Abstract
Purpose
The relationship between depression and long-term clinical outcomes in peritoneal dialysis is unclear. This study was to explore the effect of depressive symptoms on patient survival and technique survival in continuous ambulatory peritoneal dialysis (CAPD) patients.
Methods
Patients who had received CAPD therapy for ≥ 3 months were recruited from January to June, 2009, with follow-up until June, 2019. The Beck Depression Inventory-II (BDI-II) was used to evaluate depressive symptoms (BDI scores ≥ 14) at baseline. The primary outcome was all-cause mortality, and the secondary outcome was technique failure.
Results
Participants were 275 CAPD patients (mean age 49.6 ± 15.9 years, male 54.2%). Of these, 86 (31.3%) experienced depressive symptoms. The depressive group had fewer males, longer PD duration at enrollment, higher calcium levels, and lower residual glomerular filtration rates (all P < 0.05) than the non-depressive group. Long-term patient survival (P = 0.037) and technique survival (P = 0.003) were significantly poorer in depressive group than in non-depressive group. After adjustment for confounders in multivariate Cox proportional hazard regression models, depressive symptoms remained independent predictors of mortality risk [hazard ratio (HR) 1.60, 95% confidence interval (CI) 1.03–2.48; P = 0.035] and technique failure (HR 1.92, 95% CI 1.07–3.47; P = 0.029).
Conclusion
The prevalence of patients with depressive symptoms was 31.3% in this cohort. The patient survival rate and technique survival rate in depressive group were lower than in non-depressive group. Depressive symptoms were independent risk factors for long-term mortality and technique failure in CAPD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of psychological disorders in end-stage renal disease (ESRD) patients is higher than in other chronic disease patients [1]. Depression is one of the most common psychological problems in ESRD [2]; the prevalence of depression is 25–53.8% in peritoneal dialysis (PD) patients [3, 4]. Several factors are associated with depression in PD patients, including older age, long dialysis duration, diabetes, fatigue, poor sleep quality, social support level, and negative coping styles [5, 6].
Depression has several negative effects on dialysis patients, including malnutrition [7], poor treatment adherence [8], decreased quality of life (QOL) [9], increased rate of peritonitis [10], and mortality [11, 12]. Previous studies have identified the negative effects of depression on patient mortality in dialysis patients [1, 13]. However, few studies on the effect of depression on long-term survival in PD patients have featured > 5 year follow-up [13]. Additionally, the effect of depression on technical failure in PD patients is unclear. Owing to the difficulties in making a depression diagnosis in dialysis patients in clinical practice, our study focused on the depressive symptoms of PD patients. Therefore, we conducted this prospective cohort study with a large sample of PD patients to explore factors associated with depressive symptoms, and the long-term impacts of depressive symptoms on all-cause mortality and technique failure.
Methods
Patient recruitment
This was a prospective cohort study. We recruited patients receiving continuous ambulatory PD (CAPD) treatment at our PD center between January 1 and June 30, 2009. The inclusion criteria were (1) has received CAPD for ≥ 3 months; (2) age ≥ 18 years; (3) willingness to participate in the study and provide written informed consent. The exclusion criteria were (1) age < 18 years; (2) PD duration < 3 months; (3) history of tumor; (4) patients who were transferred from other PD centers; (5) acute infection or hospitalization in the last month; (6) dementia or psychotic disorder. We conducted this study in compliance with the Declaration of Helsinki guidelines. The study protocol was approved by the ethics committee of The First Affiliated Hospital of Sun Yat-sen University. We obtained written informed consent from all patients at recruitment.
Assessment of depressive symptoms
The Beck Depression Inventory-II (BDI-II) was used to assess patient depression status at baseline. The BDI is the most widely used self-rating scale for depression [14]. The scale comprises 21 items graded from 0 to 3; total scores range from 0 to 63. The Chinese version of the scale has been used to evaluate the psychological status of Chinese patients [5, 15]. For the purposes of this study, BDI-II score ≥ 14 was considered for significant depressive symptoms, which referred to previous published studies related to depression in ESRD patients [5, 16,17,18]. In addition, a sensitivity analysis using different cutoffs (BDI score > 10, > 11, > 12, respectively) was done to confirm which cutoff was better in this study. BDI-II was used to assess depressive symptoms by a well-trained nurse during clinic follow-up.
