Abstract
Purpose
Vascular calcification (VC) is an independent risk factor for cardiovascular disease in hemodialysis patients while Matrix GLA protein (MGP) is one of the most potent inhibitors of VC and its activation is vitamin K dependent. The aim of this study is to investigate the role of oral vitamin K2 supplementation in the prevention of VC progression in haemodialysis patients.
Methods
We conducted a prospective randomized interventional study in patients on hemodialysis. Patients were randomly assigned to either receiving orally 200 μgr of vitamin K2 (vitamin K2/MK-7, Solgar) every day for 1 year or no treatment. Uncarboxylated MGP (uc-MGP) concentrations were quantified using ELISA at randomization, at 3 and at 12 months. Aortic calcification was evaluated using Agatston score after an abdominal computed tomography scan that was performed at the beginning and at 12 months of follow-up.
Results
There were 102 patients that were randomized. After 1 year of follow-up, 22 patients from the vitamin K2 group and 30 patients from the control group were included in the analysis. After 3 months of treatment, uc-MGP values remained unchanged in the vitK2 group but after 1 year were reduced by 47% (p = 0.005). Furthermore, uc-MGP at 1 year was increased by 12% in the control group. At 1 year, vitK2 group had significantly lower values of uc-MGP in comparison to controls (p = 0.03). Agatston score was increased significantly both in vitamin K2 and control group at 1 year with no difference between groups.
Conclusions
Oral administration of vitamin K2 in patients on haemodialysis reduced serum uc-MGP levels but did not have an effect in the progression of aortic calcification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vascular calcification (VC) represents an independent risk factor for cardiovascular disease in hemodialysis patients [1, 2]. Vascular calcification is a complicated and multifactorial process [3] which can occur with normal aging, but is accelerated in certain disease states, including diabetes mellitus, cardiovascular disease, and specific genetic diseases [1, 4]. This process involves differentiation of contractile vascular smooth muscle cells (VSMCs), and pericytes into distinct, ‘osteoblast-like’ cells with a secretory phenotype [5]. These cells are responsible for “osteoid” such as material deposition (bone matrix), which consists primarily of collagen I and other non-collagenous bone matrix proteins, including osteopontin, bone sialoprotein, osteocalcin, fetuin, and matrix GLA protein. Cellular components include osteoblasts, osteoclasts, chondroblasts, chondroclasts, osteocytes, lymphocytes, and vascular cells [6, 7]. VC is currently recognized as an actively regulated process dependent on the balance between its inducers and inhibitors [8].
Matrix GLA protein (MGP) is one of the most potent inhibitors of VC and its activation is vitamin K dependent [9]. Matrix Gla protein (MGP) is an 11-kD protein synthesized by vascular smooth muscle (VSMC) and endothelial cells [10]. Activation of MGP requires two post-translational modifications: vitamin K-dependent γ-glutamate carboxylation and serine phosphorylation [11]. MGP acts as a potent local inhibitor of vascular calcification by directly inhibiting calcium precipitation and crystallization and/or by antagonizing bone morphogenetic protein (BMP2), which itself promotes osteoblastic differentiation of VSMCs [12]. Nevertheless, vitamin K (K1 and K2) intake [13] is low in hemodialysis (HD) patients [14]. Additionally, patients on HD have high levels of uncarboxylated-MGP, an inactive form of MGP [11, 15], suggesting a vitamin K deficiency.
Vitamin K is a group name for several structurally related compounds including phylloquinone (vitamin K1) and menaquinones (K2 vitamins) [16]. Menaquinones are classified according to the length of their aliphatic side chain and are designated as MK-n, where n stands for the number of isoprenoid residues in that chain. The synthetic short-chain vitamin K(1), as well as the natural long-chain menaquinone-7 (MK-7) are commonly used in food supplements. Both, K(1) and MK-7 are absorbed well, with peak serum concentrations at 4 h after intake. A major difference between the two vitamin K species is the very long half-life time of MK-7, resulting in much more stable serum levels, and the accumulation of MK-7 to higher levels (7–8-fold) during prolonged intake [17].
