcomes of lupus nephritis with antineutrophil cytoplasmic antibody: a retrospective study. Medicine (Baltimore) 95:e2580
Boils CL, Nasr SH, Walker PD, Couser WG, Larsen CP (2015) Update on endocarditis-assocaited glomerulonephritis. Kidney Int 87:1241–1249
Vercelone J, Cohen L, Mansuri S, Zhang PL, Kellerman PS (2018) Bartonella endocarditis mimicking crescentic glomerulonephritis with PR3-ANCA positivity. Case Rep Nephrol. https://doi.org/10.1155/2018/9607582
Acknowledgements
Authors thank Dr. Oalf Kroneman for his critical review of the manuscript.
Author information
Authors and Affiliations
Contributions
VLG, MSM, HDK and PLZ have mainly involved in research idea, data collection and study design. VLG, KML and PLZ have contributed to manuscript drafing, figure construction and the completion of tables. WL and MGM contributed to reading proof and suggestions for improving the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Golas, V.L., Lao, K.M., Misuraca, M.S. et al. The clinical features of overlap syndrome (ANCA-associated crescentic glomerulonephritis [AACGN] and immune complex-mediated glomerulonephritis) are similar to those of AACGN alone. Int Urol Nephrol 53, 515–521 (2021). https://doi.org/10.1007/s11255-020-02654-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-020-02654-0