Demographic and baseline laboratory data
The following demographic data were collected at baseline: age, gender, education level, comorbidity conditions, PD duration at enrollment, and primary cause of renal disease. The following baseline laboratory data were collected from patient charts at recruitment: hemoglobin, serum albumin, serum calcium, serum phosphorus, intact parathyroid hormone (iPTH), serum sodium, serum potassium, high-sensitivity C-reactive protein, and dialysis adequacy, including Kt/V (reflects the total urea clearance both in peritoneal dialysis and kidney) and rGFR (residual glomerular filtration).
Study outcomes and definitions
The primary outcome was all-cause mortality and the secondary outcome was technique failure. All patients were followed up until death, PD drop-out, or June 30, 2019. Causes of PD drop-out included switching to hemodialysis, kidney transplantation, transference to other PD centers, loss to follow-up, and other causes. Technique failure referred to any cause of switching to hemodialysis or death from peritonitis or encapsulating peritoneal sclerosis (EPS); other causes of death were recorded as censored.
Statistical analysis
Continuous variables were described using mean and standard deviation or median and interquartile range (IQR) as appropriate. Data were compared using independent samples t test or the Wilcoxon rank sum test for two independent samples, depending on data distribution. Categorical variables were described using frequencies and percentages, and compared using Chi-square test or Fisher’s exact test, as appropriate. The two groups (depressive vs. non-depressive) were compared using Kaplan–Meier survival analysis. Binary logistic regression models were used to explore factors associated with depressive symptoms. The relationship between depressive symptoms and long-term outcomes was evaluated using Cox proportional hazard models. Two-tailed significance tests with P < 0.05 were used for all estimated variables. IBM SPSS Statistics for Windows version 20.0 (IBM Corp, Armonk, NY, USA) was used for statistical analysis.
Results
Study population and baseline clinical data
Figure 1 is a flowchart showing patients recruitment. The final sample comprised 275 CAPD patients (average age 49.6 ± 15.9 years, male 54.2%). The percentage of diabetic patients was 25.5% (70 cases). The median PD duration of patients at baseline was 15.2 (IQR: 8.3, 28.2) months. The average BDI score was 11.6 ± 9.3 in the whole recruited population. A total of 86 patients (31.3%) were defined as experiencing depressive symptoms according to the definition.
Comparisons of baseline demographic and clinical factors between depressive and non-depressive groups
Compared with the non-depressive group, the depressive group had fewer males (43.0% vs. 59.3%, P = 0.013), longer PD duration at recruitment (20.3 months vs. 13.5 months, P = 0.001), higher calcium levels (2.40 ± 0.19 mmol/l vs. 2.35 ± 0.21 mmol/l, P = 0.048), and lower residual glomerular filtration rate (rGFR) (1.12 ml/min/1.73 m2 vs. 1.68 ml/min/1.73 m2, P = 0.019). There were no significant differences in age, proportion of diabetes, cardiovascular disease (CVD) history, education level, hemoglobin, serum albumin, serum phosphorus, serum sodium, serum potassium, high-sensitivity C-reactive protein (hsCRP), and Kt/V between the two groups (Table 1).
Factors independently associated with depressive symptoms
Variables associated with depressive symptoms in the univariate analysis and general confounding factors (age, gender, diabetes, PD duration at enrollment, serum calcium, and rGFR) were included in the binary logistic regression models. After adjusting for confounders, we found that being female [odds ratio (OR) 1.77, 95% confidence interval (CI) 1.03–3.05; P = 0.038], diabetes (OR: 2.38, 95% CI 1.20–4.72; P = 0.013), and lower rGFR (OR: 0.87, 95% CI 0.77–0.99; P = 0.035) were independently correlated with depressive symptoms (Table 2).
