Abstract
Purpose
Scientific data regarding intravenous iron supplementation in peritoneal dialysis (PD) patients are scarce. In attempting to administer the minimum monthly IV iron dose that could improve erythropoiesis, we wanted to assess the safety and efficacy of monthly maintenance intravenous administration of 100 mg iron sucrose in PD patients.
Methods
In a 9-month prospective study, all clinically stable PD patients received intravenously 200 mg of iron sucrose as a loading dose, followed by monthly doses of 100 mg for five consecutive months. Levels of hemoglobin (Hb), ferritin, transferrin saturation (TSAT), reticulocyte hemoglobin content (CHr) and C-reactive protein (CRP) were measured before each administration and 3 months after the last iron infusion. Also, doses of concurrent erythropoietin administration were recorded.
Results
Eighteen patients were eligible for the study. Mean levels of Hb and ferritin increased significantly (from 10.0 to 10.9 mg/dL, p = 0.01 and from 143 to 260 ng/mL, p = 0.005), as well as the increase in TSAT levels approached borderline significance (from 26.2 to 33.1%, p = 0.07). During the 6 months of iron administration, the erythropoietin dose was reduced in five patients and discontinued in one. During the 3 months following the last iron infusion, three of them again raised the erythropoietin dose to previous levels. None of the patients experienced any side effects related to IV iron administration.
Conclusions
A monthly maintenance intravenous dose of 100 mg iron sucrose may be a practical, effective, and safe in the short term, treatment of anemia in PD patients resulting in improved hemoglobin levels, iron indices, and erythropoietin response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anemia treatment in peritoneal dialysis (PD) patients usually requires erythropoietin (EPO) to be supported by iron administration. Compared to oral administration and regardless of iron status, intravenous (IV) iron supplementation has proven to be more effective, allowing for EPO dose reduction [1,2,3]. Furthermore, regular IV iron administration is currently regarded to be practical and safe, especially after the introduction of iron sucrose and other newer IV iron preparations that allow for rapid or even bolus injection.
However, even though IV iron is supported as standard practice by current guidelines, such as Kidney Disease Improving Global Outcomes (KDIGO), there are still concerns regarding potential adverse effects, including oxidative stress from labile iron and increased risk of infection [4]. In a recent study from China, only 18.3% of PD patients received IV iron [5]. Data from the US show that between 2007 and 2011, the percentage of PD patients on IV iron was low, even though it was increased from 19.7% in the first quarter of 2007 to 36.7% in the last quarter of 2011. In these patients, the monthly IV iron dose ranged from 127 to 151 mg and the mean EPO dose was reduced by 76.5%, probably resulting from the increase of IV iron use by 39.3% [6].
Data regarding IV iron supplementation in PD patients are limited. The determination of the lowest effective IV iron dose and its frequency of administration remains a challenge for these patients. A weekly low dose administration, which is feasible in hemodialysis patients, is rather impractical in PD patients, due to the monthly outpatient visits. As a result, relatively higher doses should be administered at monthly or even longer intervals.
In this study, we first investigated the efficacy and safety of a maintenance monthly IV dose of 100 mg iron sucrose in PD patients.
Methods
This is a prospective study conducted in one dialysis center over a period of 9 months. Inclusion criteria for enrolment into the study were: age greater than 18 years and duration on PD longer than 3 months, hemoglobin (Hb) levels between 9 and 11.5 g/dL, stable EPO dose, and no iron therapy (oral or IV or phosphate binders containing iron) for at least 8 weeks, ferritin and transferrin saturation (TSAT) levels less than 500 ng/mL and 45%, respectively, and no hematological disorder except anemia due to chronic renal failure. Patients with chronic or serious acute infection, malignancy, major surgery, clinically significant bleeding or blood transfusion within 3 months before enrolment were excluded. The study protocol was approved by the institutional review board of the Papageorgiou General Hospital. Written informed consent was obtained from each subject prior to enrolment in the study.
However, most clinical guidelines recommend iron therapy for dialysis patients, when TSAT is < 30% and ferritin is < 300 or < 500 ng/mL [7]. In the present study, the upper limit of TSAT was higher (< 45%) for the following reasons. First, because the above guidelines mainly concern the common practice of administering a course of IV iron amounting to approximately 1000 mg, while the monthly dose of iron given in our study was significantly lower (100 mg) and second because, according to the literature, TSAT and ferritin levels were most often unchanged from before to after IV iron dosing of < 300 mg/month [8].