Increasing evidence suggest that patients on dialysis may benefit from vitamin K2 (MK-7) supplementation [18,19,20]. Thus, the aim of this study is to investigate the role of oral vitamin K2 supplementation in the prevention of vascular calcification progression among haemodialysis patients.
Patients and methods
This was a prospective randomized interventional study, in end stage renal disease (ESRD) patients on hemodialysis.
Inclusion and exclusion criteria
All patients over age of 18 with ESRD on hemodialysis were considered for the study. Patients with a medical history of abdominal aortic surgery, hemodynamically unstable patients, patients with mental function impairment, atrial fibrillation or mechanical heart valve receiving acenocoumarol and those with chronic gastrointestinal disorders, were excluded.
Examined parameters
Examined comorbidities included; peripheral artery disease as defined by a history of claudication or relevant medical or interventional treatment, diabetes, coronary arterial disease in patients with a history of myocardial infarction or positive functional cardiac assessment test and hypertension in patients on relevant antihypertensive treatment.
Blood samples were drawn at randomization, at 3 and at 12 months. Blood samples were taken at the start of dialysis treatment, in a mid-week session interval at the dialyzer inlet line. Citrated plasma was obtained after a 10 min centrifugation at 3000 rounds per minute and stored at − 20 °C until the analysis. Uncarboxylated MGP (uc-MGP) concentrations were quantified using ELISA (Human Uncarboxylated Matrix Gla Protein (ucMGP) ELISA kit, Cusabio, Catalog No. CSB-EC013789HU). All measurements were carried out following manufacturer’s instructions. Ηaemoglobin and serum calcium, phosphorus, albumin, total cholesterol, LDL, HDL, ferritin and intact parathyroid hormone were measured using standard laboratory techniques. The laboratory staff carrying the procedures was blinded to the study set up.
Randomization and vitamin K2 administration
This was an open label trial. All patients in our hemodialysis unit were screened. Patients who accepted to take part in the study were randomly assigned into two groups (either to receive vitamin K2 or no treatment) in 1:1 ratio. Those in the intervention group (vitamin K2 group) received 200 μgr of vitamin K2 orally (vitamin K2/MK-7, Solgar) every day for 1 year. Vitamin K2 supplementation was assigned at least 2 h away from any other orally prescribed drug. Patients in the control group did not receive vitamin K2 supplementation or placebo. Some patients who were finally assigned to the vitamin K2 group decided to participate in the study (with laboratory/imaging control) but refused receiving vitamin K2 supplementation, they were shifted to the control group (n = 7 patients). No patient received anti-vitamin K anticoagulant during follow-up.
All patients included in the study provided a written informed consent. The study protocol was approved by the local ethic committee and was in accordance with the Helsinki declaration as revised in 2000.
Imaging procedures
An abdominal computed tomography scan (CT) was performed in all patients at the beginning and at 12 months of follow-up for the quantification of the calcification of the abdominal aorta. The aortic calcification was evaluated based on the Agatston score, using Hounsfield units scale as previously described [21].
Statistical analysis
All statistical analyses were conducted using SPSS Statistics 21.0, for Windows. A p value of < 0.05 was considered to be statistically significant. Continuous variables are presented as mean ± standard deviation and skewed continuous data are presented as medians (first to third quartile). Differences between the two study groups were tested using independent sample t test and for skewed continuous data with Mann–Whitney test. Differences of each variable in the same group of patients over the study period were tested using paired sample t test and for skewed continuous data with Wilcoxon test. Bivariate correlation analysis was performed using the Pearson’s correlation coefficient. Categorical data were tested with Chi-square test.