Relationship between depressive symptoms and all-cause mortality
After a median of 45.8 (IQR: 20.3, 87.2) months follow-up, 111 (45.5%) patients in the cohort died. A total of 133 (48.4%) patients dropped out of PD for the following reasons: 55 (22.5%) switched to hemodialysis, 59 (24.2%) underwent renal transplantation, 10 (4.1%) transferred to other centers, 7 (2.9%) were lost to follow-up, and 2 (0.8%) withdrew for other reasons. Figure 1 shows the status of the two groups at the end of the study. Of the 111 patients who died, 55 (49.5%) died from CVD, 32 (28.8%) (peritonitis in 16 cases) from infection, 4 (3.6%) from tumor, 7 (6.3%) from cachexy, 4 (3.6%) from gastrointestinal bleeding, 4 (3.6%) from unknown causes, and 5 (4.5%) from other causes. However, no patients died from suicide.
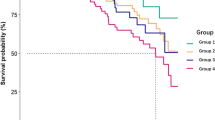
We compared patient survival in depressive and non-depressive patients using Kaplan–Meier analysis. As shown in Fig. 2, the 1-, 3-, 5-, and 10-year patient survival rates in the depressive group were 87.5%, 65.5%, 55.2%, and 33.1%, respectively, which was significantly poorer than in the non-depressive group, whose survival rates were 95.5%, 82.3%, 69.8%, and 43.4%, respectively. Univariate Cox regression analysis indicated that depressive symptoms were risk factors for all-cause mortality (HR 1.51, 95% CI 1.02–2.23). After adjusting for age, gender, diabetes, PD duration at enrollment, CVD history, serum albumin, serum sodium, hsCRP, total weekly Kt/V, and rGFR in the multivariate Cox regression model 2, depressive symptoms remained independently correlated with all-cause mortality (HR 1.60, 95% CI 1.03–2.48) (Table 3). Furthermore, the results of sensitivity analysis showed that only the cutoff of BDI score ≥ 14 showed a better performance, which reveals a close correlation between depressive symptoms and all-cause mortality (Table 4).
Relationship between depressive symptoms and technique failure
Until the end of the study, there were 76 patients who experienced technique failure according to the definition. Fifty-six patients switched to hemodialysis for the following reasons: 29 (51.8%): peritonitis; 13 (23.2%): ultrafiltration failure; 6 (10.7%): inadequate dialysis; 1 (1.8%): catheter dysfunction; and 7 (12.5%): other causes. Additionally, 19 patients died owing to peritonitis-related problems and 1 patient died from EPS.
After the long-term follow-up, the 1-, 3-, 5-, and 10-year technique survival rates were 91.3%, 80.0%, 70.0%, and 37.7%, respectively, in depressive patients and 98.3%, 91.0%, 83.2%, and 54.4%, respectively, in non-depressive patients (Fig. 3). Univariate Cox regression analysis showed that depressive symptoms were predictive risk factors for technical failure (HR 1.79, 95% CI 1.04–3.09). After adjusting for age, gender and diabetes, a multivariate Cox regression model showed that depressive symptoms remained independent predictive risk factors for technical failure (HR 1.92, 95% CI 1.07–3.47) (Table 5).
Discussion
This cohort study showed that the prevalence of depressive symptoms was 31.3% in CAPD patients. Female gender, diabetes, and lower rGFR were independently associated with depressive symptoms in this cohort. The long-term patient survival rate and technique survival rate in the depressive group were significantly lower than those in the non-depressive group. Depressive symptoms were independently correlated with increased risk of all-cause mortality and technique failure.