According to the treatment protocol, patients received a total of six monthly IV doses of iron sucrose, during their regular visit at the PD outpatient clinic. The first dose was 200 mg and was followed by five monthly doses of 100 mg. Iron sucrose was diluted in 100 ml of normal saline and infused in a peripheral vein over 15–30 min.
Our primary outcomes were the change in Hb, ferritin, and TSAT levels and the secondary outcome was the change in EPO dose. Levels of Hb, ferritin, TSAT, reticulocyte hemoglobin content (CHr), and C-reactive protein (CRP) were measured before each dose administration and at 3 months after the last (sixth) iron infusion, along with the routine monthly blood test (urea, creatinine, electrolytes, albumin, intact parathyroid hormone [iPTH]). Also, the dosage of EPO and the EPO resistance index (quotient of EPO dose in units/kg/week and hemoglobin in g/dL) were recorded. Moreover, we recorded information on PD modality (continuous ambulatory vs. cycler-assisted PD); PD solutions and total volume exchanges on dialysis, residual urine output, transport status of peritoneal membrane, and adequacy parameters on PD (total and peritoneal Kt/V).
Iron supplementation was withdrawn before the sixth dose either if Hb exceeded 12.5 g/dL or two consecutive levels of ferritin or TSAT exceeded 800 ng/mL or 55%, respectively. Patients dropped out of the study in case of a severe infection or clinically significant blood loss. EPO was administered subcutaneously and the EPO dose was adjusted monthly, if Hb levels were increased or decreased by more than 1.5 g/dL or exceeded 12.5 g/dL or reduced below 9 g/dL.
To estimate the daily exposure to glucose due to peritoneal dialysis solutions (g/day), we summed the glucose content [product of dialysate volume (L) and dialysate glucose concentration (g/L)] of each exchange performed over a 24-h period. Patient peritoneal transporter status was determined by 4-h peritoneal dialysate to plasma (D/P) creatinine ratio. CHr and standard hematology parameters were determined using the ADVIA®120 Hematology System (Bayer Corporation, Tarrytown, NY, USA). Serum levels of CRP were determined quantitatively by rate nephelometry in the IMMAGE Beckman Coulter analyte with normal range < 0.8 mg/dL.
Statistical analysis
Continuous data are presented as mean ± SD. Differences in patients’ characteristics at baseline, 1 month after the last iron infusion and 3 months later were tested by Wilcoxon signed-rank test or Mann–Whitney U test, as appropriate. The Chi-squared test was used to examine differences between categorical variables. Pearson’s correlation coefficients were calculated to investigate a possible relationship between the baseline clinical characteristics and laboratory parameters and the actual Hb change before and after the iron supplementation. A p value of less than 0.05 was considered significant.
Results
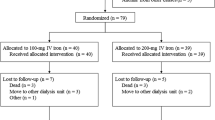
There were 18 patients eligible for this study. Three patients dropped out of the study in the first trimester, due to gastrointestinal bleeding, severe peritonitis, and foot ulcer with CRP increase at levels higher than 5 mg/dL. None of these disorders were attributed to iron administration, but rather to other causes.
Fifteen patients (seven males, three diabetics) were included in the final analysis. Among them, four were on continuous ambulatory PD and 11 on cycler-assisted PD. All patients were dialyzed with a standard glucose-based dialysis fluids and six of them had an additional exchange with icodextrin dialysate. The total daily exchanged volume during PD was 9.5 ± 0.9 L and the daily glucose exposure load was 170 ± 44 g. Their mean age was 70 ± 15 years and their mean PD duration was 25 ± 19 months. Twelve of them had residual diuresis greater than 300 mL per day and no one had a total Kt/V of less than 1.7. At study entry, the mean iPTH level was 274 ± 181 ng/mL and the mean EPO dose was 72.2 ± 88.8 units/kg/week. Three patients were not on EPO treatment and remained so, until the end of the study. Iron supplementation was withdrawn after three doses in two patients due to an increase in Hb levels above 12.5 mg/dL. The remaining 13 patients received all six monthly doses. No adverse reactions related to IV iron administration were observed, either during the treatment or the later follow-up period.
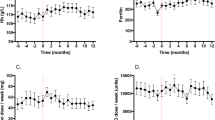
By the end of iron treatment, there was a significant elevation of the mean Hb level, from 10.0 to 10.9 mg/dL (p = 0.01) and the mean ferritin level from 143 to 260 ng/mL (p = 0.005). There was also an increase in the mean level of TSAT from 26.2 to 33.1% that approached borderline significance (p = 0.07). Also, mean levels of CHr and CRP showed an increase from 31.1 to 31.3 pg and from 1.1 to 1.3 mg/dL, although it was not statistically significant, p = 0.63 and p = 0.76, respectively (Table 1).
