Abstract
Background
The rehabilitation of post-prostatectomy urinary incontinence has traditionally focused on pelvic floor strengthening exercise. The goal of this study was to determine whether an individualized pelvic physical therapy (PT) program aimed at normalizing both underactive and overactive pelvic floor dysfunction (PFD) can result in improvement in post-prostatectomy stress urinary incontinence (SUI) and pelvic pain.
Methods
A retrospective chart review of 136 patients with post-prostatectomy SUI and treated with pelvic PT. Patients were identified as having either underactive, overactive, or mixed-type PFD and treated accordingly with a tailored program to normalize pelvic floor function. Outcomes including decrease in SUI as measured in pad usage per day and pain rated on the numeric pain rating scale.
Results
Twenty five patients were found to have underactive PFD and were treated with strengthening. Thirteen patients had overactive PFD and were treated with relaxation training. Ninety eight patients had mixed-type PFD and were treated with a combination of relaxation training followed by strengthening. Patients demonstrated statistically significant decrease in pad usage per day (p < 0.001), decreased pelvic pain (p < 0.001), and increased pelvic floor strength (p = 0.049), even in patients who received predominantly pelvic floor relaxation training to normalize pelvic floor overactivity.
Conclusions
A majority of post-prostatectomy men with SUI have pelvic floor overactivity in addition to pelvic floor underactivity. An individualized pelvic PT program aimed at normalizing pelvic floor function (as opposed to a pure Kegel strengthening program) can be helpful in reducing SUI and pelvic pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As defined by the International Continence Society, stress urinary incontinence (SUI) is the involuntary leakage of urine with effort or exertion, including sneezing and coughing [1]. It is one of the most feared complications of a prostatectomy and has been shown to be an independent predictor of global quality of life (QOL) with a prevalence ranging from 2 to 90% [2,3,4,5,6,7,8]. This incontinence rate typically decreases over time; however 5–20% of men will continue to have some degree of incontinence 1–2 years after surgery [2,3,4,5,6,7,8]. A number of conservative and invasive methods have been developed to address SUI, including: lifestyle adjustment, pelvic floor muscle training (PFMT) with or without the use of biofeedback (BF), extracorporeal magnetic innervation, external pelvic compression devices, oral pharmacotherapy, injectable bulking agents, and surgical implantation of a male sling or an artificial sphincter [4, 9, 10].
PFMT is thought to be beneficial, because it normalizes pelvic muscle function and teaches the patient to compensate for the loss of urethral closing pressure which results after surgery. Current published pelvic physical therapy (PT) protocols for SUI following prostatectomy focus solely on strengthening exercises (often termed Kegel exercises or “uptraining”) [4, 5, 8, 11]. This method, however, only addresses one of the possible types of pelvic floor dysfunction (PFD)—weakness. The types of PFD can be broadly divided into three main categories: weakness (termed “underactivity”), tightness or muscle spasm (termed “overactivity” and often accompanied by muscle shortening), and abnormal coordination with poorly timed or inappropriate movement (termed “dyssynergia”) [13]. There is a growing trend in pelvic rehabilitation to avoid a one-size-fits-all approach to treatment for any particular diagnosis, with pelvic floor physical therapists instead focusing on the normalization each patient’s individual type PFD to achieve maximal functional results [12]. To date, however, there are no studies of rehabilitation for post-prostatectomy SUI that document the efficacy of therapies that are tailored for the patient based on pelvic floor physical examination findings.
We theorized that an individualized pelvic floor therapy program aimed at normalizing pelvic floor function would improve incontinence and also reduce post-prostatectomy pain (the presence of which is rarely reported in the pelvic rehabilitation literature for this patient population). We further hypothesized that there is a subset of post-prostatectomy patients for whom Kegel exercises alone may be ineffective due to the presence of overactivity as the chief form of PFD. To this end, a retrospective review was conducted to determine the types of PFD seen in post-prostatectomy men with SUI and to evaluate their response to individualized treatment regimens.
Methods
Approval was obtained from our institution’s IRB for a retrospective chart review of patients who received robotic-assisted prostatectomies (performed by the same urologist, CR) and were referred for PT at our institution. Inclusion criteria captured any patient who underwent a robotic-assisted prostatectomy by a single urologist at our facility which was followed by a course of pelvic PT for urinary incontinence between 1/1/2009 and 1/31/2014. Exclusion criteria included participating in PT for any reason unrelated to the prostatectomy, concurrent neurologic disease, which might impact bladder function, and the inability to describe their amount of urine leakage or other lower urinary tract symptoms (LUTS).