A previous study showed that the average prevalence of depression was about 39% in dialysis patients [19], which is higher than in other chronic disease patients, such as those with diabetes [1]. Using the BDI, Simic Ogrizovic et al. found a depression rate of 28.2% in PD patients [20], which is similar to the present findings (prevalence: 31.3%). Similarly, a recent Australian study involving 110 PD and hemodialysis patients showed that 41% had substantial depressive symptoms [21]. These results suggest that more attention should be paid to the psychological status of patients during our follow-up routine. The mechanism underlying the high prevalence of depression in dialysis patients is likely to be complex. According to Katon’s model [22], genetic vulnerability, childhood adversity (including loss, abuse, and neglect), maladaptive attachment, and consequences of chronic illness (symptom burden, functional impairment, and biological changes in the brain secondary to chronic illness) may result in depressive and anxiety disorders. The aversive symptoms and functional impairments associated with the consequences of chronic illness may also precipitate or worsen depression. For chronic kidney disease (CKD) patients, in addition to the factors mentioned above, biological aspects such as immune responses, inflammatory pathways, disturbances of the hypothalamic pituitary axis, changes in the parasympathetic and sympathetic nervous systems, and chronic pro-inflammatory states are associated with depression [23, 24]. In addition, kidney disease-related loss may contribute to the high level of depression in dialysis patients and, in turn, may hinder coping and QOL in dialysis patients [25]. Dialysis patients, particularly those who have lost their jobs, often have ambivalent and complex feelings towards treatment because of life style changes, loss of autonomy and control, and reliance on dialysis. Some patients even experience feelings of guilt about being a burden to their caregivers, which may result from loss of identity and impaired social function. This may explain why dialysis patients show a higher prevalence of depression than patients with other chronic illnesses. Therefore, it is important to encourage patients to return to work and/or participate in social activities, which may help them to recognize their social roles and rebuild their confidence.
In this study, we also found that being female, diabetes, and rGFR were strongly associated with depressive symptoms in CAPD patients, which is consistent with the recent study findings. For example, Gerogianni et al. found that depression and anxiety were significantly associated with being female and with some comorbidities [26]. Depression is also more common in diabetes patients (OR 2.03, 95% CI 1.09–3.81) [3]. Residual renal function also plays an important role in the association between depression and impaired health-related QOL in PD patients [27]. Because of these risk factors, we paid more attention to female patients, helped diabetic patients control blood glucose and complications, and took active measures to protect residual renal function during the follow-up period.
Depression correlates with adverse clinical outcomes in dialysis patients, including malnutrition [7], poor treatment adherence [8], reduced QOL [9], increased rate of peritonitis [10], and mortality [11, 12, 20]. The between-group differences in 1-, 3-, 5-, and 10-year survival rates in our study indicated that depressive symptoms had significant impacts on both short-term and long-term survival rates of patients with CAPD. Riezebos et al. found that the 1-year mortality of dialysis patients with depression was higher than in those without depression (26% and 8%, respectively) [28]. Chilcot et al. showed that the survival rate at 18 months was 74.9% for depressed patients and 87.6% for non-depressed patients [12]. Because there is less research on depression in PD patients than in hemodialysis patients, there are a few reports about the survival of PD patients followed up for more than 5 years. In this study, depressive symptoms remained independent predictive risk factors for death in CAPD patients after adjusting for age, gender, diabetes, PD duration at enrollment, CVD history, serum albumin, serum sodium, hsCRP, total weekly Kt/V, and rGFR. This is consistent with some previous findings [11, 12, 28]. Riezebos et al. found that depressive symptoms were negatively correlated with patient survival [28]. However, the mechanism by which depression increases the risk of death in PD patients is unclear. Kimmel has suggested that the association between accelerated death and depression may be related to medical care, treatment adherence, nutritional status, immune function, interpersonal relationships, and medication effects, as well as suicide [29]. Simic Ogrizovic et al. [20] found that depression was closely related to inflammatory indicators, malnutrition, and cardiovascular death. Some scholars have also found that depression affects albumin levels, and poor nutritional status may mediate the relationship between depression and mortality in ESRD [7]. The sensitivity analysis shows that while depressive symptoms are numerically associated with all-cause mortality also at lower cutoff values on the BDI, the higher cutoff used in the manuscript clearly showed the best predictive effect. This may indicate that more severe depressive symptoms are likely to be stronger indicators of increased mortality risk than milder symptoms.