Results
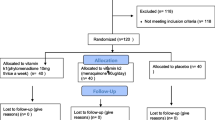
There were 102 patients that were randomized to receive either vitamin K2 or no treatment. After 1 year of follow up, 22 patients from the vitamin K2 group (mean age 70.09 ± 12.68 years) and 30 patients from the control group (mean age 64.74 ± 16.95 years) were included in the study (Fig. 1). Patients’ compliance was estimated at each hemodialysis session where the patients who participated reported their daily schedule (habits—treatment—possible complications—problems) and returned the drug package when it was empty and then a new one was provided. Drop-outs from non compliance were considered for those who did not follow the protocol in terms of dose or frequency. The drop-outs during the follow-up were similar between the two study groups. In the vitamin K2 group, 22 patients dropped-out due to; myocardial infarction (n = 7), non compliance (n = 4), pulmonary infection (n = 6) and kidney transplantation (n = 5). In the control group 28 patients dropped-out due to; myocardial infarction (n = 6), non compliance (n = 12), pulmonary infection (n = 3), kidney transplantation (n = 5) and neoplastic disease (n = 2).
There were no significant differences in baseline demographics, comorbidities and standard lab values between the study groups. Demographics and comorbidities from both groups of patients at the beginning of follow-up are shown in Table 1. Laboratory parameters for both groups of patients, at the beginning, at 3 months and at the end of the study are presented in Table 2. Data on use of calcium-based phosphate binders, vitamin D analogs and cinacalcet during follow-up are presented in Table 3. All patients received adequate dialysis dose and had a Kt/V value of over 1.2 in consecutive monthly measurements. Moreover, nutrition status was considered adequate as mean serum albumin values were above 3.5 g/dl in both groups of patients.
Uc-MGP measurements
Baseline uc-MGP did not differ between study groups (p = ns). After 3 months of treatment, uc-MGP median values remained approximately the same in the vitK2 group (from 8342 ± 10,047 to 9059 ± 8192 ng/ml, p = ns) while after 1 year of treatment, uc-MGP median values were reduced by 46.56% from the baseline value (p = 0.007) and by 53% from the 3 months value (p = 0.005). Furthermore, uc-MGP median values in the control group remained the same during the 1 year of follow-up (from 8903 ± 10,517 to 8050 ± 12,155 ng/ml, p = ns) (Fig. 2). Baseline and 3 months median values of uc-MGP between vitK2 and control group showed no significant difference. Instead, at 1 year, vitK2 group had significantly lower values of uc-MGP in comparison to controls (p = 0.03). The differences of the medians of the uc-MGP values between the two groups of patients during follow-up are shown in Table 4.
Aortic calcification
Aortic calcification (Agatston score) was estimated in all patients included in the study at the beginning and at 12 months of follow-up. In the control group, Agatston score increased from 8253 ± 6298.94 to 11,036.58 ± 9053.34 (p = 0.01). Likewise, in the vitamin K2 group, Agatston score was increased from 7827.88 ± 5493.38 to 10,412.53 ± 7227.2 (p = 0.02). There was no significant difference of aortic calcification estimation score between vitamin K2 and control group either at the start or at on 1 year of follow-up. All measurements and comparisons between groups of patients are presented in Table 5 and Fig. 3.
Side effects
During the 1 year of follow-up no side-effects were observed or described and no drop-outs were attributed to gastrointestinal or other disorders.
Discussion
The concept of correcting Vitamin K deficiency physiologically may help reduce the risk of vascular calcification in ESRD patients. Thus, the main finding of this study is that K2 administration in dialysis depended ESRD patients reduces uc-MGP serum levels. Nevertheless, this reduction does not seem to adequately withhold or subvert vascular calcification of abdominal aorta as its shown with Agatston score deterioration in both study groups. Finally, this is the first trial in humans that the effect of Vitamin K2 supplementation on vascular calcification is estimated with Agatston score of abdominal aorta.