Each patient was evaluated by one of the five physical therapists with specialized training in the assessment and treatment of PFD. The pelvic floor physical therapists were all certified through the Certificate of Achievement in Pelvic Physical Therapy (CAPP-pelvic) program offered by the American Physical Therapy Association Section on Women’s Health. Three of the five physical therapists also possessed the Women’s Health Clinical Specialist Certification (WCS).
On the initial evaluation using physical examination and BF, the physical therapist determined the specific nature of the patient’s PFD—underactive, overactive, or mixed (both underactive and overactive qualities). Underactivity was diagnosed when the patient presented with weakness or lack of endurance of pelvic floor contraction. Overactivity was diagnosed when there was an inability to relax the pelvic floor muscles after a contraction, which was often but not necessarily accompanied by the presence of spasm and shortening of the pelvic floor musculature. The presence of dyssynergia, scar tissue restriction, and extra-pelvic biomechanical abnormalities was also noted. Pelvic pain scores were obtained using the numeric pain rating scale (NPRS) of 1–10/10. All patients received basic bladder education, including dietary recommendations, information on timed voiding, and urge suppression techniques.
The total number of pelvic PT treatment sessions depended on patient’s progress and was individualized for each patient. Sessions were generally held once per week. Treatment protocols depended on the type of presenting PFD. Patients with underactive PFD were instructed in an “uptraining” program consisting of strength, endurance, and coordination training in supine, sitting, and with functional movements using fast- and long-hold Kegels. The timing of a contraction prior to transitional movements or a cough/sneeze, otherwise known as “the Knack”, was also emphasized [14]. Patients with overactive PFD were instructed in a “downtraining” program consisting of relaxation training with the use of diaphragmatic breathing, stretches, and perineal bulges (also termed “reverse Kegels”). Manual therapy was also utilized in these patients, in the form of external and intrarectal myofascial release to release trigger points and to manually lengthen shortened tissues and mobilize scar tissue. It is important to note that patients with overactive PFD were never taught Kegel or other strengthening exercises. Patients with the mixed type of PFD were given a combination program, with an initial emphasis on downtraining, followed by uptraining protocols, once the patient understood how to properly relax their pelvic floor muscles after a contraction. BF guidance via surface electromyography (sEMG) was used in all treatment groups when the physical therapist determined, it would be beneficial for the patient to aide in learning the techniques.
The primary outcome measure was improvement in urinary incontinence as measured by the decrease in the total number of pads utilized per day. Physical therapists recorded the number and type of pads used per day in each patient encounter. The therapy was considered optimally successful if the patient was able to reduce pad usage to 0–2 regular pads per day by the end of treatment. The Incontinence Grading Scale was originally intended as the primary outcome measure, but the IGS was too infrequently documented to obtain useful information from reporting it in the minority of patients for whom it was available.
Secondary outcomes included the type of presenting PFD—underactive, overactive, or mixed, as well as the change in pelvic pain scores from initial to final treatment session. The number of therapy sessions and compliance with PT treatment recommendations was also evaluated. Reasons for non-compliance included only attending less than the recommended number of therapy sessions or not performing the prescribed home exercise program.
The data were analyzed with one-way ANOVA, Mann–Whitney test, χ2 analysis, paired t tests, and the General Linear Model.
Results
Two hundred and thirty two patient charts were initially evaluated and 136 met all inclusion criteria. The mean age of patients was 66.2 years, with a mean time between surgery and the start of PT of 6.8 months. The mean number of PT treatment sessions required was 4, with the maximum number being 18. Other demographics and their association with the type of presenting PFD are summarized in Table 1.