The present study showed that patients in the depressive group had poorer technical survival rates than those in the non-depressive group. Such a relationship between depressive symptoms and technique failure in PD has rarely been reported. Similarly, Griva et al. showed that anxiety affects the technical survival rate of PD patients [30]. Therefore, psychological status may affect technical survival in PD patients; however, the reasons for this are unclear. Troidle et al. found that a BDI score > 11 increases the risk of peritonitis in long-term PD patients, and peritonitis is the main cause of technical failure for these patients [10]. In the present study, the prevalence of peritonitis was higher in the depressive group than in the non-depressive group, although the difference was not significant (0.15 episodes/patient year vs. 0.12 episodes/patient year, P = 0.499). The percentage of technique failure was higher in the depressive group than in the non-depressive group (36.0% vs. 23.8%, P = 0.042) and the main cause of technique failure was peritonitis in both groups (71.0% vs. 64.4%, P = 0.550). Depression may reduce treatment and medication compliance [8, 31, 32], which may weaken therapeutic effects and result in inadequate dialysis or ultrafiltration problems. However, these inferences cannot be confirmed by the additional analysis in the present study. Further studies are needed to confirm the present findings, and we should pay more attention to technique failure in PD patients with depressive symptoms.
The present study had some limitations. First, this was a single-center cohort study in which selection bias could not be avoided. Second, this was an observational study, so we cannot predict whether the clinical outcomes would be improved by a depression intervention. Third, owing to the high drop-out rate during the follow-up, we did not obtain a regular evaluation of depression status and, therefore, could not obtain a longitudinal depression assessment. In addition, our study only focused on the assessment of depressive symptoms, while there was no complete diagnosis of depression, may limit the understanding of the significance of depression in this population.
Conclusions
The prevalence of patients with depressive symptoms was high in CAPD patients in our study population. Long-term patient survival and technique survival were poor in CAPD patients with depressive symptoms. Depressive symptoms were independent risk factors of all-cause mortality and technique failure. More attention needs to be paid in the clinic to patients’ psychological problems, regular screening to identify depressive symptoms, and the provision of timely intervention and treatment. These measures may improve clinical outcomes in CAPD patients.
References
Palmer SC, Vecchio M, Craig JC, Tonelli M, Johnson DW, Nicolucci A, Pellegrini F, Saglimbene V, Logroscino G, Hedayati SS, Strippoli GF (2013) Association between depression and death in people with CKD: a meta-analysis of cohort studies. Am J Kidney Dis 62(3):493–505. https://doi.org/10.1053/j.ajkd.2013.02.369
Finkelstein FO, Finkelstein SH (2000) Depression in chronic dialysis patients: assessment and treatment. Nephrol Dial Transplant 15(12):1911–1913. https://doi.org/10.1093/ndt/15.12.1911
Mok MMY, Liu CKM, Lam MF, Kwan LPY, Chan GCW, Ma MKM, Yap DYH, Chiu F, Choy CBY, Tang SCW, Chan TM (2019) A longitudinal study on the prevalence and risk factors for depression and anxiety, quality of life, and clinical outcomes in incident peritoneal dialysis patients. Perit Dial Int 39(1):74–82. https://doi.org/10.3747/pdi.2017.00168
Ko GJ, Kim MG, Yu YM, Jo SK, Cho WY, Kim HK (2010) Association between depression symptoms with inflammation and cardiovascular risk factors in patients undergoing peritoneal dialysis. Nephron Clin Pract 116(1):c29–35. https://doi.org/10.1159/000314548