Vascular calcification is an energetic, complex and controlled process, where vascular smooth muscle cells (vSMC) have a central role. Transcription factors, cytokines, proteins and vesicles characteristic of mature osteoblasts and chondrocytes are found in biopsy sections of calcified vessels, while calcification is made up by inorganic hydroxyapatite. During this process, vSMC, stem cells from the circulation and nascent multivalent fibroblasts are phenotypically transformed to osteocyte like cells, leading eventually to vascular calcification. This conversion is achieved by the expression of osteopoetic factors such as Cbfα/Runx2, Msx2, osterix and is favored by factors that are prevalent in the uremic milieu. In particular, in this active process a variety of factors can either act as protective agents by inhibiting the calcification of the arterial wall (such as fetuin, Matrix Gla Protein, osteopontin and inorganic pyrophosphate), or can promote it (such as high serum concentrations of calcium, phosphorus and parathormone). In addition, the loss of calcification limiting factors observed in CKD further facilitates vascular calcification [22]. Finally, vascular calcification may affect different blood vessels in different degree and in a way that some blood vessels calcify more than others [23, 24].
As mentioned before, vascular calcification in hemodialysis patients is an active process that in part is regulated and inhibited by Matrix GLA protein (MGP) which in turn is activated by vitamin K. Moreover, it is well known that patients on dialysis have vitamin K deficiency and high uncarboxylated-MGP levels, an inactive form of MGP [25]. The immunohistochemical data reported by Schurgers et al. demonstrated that uncarboxylated MGP is abundantly present in atherosclerotic intima and in media vascular sclerosis, suggesting local vitamin K deficiency and impaired protection attributable to poor MGP carboxylation [26]. The findings in this study support all the aforementioned data, as the serum uc-MGP values were significantly lower in patients who received vitamin K2 supplementation for 1 year compared to controls. After 1 year of daily oral administration of 200 μgr of vitamin K2, we observed a 46.56% reduction of serum uc-MGP from the baseline value. On the contrary, serum uc-MGP levels remained stable at 1 year in the control group. This is consistent with the previous observations of Aoun et al. where the administration of 360 μgr Menaquinone-7 daily, for 4 weeks, effectively reduced uc-MGP up to 86% [27]. Similarly, Caluwé et al. proved that pharmacological doses of MK-7, dose-dependently reduced uc-MGP in patients on dialysis [19].
Puzantian et al. studied large artery stiffening in advanced CKD and pointed out that CKD is associated with increased (inactive) uc-MGP [28]. In a murine model, Scheiber et al. showed that high dose of MK-7 supplementation inhibits the development of cardiovascular calcification [29]. The KING trial (vitamin K2 In reNal Graft) was a single-arm study that evaluated the association between the change in vitamin K status and indices of arterial stiffness following 8 weeks of supplementation (360 μg once daily) among renal transplant recipients. In this study, supplementation of vitamin K2 was associated with a 14.2% reduction in mean carotid-femoral pulse wave velocity (cfPWV) at 8 weeks [30]. Despite that, there are not many studies indicating the direct effect of vitamin K2 supplementation on vascular calcification inhibition. Vossen et al., in the ongoing Vita-K CAC trial is expected to show the effect of vitamin K2 supplementation on progression of CAC score in a randomized, placebo-controlled trial [31]. In our study, although uc-MGP values were significantly reduced with vitamin K2 supplementation, vascular calcification as measured with Agatston score did not shown any significant improvement. Furthermore, the aortic calcification measured in patients treated with vitamin K2, progressed in the same way, without any significant difference from the controls.
In our study there are limitations that must be acknowledged. First of all, although the number of participants at the beginning of the study was sufficient, a significant proportion of them dropped out. In addition, a longer observational period and higher vitamin K2 dose would allow for safer conclusions related especially to the progress of vascular calcification and hard end points such as cardiovascular events and mortality.
Although Vitamin K2 supplementation did not show clinically significant regression of vascular calcification, its safe administration profile renders it a candidate for systematic dietary supplement in all dialysis patients. Notably, Vitamin K2 was well tolerated in all patients in this study.
In conclusion, oral administration of vitamin K2 in haemodialysis patients reduced serum uc-MGP levels. This effect though, theoretically positive, seems not to be sufficient to withhold vascular calcification progression which is a multi-factorial pathophysiologic process. Larger studies are needed to confirm whether preventive vitamin K2 supplementation is warranted in ESRD patients.