During the treatment period, nine patients showed an increase in Hb levels of greater than 1 g/dL and were considered to be iron responders. In one of these nine patients, treatment with EPO (2000 units/week) was discontinued and in five patients, the EPO dose was reduced by 27%. One patient was maintained on a stable EPO dose. The remaining two iron responders were not on EPO. In total, EPO dose was significantly reduced from a mean dose of 92.3 units/kg/week before iron supplementation to 71.1 units/kg/week, 1 month following the last iron infusion, p = 0.03. Also, the mean EPO resistance index was significantly reduced from 10.6 to 7.2, p = 0.02 (Table 1).
In the remaining six patients, Hb levels remained stable at a mean value of 10.4 g/dL before and after iron administration and they were considered to be non-responders. Regarding EPO treatment, one of them was not on EPO, in two patients, EPO dose remained unchanged, and in the remaining three, a low mean dose of 28.5 units/kg/week at baseline was increased to 65.2 units/kg/week by the end of the 6 months of iron treatment (Table 1).
During the 3-month follow-up throughout which no iron was administered, the dose of EPO was adjusted as follows: one patient who had stopped EPO treatment resumed it and in two patients who had reduced their EPO dose, it increased to previous levels.
Dividing patients by baseline ferritin and TSAT levels, we had four groups: those with absolute iron deficiency (ferritin ≤ 100 and TSAT ≤ 20%, n = 3), those with functional iron deficiency (ferritin > 100 and TSAT ≤ 20, n = 1), those who had replete iron stores (ferritin > 100 and TSAT > 20, n = 7), and a group with TSAT > 20 and ferritin ≤ 100 (n = 4). From the nine patients who responded to iron administration, two had absolute iron deficiency, four had replete iron stores (with TSAT levels of 25%, 34%, 37%, 41%, and ferritin levels of 264, 360, 147, 246 ng/mL, respectively) and the remaining three were in the 4th group. If patients in the 4th group, due to low ferritin levels, are considered to be iron deficient as those in the 1st and 2nd groups, then five out of eight (63%) iron-deficient patients responded to treatment and four out of seven (57%) non-iron deficient patients also responded to treatment (p = 1.00).
Baseline patients’ characteristics in iron responders and non-responders are summarized in Table 2. Only serum albumin levels were significantly higher in the iron responder group compared to the non-responder group. Also, correlations between baseline patients’ characteristics and the actual changes in Hb levels before and after iron supplementation as continuous variable were not significant. However, serum albumin levels again had the highest correlation coefficient (r = 0.36, p = 0.19).
Discussion
The current study is the first one examining the effect of maintenance monthly IV dose of 100 mg iron sucrose in chronic PD patients. According to our results, 100 mg of iron sucrose administered monthly are sufficient to significantly raise Hb levels, while simultaneously reducing EPO requirements in these patients. Furthermore, this small monthly dose was able to increase ferritin levels significantly, as well as TSAT levels with borderline significance. These results are important, since they may provide guidance for determining an intravenous iron supplementation strategy in PD patients.
Compared to patients on chronic hemodialysis, far fewer patients on PD receive IV iron supplementation. Accordingly, there are very few studies regarding IV iron administration in PD patients. In those studies, the monthly iron dose is usually well above 100 mg, reaching up to 1200 mg of iron dextran [2, 9,10,11,12,13,14]. Also, many studies, mainly on hemodialysis patients, report on the potential hazards associated with IV iron overdosage, due to oxidative stress, endothelial dysfunction, immune dysfunction, and risk of infection. Particularly in PD patients, there are concerns as to whether the proinflammatory state caused by iron administration could alter peritoneal clearance rates or affect residual renal clearance [15, 16]. Because of that, in PD patients, it is very crucial to determine the lowest effective IV iron dose, to minimize potential risks associated with iron toxicity.