Lin J, Guo Q, Ye X, Li J, Yi C, Zhang X, Wu X, Cao P, Yu X, Zhu L, Lin X, Yang X (2013) The effect of social support and coping style on depression in patients with continuous ambulatory peritoneal dialysis in southern China. Int Urol Nephrol 45(2):527–535. https://doi.org/10.1007/s11255-012-0309-7
Ibrahim N, Chiew-Thong NK, Desa A, Razali R (2013) Depression and coping in adults undergoing dialysis for end-stage renal disease. Asia Pac Psychiatry 5(Suppl 1):35–40. https://doi.org/10.1111/appy.12042
Friend R, Hatchett L, Wadhwa NK, Suh H (1997) Serum albumin and depression in end-stage renal disease. Adv Perit Dial 13:155–157
Kauric-Klein Z (2017) Depression and medication adherence in patients on hemodialysis. Curr Hypertens Rev 13(2):138–143. https://doi.org/10.2174/1573402113666171129182611
John MM, Gupta A, Sharma RK, Kaul A (2017) Impact of residual renal function on clinical outcome and quality of life in patients on peritoneal dialysis. Saudi J Kidney Dis Transpl 28(1):30–35. https://doi.org/10.4103/1319-2442.198109
Troidle L, Watnick S, Wuerth DB, Gorban-Brennan N, Kliger AS, Finkelstein FO (2003) Depression and its association with peritonitis in long-term peritoneal dialysis patients. Am J Kidney Dis 42(2):350–354. https://doi.org/10.1016/s0272-6386(03)00661-9
Einwohner R, Bernardini J, Fried L, Piraino B (2004) The effect of depressive symptoms on survival in peritoneal dialysis patients. Perit Dial Int 24(3):256–263
Chilcot J, Davenport A, Wellsted D, Firth J, Farrington K (2011) An association between depressive symptoms and survival in incident dialysis patients. Nephrol Dial Transplant 26(5):1628–1634. https://doi.org/10.1093/ndt/gfq611
Farrokhi F, Abedi N, Beyene J, Kurdyak P, Jassal SV (2014) Association between depression and mortality in patients receiving long-term dialysis: a systematic review and meta-analysis. Am J Kidney Dis 63(4):623–635. https://doi.org/10.1053/j.ajkd.2013.08.024
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J (1961) An inventory for measuring depression. Arch Gen Psychiatry 4:561–571. https://doi.org/10.1001/archpsyc.1961.01710120031004
Yang JY, Huang JW, Peng YS, Chiang SS, Yang CS, Yang CC, Chen HW, Wu MS, Wu KD, Tsai TJ, Chen WY (2007) Quality of sleep and psychosocial factors for patients undergoing peritoneal dialysis. Perit Dial Int 27(6):675–680
Wilson B, Spittal J, Heidenheim P, Herman M, Leonard M, Johnston A, Lindsay R, Moist L (2006) Screening for depression in chronic hemodialysis patients: comparison of the Beck Depression Inventory, primary nurse, and nephrology team. Hemodial Int 10(1):35–41. https://doi.org/10.1111/j.1542-4758.2006.01172.x
O'Donnell K, Chung JY (1997) The diagnosis of major depression in end-stage renal disease. Psychother Psychosom 66(1):38–43. https://doi.org/10.1159/000289104
King-Wing Ma T, Kam-Tao Li P (2016) Depression in dialysis patients. Nephrology 21(8):639–646. https://doi.org/10.1111/nep.12742
Palmer S, Vecchio M, Craig JC, Tonelli M, Johnson DW, Nicolucci A, Pellegrini F, Saglimbene V, Logroscino G, Fishbane S, Strippoli GF (2013) Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int 84(1):179–191. https://doi.org/10.1038/ki.2013.77
Simic Ogrizovic S, Jovanovic D, Dopsaj V, Radovic M, Sumarac Z, Bogavac SN, Stosovic M, Stanojevic M, Nesic V (2009) Could depression be a new branch of MIA syndrome? Clin Nephrol 71(2):164–172. https://doi.org/10.5414/cnp71164
Kwan E, Draper B, Harvey SB, Endre ZH, Brown MA (2019) Prevalence, detection and associations of depression in Australian dialysis patients. Australas Psychiatry 27(5):444–449. https://doi.org/10.1177/1039856219859281
Katon WJ (2011) Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin Neurosci 13(1):7–23
Raison CL, Capuron L, Miller AH (2006) Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 27(1):24–31. https://doi.org/10.1016/j.it.2005.11.006
Zunszain PA, Hepgul N, Pariante CM (2013) Inflammation and depression. Curr Top Behav Neurosci 14:135–151. https://doi.org/10.1007/7854_2012_211