References
London G, Guerin A, Marchais S et al (2003) Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. NDT 18(9):1731–1740. https://doi.org/10.1093/ndt/gfg414
Russo D, Corrao S, Battaglia Y et al (2011) Progression of coronary artery calcification and cardiac events in patients with chronic renal disease not receiving dialysis. Kidney Int 80(1):112–118. https://doi.org/10.1038/ki.2011.69
Paloian N, Giachelli C (2014) A current understanding of vascular calcification in CKD. Am J Physiol Renal Physiol 307(8):F891–F900. https://doi.org/10.1152/ajprenal.00163.2014
Speer M, Giachelli C (2004) Regulation of cardiovascular calcification. Cardiovasc Pathol 13(2):63–70. https://doi.org/10.1016/S1054-8807(03)00130-3
Sage A, Tintut Y, Demer L (2010) Regulatory mechanisms in vascular calcification. Nat Rev Cardiol 7:528–536. https://doi.org/10.1038/nrcardio.2010.115
Boström K, Watson K, Horn S et al (1993) Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest 91(4):1800–1809. https://doi.org/10.1172/JCI116391
Ewence A, Bootman M, Roderick L et al (2008) Calcium phosphate crystals induce cell death in human vascular smooth muscle cells: a potential mechanism in atherosclerotic plaque destabilization. Circ Res 103(5):28–34. https://doi.org/10.1161/CIRCRESAHA.108.181305
Shroff R, Shanahan C (2007) The vascular biology of calcification. Semin Dial 20:103–109. https://doi.org/10.1111/j.1515-139X.2007.00255.x
Delanaye P, Krzesinski JM, Warling X et al (2014) Dephosphorylated-uncarboxylated Matrix Gla protein concentration is predictive of vitamin K status and is correlated with vascular calcification in a cohort of hemodialysis patients. BMC Nephrol 15:145. https://doi.org/10.1186/1471-2369-15-145
Schurgers J, Cranenburg C, Vermeer C (2008) Matrix Gla-protein: the calcification inhibitor in need of vitamin K. Thromb Haemost 100(4):593–603. https://doi.org/10.1160/TH08-02-0087
Fang-Fei W, Trenson S, Thijs L et al (2018) Desphospho-uncarboxylated matrix Gla protein is a novel circulating biomarker predicting deterioration of renal function in the general population. Nephrol Dial Transplant 33(7):1122–1128. https://doi.org/10.1093/ndt/gfx258
Zebboudj F, Imura M, Bostrom K (2002) Matrix GLA protein, a regulatory protein for bone morphogenetic protein-2. J Biol Chem 277(6):4388–4394. https://doi.org/10.1074/jbc.M109683200
Stafford D (2005) The vitamin K cycle. J Thromb Haemost 3(8):1873–1878. https://doi.org/10.1111/j.1538-7836.2005.01419
Cranenburg E, Schurgers J, Uiterwijk H et al (2012) Vitamin K intake and status are low in hemodialysis patients. Kidney Int 82(5):605–610. https://doi.org/10.1038/ki.2012.191
Dalmeijer G, van der Schouw Y, Magdeleyns E et al (2012) The effect of menaquinone-7 supplementation on circulating species. Atherosclerosis 225(2):397–402. https://doi.org/10.1016/j.atherosclerosis.2012.09.019
Berkner K, Runge K (2004) The physiology of vitamin K nutriture and vitamin K-dependent protein function in atherosclerosis. J Thromb Haemost 2(12):2118–2132. https://doi.org/10.1111/j.1538-7836.2004.00968.x
Schurgers J, Teunissen J, Hamulyak K et al (2007) Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood 109(8):3279–3283. https://doi.org/10.1182/blood-2006-08-040709
Westenfeld R, Krueger T, Schlieper G et al (2012) Effect of vitamin K2 supplementation on functional vitamin K deficiency in hemodialysis patients: a randomized trial. Am J Kidney Dis 59(2):186–195. https://doi.org/10.1053/j.ajkd.2011.10.041