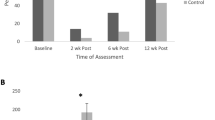
87% of patients achieved improvement in urinary incontinence, recorded as a decrease in pad usage per day (p < 0.001). 58% of patients achieved the optimal outcome of a decrease in urinary incontinence to the point where they required pad utilization of two or less per day. Among the 136 patients, 25 had underactive PFD requiring only uptraining treatment, while 13 had only overactive PFD and were treated with only downtraining protocols. Ninety eight had mixed-type PFD with components of both underactivity and overactivity and were treated with both uptraining and downtraining. Overall, 90% of patients had pelvic floor underactivity and 82% had overactivity. Both those with uptraining and downtraining protocols showed significant improvement in the number of pads used per day (p < 0.001). Patients treated with downtraining (those in the overactive and mixed-type groups) attended significantly more therapy sessions (p = 0.004), but tended to improve urinary incontinence more quickly during the course of treatment (p < 0.001). BF was used more frequently in patients who required uptraining (p = 0.001). For the group of patients as a whole, there was a mean decrease in the initial resting tone, while side-laying, as determined by BF, from 3.30 to 2.30 (p = 0.032) and an increase in maximal contraction strength in the side-laying position from 22.49 to 28.97 (p = 0.049). Among the patients who had not improved with prior standard pelvic PT regimens, six out of seven had pelvic floor overactivity requiring downtraining (p = 0.028).
There was also significant improvement in pelvic pain for the patients after participation in pelvic PT. For the entire sample of 136 patients, the mean pain score decreased with treatment from 0.88 to 0.30 (p < 0.001). On the initial evaluation, 27% of patients reported having pelvic pain. Patients with pelvic floor overactivity were more likely to present with pain than those without overactivity. For those with pain, the mean initial pain score was 3.62 and the final mean pain rating was 1.08. On the final evaluation, 86% reported no pain and 14% reported still having some pain. Compliance to treatment protocols was also assessed for each patient. 79% of patients were deemed compliant with all treatment recommendations. Treatment outcomes are summarized in Table 2.
Discussion
To our knowledge, this is the first study that attempts to determine the types of PFD present in post-prostatectomy men with SUI. Counter to the prevailing view that men in this population likely have underactive PFD requiring Kegel uptraining, this study showed that a majority of patients (82%) had pelvic floor overactivity present, most but not all in addition to pelvic floor underactivity. As the patients did not have a pelvic floor examination prior to or immediately after surgery, it is not known whether the PFD developed in these patients as a result of the prostatectomy or in response to significant and prolonged post-operative SUI. It is also not known whether some of these men may have had PFD prior to undergoing surgery. The prevalence of the various types of PFD in a normal male population has not been reported in the medical literature to date. Conceptually, however, overactive pelvic floor muscles would be expected in a post-surgical population, as the muscles are influenced by the viscerosomatic convergence phenomenon (manifested as the “guarding reflex”). There is a growing understanding in the pelvic PT community that rote uptraining in patients with pelvic floor overactivity may in fact worsen overactivity, leading to worsened pain and potentially even urinary incontinence [15]. This study suggests that there may be a subset of patients for whom downtraining instead of Kegel uptraining is required for maximal improvement of post-prostatectomy incontinence.
Although the findings did not reach statistical significance (possibly due to the small number of patients) both patients who received only downtraining and who received only uptraining showed approximately the same amount of improvement in pelvic floor contraction strength as measured by BF. We theorize that the improvement in strength in the patients who received downtraining (including the downtraining-only group) may be a function of the muscle length-tension relationship. The patients with overactivity have functionally shortened muscles, thus decreasing the number of cross-links available to generate the desired contraction strength to prevent incontinence. By relaxing the pelvic floor and functionally increasing the muscle length, more cross-links are available to generate a stronger and more functional contraction [12].
Overactive pelvic floor muscles frequently result in the development of trigger points. Returning the muscle to its optimal resting length can not only improve the strength and coordination of pelvic muscle contractions, but it can also result in decreased pain [12]. To our knowledge, this is the first study to demonstrate that there is a significant portion of the post-prostatectomy population who has pain after prostatectomy, which can be improved with pelvic PT. While there is inherent subjectivity in the report of pain with the NPRS, our findings suggest that an individualized pelvic PT program can result in a statistically significant improvement in post-prostatectomy pain.
21% of patients in this study were not fully compliant with treatment recommendations, either by attending fewer PT sessions than recommended or by not performing the home exercise program prescribed by the therapist. It may be that some patients felt it was difficult to engage fully with the pelvic PT process due to the invasive nature of the therapy and the embarrassment which may come with performing some of the therapeutic techniques.
The limitations of this study include that it was a retrospective review, with inherent risk of selection bias. Not all patients were compliant with treatment protocols, which may have lessened the potential effectiveness of the treatment. Another shortcoming of this study is that some of the desired data were too inconsistently documented in the chart to be included as originally intended—this includes the validated functional outcome scales which we had hoped to capture. The number of pads per day is a less desirable way to measure improvement in SUI, as patients can opt to change pads for a variety of reasons besides wetness level. Finally, lack of long-term follow up of this patient sample makes it impossible to know if the improvement in urinary incontinence was sustained beyond the end of treatment.