Chan R, Brooks R, Erlich J, Chow J, Suranyi M (2009) The effects of kidney-disease-related loss on long-term dialysis patients' depression and quality of life: positive affect as a mediator. Clin J Am Soc Nephrol 4(1):160–167. https://doi.org/10.2215/CJN.01520308
Gerogianni G, Lianos E, Kouzoupis A, Polikandrioti M, Grapsa E (2018) The role of socio-demographic factors in depression and anxiety of patients on hemodialysis: an observational cross-sectional study. Int Urol Nephrol 50(1):143–154. https://doi.org/10.1007/s11255-017-1738-0
Park HC, Lee H, Lee JP, Kim DK, Oh KH, Joo KW, Lim CS, Kim YS, Ahn C, Oh YK (2012) Lower residual renal function is a risk factor for depression and impaired health-related quality of life in Korean peritoneal dialysis patients. J Korean Med Sci 27(1):64–71. https://doi.org/10.3346/jkms.2012.27.1.64
Riezebos RK, Nauta KJ, Honig A, Dekker FW, Siegert CE (2010) The association of depressive symptoms with survival in a Dutch cohort of patients with end-stage renal disease. Nephrol Dial Transplant 25(1):231–236. https://doi.org/10.1093/ndt/gfp383
Kimmel PL (2002) Depression in patients with chronic renal disease: what we know and what we need to know. J Psychosom Res 53(4):951–956. https://doi.org/10.1016/s0022-3999(02)00310-0
Griva K, Kang AW, Yu ZL, Lee VY, Zarogianis S, Chan MC, Foo M (2016) Predicting technique and patient survival over 12 months in peritoneal dialysis: the role of anxiety and depression. Int Urol Nephrol 48(5):791–796. https://doi.org/10.1007/s11255-015-1191-x
Loghman-Adham M (2003) Medication noncompliance in patients with chronic disease: issues in dialysis and renal transplantation. Am J Manag Care 9(2):155–171
Yu ZL, Yeoh LY, Seow YY, Luo XC, Griva K (2012) Evaluation of adherence and depression among patients on peritoneal dialysis. Singap Med J 53(7):474–480
Acknowledgements
We thank all the doctors and nurses in our PD center for their patient care and data collection. We also thank Diane Williams, PhD, from Liwen Bianji, Edanz Group China (https://www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
This work was supported by the Natural Science Foundation of China (Grant no. 81774069), the National Key R&D Program of China (2016YFC0906101), an Operational Grant of Guangdong Provincial Key Laboratory (2017B030314019), the Science and Technology Planning Project of Guangdong Province of China (A2017054), and Guangdong Provincial Programme of Science and Technology (2017A050503003).
Author information
Authors and Affiliations
Contributions
JXL and HJY contributed equally to this work for the protocol development, data collection and analysis, drafting of the MS. JYL, CYY, XLY, and HPM were involved in protocol development, gaining ethical approval, patient recruitment and data analysis. XQY and XY are the senior authors and main contributor to the design, interpretation of data, and final approval of the version to be published. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We have read and understood International Urology and Nephrology's policy on conflicts of interest disclosure and declare that we have none.
Ethical approval
The study was conducted in compliance with the Declaration of Helsinki guidelines. The study protocol was approved by the ethics committee of The First Affiliated Hospital of Sun Yat-sen University. We obtained written informed consent from all patients at recruitment.
Research involving human participants
All participants provided written informed consent before participation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, J., Ye, H., Yi, C. et al. The negative impact of depressive symptoms on patient and technique survival in peritoneal dialysis: a prospective cohort study. Int Urol Nephrol 52, 2393–2401 (2020). https://doi.org/10.1007/s11255-020-02593-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-020-02593-w