Caluwé R, Vandecasteele S, Van Vlem B et al (2014) Vitamin K2 supplementation in haemodialysis patients: a randomized dose-finding study. Nephrol Dial Transplant 29(7):1385–1390. https://doi.org/10.1093/ndt/gft464
Braam A, Hoeks P, Brouns F et al (2004) Beneficial effects of vitamins D and K on the elastic properties of the vessel wall in postmenopausal women: a follow-up study. Thromb Haemost 91(2):373–380. https://doi.org/10.1160/TH03-07-0423
Agatston A, Janowitz W, Hildner F et al (1990) Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15(4):827–832. https://doi.org/10.1016/0735-1097(90)90282-T
Giachelli C (2009) The emerging role of phosphate in vascular calcification. Kidney Int 75:890–897. https://doi.org/10.1038/ki.2008.644
Schlieper G, Brandenburg V, Djuric Z et al (2009) Risk factors for cardiovascular calcifications in non diabetic Caucasian hemodialysis patients. Kidney Blood Press Res 32:161–168. https://doi.org/10.1159/000221064
Blacher J, Guerin A, Pannier B et al (2001) Arterial calcifications, arterial stoffness and cardiovascular risk in end-stage renal disease. Hypertension 38:938–942. https://doi.org/10.1161/hy1001.096358
Fusaro M, D’Alessandro C, Noale M et al (2017) Low vitamin K1 intake in haemodialysis patients. Clin Nutr 36(2):601–607. https://doi.org/10.1016/j.clnu.2016.04.024
Schurgers L, Teunissen K, Knapen M et al (2005) Novel conformation-specific antibodies against matrix γ-carboxyglutamic acid (Gla) protein. Arterioscler Thromb Vasc Biol 25:1629–1633. https://doi.org/10.1161/01.ATV.0000173313.46222.43
Aoun M, Makki M, Azar H et al (2017) High dephosphorylated-uncarboxylated MGP in hemodialysis patients: risk factors and response to vitamin K2, A pre-post intervention clinical trial. BMC Nephrol 18(1):191. https://doi.org/10.1186/s12882-017-0609-3
Puzantian H, Akers S, Oldland S et al (2018) Circulating dephospho-uncarboxylated matrix gla-protein is associated with kidney dysfunction and arterial stiffness. Am J Hypertens 31(9):988–994. https://doi.org/10.1093/ajh/hpy079
Scheiber D, Veulemans V, Horn P et al (2015) High-dose menaquinone-7 supplementation reduces cardiovascular calcification in a murine model of extraosseous calcification. Nutrients. 7(8):6991–7011. https://doi.org/10.3390/nu7085318
Mansour A, Hariri E, Daaboul Y et al (2017) Vitamin K2 supplementation and arterial stiffness among renal transplant recipients-a single-arm, single-center clinical trial. J Am Soc Hypertens 11(9):589–597. https://doi.org/10.1016/j.jash.2017.07.001
Vossen L, Schurgers L, vanVarik B et al (2015) Menaquinone-7 supplementation to reduce vascular calcification in patients with coronary artery disease: rationale and study protocol (VitaK-CAC trial). Nutrients 7(11):8905–8915. https://doi.org/10.3390/nu7115443
Acknowledgements
We would like to thank Solgar Inc., USA, for kindly providing to all participants in the study, the Solgar Vitamin K2 100 mg preparation, free of charge.
Funding
Authors received no funding for this study. Solgar Inc. USA provided the Vitamin K2 preparation for free.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oikonomaki, T., Papasotiriou, M., Ntrinias, T. et al. The effect of vitamin K2 supplementation on vascular calcification in haemodialysis patients: a 1-year follow-up randomized trial. Int Urol Nephrol 51, 2037–2044 (2019). https://doi.org/10.1007/s11255-019-02275-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-019-02275-2