To our best knowledge there are three studies investigating IV iron supplementation in PD patients with a dosing scheme similar to that of our study. Johnson et al. [3] were able to demonstrate improved body iron stores and an enhanced hemopoietic response to EPO in 16 iron replete PD outpatients, with a 200 mg IV iron infusion every second month over 4 months (in total two doses were administered). There was a significant increase in Hb levels (+ 0.6 g/dL) during the IV iron administration phase, even though a significant reduction in EPO requirements was not apparent in the relatively short follow-up period. The total duration of iron infusion was 1 h and 45 min. Because, the 200 mg dosage every 2 months requires at least twice the time spent in the clinic, we consider that a scheme of 100 mg per month is more practical and with fewer side effects. In the second study, involving 17 stable peritoneal dialysis patients [17] administered monthly IV iron saccharate over 6 months. The dose was adjusted according to TSAT (100 mg if TSAT was greater than 20% or 200 mg if TSAT was less than 20%). There was a significant increase in TSAT levels from 12.1 to 20.9% and EPO requirements decreased significantly from 148 to 69 units/kg/week, but there was no significant change in ferritin and hematocrit levels. Adverse events such as vertigo, hypotension, and vomiting occurred in 0.9% of the 100 mg iron infusions and in 5.9% of the 200 mg iron infusions. Even though the exact number of patients receiving 200 mg is not mentioned in the study, it is implied that they were the majority, since most of the study subjects demonstrated a TSAT less than 20% during the study (mean TSAT was 12.1 ± 1.6% at baseline and 20.9 ± 2.4% at the end of the study). Lastly, Dittrich et al. [18] administered low-dose iron sucrose in 45 PD patients for a year. Patients were divided into three groups: one of absolute iron deficiency (serum ferritin < 100 ng/mL), one of functional iron deficiency (ferritin ≥ 100 ng/mL and TSAT < 20%), and one of replete iron stores (ferritin ≥ 100 ng/mL and TSAT ≥ 20%). The first group received 50 mg iron sucrose IV every second week, while the two others were given 50 mg monthly. Only the absolute iron deficiency group showed a significant decrease in EPO dose and a significant increase of ferritin levels. On the contrary, in the other two groups, ferritin levels did not change significantly and there was an increase in EPO requirements. TSAT did not change significantly in any of the groups. The authors suggest that the 50 mg monthly dose was too low to be effective in patients with functional iron deficiency or replete iron stores. Respectively, in our study, eight patients without absolute iron deficiency (ferritin ≥ 100 ng/mL) showed a significant increase in hemoglobin levels (from 10.0 to 10.7 mg/dL, p = 0.02), probably due to the higher monthly dose of iron they received (100 mg). Levels of ferritin and TSAT also increased, even though this did not reach statistical significance. Therefore, since the monthly dose of 50 mg was shown to be ineffective in the above study [18], the monthly dose of 100 mg, as administered in our study, appears to be the lowest effective monthly dose tested to date in PD patients.
Finally, among the iron deficiency indices and the other parameters determined in the present study, only baseline serum albumin levels differed significantly between the patients who responded to IV iron administration and the patients who did not. Even though serum albumin concentration has been shown to predict EPO response in chronic hemodialysis patients [19], there are currently no studies associating serum albumin levels or any other parameter with a hemopoietic response to iron administration in PD patients. Indeed, in a study by Domrongkitchaiporn et al. [20], baseline EPO dose, iron indices, serum albumin, and iPTH did not differ significantly in patients who responded to intravenous iron therapy and in those who did not. Likewise, Singh et al. [10] were not able to show any association between response to iron treatment and baseline TSAT or ferritin. Similarly in our study, the response to iron administration did not differ significantly between iron deficient and non-iron deficient patients (63% vs. 57%, respectively).
In addition, although no acute side effects were observed during a 9-month follow-up, many questions regarding the safety of IV iron administration have not been addressed in the present study. These include concerns such as increasing the risk for long-term infection, enhancing oxidative stress, or the induction of other immunomodulatory effects. Another limitation of the present study is the small number of patients, although it has produced significant results in increasing Hb and ferritin levels. One more limitation is the lack of a control group. However, unfortunately, in PD patients, there are no adequate and controlled studies recommending the optimal dose of IV iron supplementation. Therefore, this is a common limitation of all similar studies yet reported. Due to that lack, the current study appears to add substantial evidence to the limited available data regarding IV iron administration in PD patients.
In conclusion, in an effort to administer the minimum monthly IV iron dose that could improve hemoglobin levels and at the same time enhance response to EPO, we demonstrated for the first time that a once monthly IV administration of 100 mg iron sucrose appears to be safe in the short term and effective in treating anemia in PD outpatients.