More research is needed into the nature of post-prostatectomy PFD and optimal treatment algorithms for SUI in this population. Prospective and randomized trials would be helpful to ascertain whether individualized pelvic PT treatment is preferable to standard Kegel prescription in this population. This study suggests that a more individualized approach, geared toward normalizing pelvic floor function, is a potentially valid way to manage post-prostatectomy urinary incontinence and pelvic pain.
References
Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U et al (2003) The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 21(2):167–178
Reynolds WS, Ahikavoc SA, Katz MH, Zagaja GP, Shalhav AL, Zorn KC (2010) Analysis of continence rates following robot-assisted radical prostatectomy strict leak-free and pad-free continence. Urology 75(2):431–438
Bauer RM, Gozzi C, Hubner W, Nitti VW, Novara G, Peterson A et al (2011) Contemporary management of postprostatectomy incontinence. Eur Urol 59(6):985–996
Anderson CA, Omar MI, Campbell SE, Hunter KF, Cody JD, Glazener CM (2015) Conservative management for postprostatectomy urinary incontinence. Cochrane Database Syst Rev 1:CD001843. https://doi.org/10.1002/14651858.CD001843.pub5
Dorey G, Glazener C, Buckley B, Cochran C, Moore K (2009) Developing a pelvic floor muscle training regimen for use in a trial intervention. Physiotherapy 95(3):199–209
Goode PS, Burgio K, Johnson TM, Clay OJ, Markland AD, Burkhardt JH et al (2011) Behavioral therapy with or without biofeedback and pelvic floor electrical stimulation for persistent postprostatectomy incontinence: a randomized controlled trial. JAMA 305(2):151–159
Manassero F, Traversi C, Ales V, Pistolesi D, Panicucci E, Valent F et al (2007) Contribution of early intensive prolonged pelvic floor exercises on urinary continence recovery after bladder neck-sparing radical prostatectomy: results of a prospective controlled randomized trial. Neurourol Urodyn 26(7):985–989
Overgard M, Angelsen A, Lydersen S, Morkved S (2008) Does physiotherapist-guided pelvic floor muscle training reduce urinary incontinence after radical prostatectomy? A randomised controlled trial. Eur Urol 54(2):438–448
Chen YC, Lin PH, Jou YY, Lin VC (2017) Surgical treatment for urinary incontinence after prostatectomy: a meta-analysis and systematic review. PLoS One 12(5):1–19
Davis NF, Kheradmand F, Creagh T (2013) Injectable biomaterials for the treatment of stress urinary incontinence: their potential and pitfalls as urethral bulking agents. Int Urogynecol J 24(6):913–919
Chughtai B, Lee R, Sandhu J, Te A, Kaplan S (2013) Conservative treatment for postprostatectomy incontinence. Rev Urol 15(2):61–66
Bradley MH, Rawlins A, Brinker CA (2017) Physical therapy treatment of pelvic pain. Phys Med Rehabil Clin N Am 28:589–601
Messelink B, Benson T, Berghmans B, Bo K, Corcos J, Fowler C et al (2005) Standardization of terminology of pelvic floor muscle function and dysfunction: report from the pelvic floor clinical assessment group of the International Continence Society. Neurourol Urodyn 24(4):374–380
Miller J, Sampselle CM, Ashton-Miller JA, Hong GR, DeLancey JO (2008) Clarification and confirmation of the effect of volitional pelvic floor muscle contraction to preempt urine loss (The Knack Maneuver) in stress incontinent women. Int Urogynecol J Pelvic Floor Dysfunct 19(6):773–782
FitzGerald MP, Kotarinos R (2003) Rehabilitation of the short pelvic floor II: treatment of the patient with the short pelvic floor. Int Urogynecol J Pelvic Floor Dysfunct 13(4):269–275
Acknowledgements
This work was supported by a grant from the David M. Crowley Foundation to the Department of Urology at UT Southwestern Medical Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors report financial conflicts of interest with this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Scott, K.M., Gosai, E., Bradley, M.H. et al. Individualized pelvic physical therapy for the treatment of post-prostatectomy stress urinary incontinence and pelvic pain. Int Urol Nephrol 52, 655–659 (2020). https://doi.org/10.1007/s11255-019-02343-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-019-02343-7