References
Pandey R, Daloul R, Coyne DW (2016) Iron treatment strategies in dialysis-dependent CKD. Semin Nephrol 36(2):105–111
Li H, Wang SX (2008) Intravenous iron sucrose in peritoneal dialysis patients with renal anemia. Perit Dial Int 28(2):149–154
Johnson DW, Herzig KA, Gissane R, Campbell SB, Hawley CM, Isbel NM (2001) A prospective crossover trial comparing intermittent intravenous and continuous oral iron supplements in peritoneal dialysis patients. Nephrol Dial Transpl 16(9):1879–1884
Locatelli F, Barany P, Covic A, De Francisco A, Del Vecchio L, Goldsmith D et al (2013) Kidney disease: improving global outcomes guidelines on anaemia management in chronic kidney disease: a European renal best practice position statement. Nephrol Dial Transpl 28(6):1346–1359
Liu H, Yao Y, Cao Y, Yang X, Huang B, Han X et al (2015) Anemia management trends in patients on peritoneal dialysis in the past 10 years. Int J Clin Exp Med 8(10):18050–18057
Wetmore JB, Peng Y, Monda KL, Kats AM, Kim DH, Bradbury BD et al (2015) Trends in anemia management practices in patients receiving hemodialysis and peritoneal dialysis: a retrospective cohort analysis. Am J Nephrol 41(4–5):354–361
Del Vecchio L, Locatelli F (2017) Clinical practice guidelines on iron therapy: a critical evaluation. Hemodial Int 21:S125–S131
Robinson BM, Larkina M, Bieber B, Kleophas W, Li Y, Locatelli F et al (2017) Evaluating the effectiveness of IV iron dosing for anemia management in common clinical practice: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). BMC Nephrol 18(1):330
Moniem KA, Bhandari S (2007) Bolus intraperitoneal iron versus intravenous iron in peritoneal dialysis patients: a prospective study. Transfus Altern Transfus Med 9(2):101–107
Singh H, Reed J, Noble S, Cangiano JL, Van Wyck DB (2006) Effect of intravenous iron sucrose in peritoneal dialysis patients who receive erythropoiesis-stimulating agents for anemia: a randomized, controlled trial. Clin J Am Soc Nephrol 1(3):475–482
Prakash S, Walele A, Dimkovic N, Bargman J, Vas S, Oreopoulos D (2001) Experience with a large dose (500 mg) of intravenous iron dextran and iron saccharine in peritoneal dialysis patients. Perit Dial Int 21(3):290–295
Ahsan Ν (1998) Intravenous infusion of total dose iron is superior to oral iron in treatment of anemia in peritoneal dialysis patients: a single center comparative study. J Am Soc Nephrol 9(4):664–668
Silverberg DS, Blum M, Peer G, Kaplan E, Iaina A (1996) Intravenous ferric saccharate as an iron supplement in dialysis patients. Nephron 72(3):413–417
Richardson D, Bartlett C, Jolly H, Will EJ (2001) Intravenous iron for CAPD populations: proactive or reactive strategies? Nephrol Dial Transpl 16(1):115–119
Zager RA (2005) Parenteral iron treatment induces MCP-1 accumulation in plasma, normal kidneys, and in experimental nephropathy. Kidney Int 68(4):1533–1542
Agarwal R, Vasavada N, Sachs NG, Chase S (2004) Oxidative stress and renal injury with intravenous iron in patients with chronic kidney disease. Kidney Int 65(6):2279–2289
Vychytil A, Haag-Weber M (1999) Iron status and iron supplementation in peritoneal dialysis patients. Kidney Int Suppl 69:S71–S78
Dittrich E, Schillinger M, Sunder-Plassmann G, Hörl WH, Vychytil A (2002) Efficacy of a low-dose intravenous iron sucrose regimen in peritoneal dialysis patients. Perit Dial Int 22(1):60–66
Agarwal R, Davis JL, Smith L (2008) Serum albumin is strongly associated with erythropoietin sensitivity in hemodialysis patients. Clin J Am Soc Nephrol 3(1):98–104
Domrongkitchaiporn S, Jirakranont B, Atamasrikul K, Ungkanont A, Bunyaratvej A (1999) Indices of iron status in continuous ambulatory peritoneal dialysis patients. Am J Kidney Dis 34(1):29–35
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mitsopoulos, E., Lysitska, A., Pateinakis, P. et al. Efficacy and safety of a low monthly dose of intravenous iron sucrose in peritoneal dialysis patients. Int Urol Nephrol 52, 387–392 (2020). https://doi.org/10.1007/s11255-019-02362-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-019-02362-4