Abstract
Introduction and aim
Hydrogen sulfide (H2S) is an endogenously produced gas-structure mediator. It is proposed to have antioxidant, anti-inflammatory and antiapoptotic effects. Acetaminophen (N-acetyl-P-aminophenol; APAP) is an antipyretic and analgesic medication known as paracetamol. When taken at therapeutic doses there are few side-effects, but at high doses APAP can cause clear liver and kidney damage in humans and experimental animals. In this study, the effects of the H2S donor of sodium hydrosulfide (NaHS) on acute renal toxicity induced by APAP in rats were researched in comparison with N-acetyl cysteine (NAC).
Method
Rats were divided into six groups (n = 7) as control. APAP, APAP + NAC, APAP + NaHS 25 µmol/kg, NaHS 50 µmol/kg and NaHS 100 µmol/kg. After oral dose of 2 g/kg APAP, NAC and NaHS were administered via the i.p. route for 7 days. In renal homogenates, KIM-1 (Kidney Injury Molecule-1), NGAL (neutrophil gelatinase-associated lipocalin), TNF-α and TGFβ levels were measured with the ELISA method for tissue injury and inflammation. In renal tissue, oxidative stress levels were identified by spectrophotometric measurement of TAS and TOS. Histopathologic investigation of renal tissue used caspase 3 staining for apoptotic changes, Masson trichrome and H&E staining for variations occurring in glomerular and tubular systems.
Results
NaHS lowered KIM-1, NGAL, TNF-α, TGF-β and TOS levels elevated in renal tissue linked to APAP and increased TAS values. NaHS prevented apoptosis in the kidney and was identified to ensure histologic amelioration in glomerular and tubular structures. NaHS at 50 µmol/kg dose was more effective, with the effect reduced with 100 µmol/kg dose.
Conclusion
H2S shows protective effect against acute renal injury linked to APAP. This protective effect reduces with high doses of H2S. The anti-inflammatory and antioxidant activity of H2S may play a role in the renoprotective effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medications containing paracetamol, and as a result acetaminophen (N-acetyl-para-amino-phenol [APAP]), are one of the commonly used non-steroidal anti-inflammatory (NSAI) drugs globally. Generally, they are used excessively as antipyretic and analgesic. Though paracetamol toxicity is only seen at very high doses, the common use causes toxicity reactions to be observed [1]. As medications containing paracetamol are seen as safe, they are included on nearly all prescriptions. They may be used simultaneously with other medications. Uncontrolled use may be present in nearly all households.
In recent decades, the hepatotoxic effects of APAP have been a focus. However, attention is drawn to other known, mainly negative, effects of APAP. One of these effects is acute renal failure. Apart from acute renal failure, it is reported it may cause pulmonary, endocrine, neurologic and neurodevelopmental toxicity [2]. The hepatotoxic effect caused by overdose of APAP is greater than the nephrotoxic effect. However, even in situations where hepatotoxicity is not observed with toxic dose intakes, nephrotoxicity is reported [3]. Of patients with APAP overdose, 1–2% experience renal failure. However, it may cause both human and animal deaths [4]. APAP is mainly metabolized by conjugation with sulfate or glucuronic acid in the liver. The remaining 2–4% portion of APAP taken at therapeutic doses is transformed by cytochrome p450 enzyme (CYP) to the toxic metabolite of N-acetyl p-benzoquinoneimine (NAPQI) [5]. This metabolite is a very reactive electrophilic molecule which is detoxified by binding to glutathione (GSH) in the liver and excreted in urine. When toxic doses are taken, the amount of NAPQI formed exceeds the binding capacity of GSH causing liver and kidney injury. APAP is deacetylated in the kidneys and transforms to the p-aminophenol metabolite which is a nephrotoxin and causes renal cortical necrosis [6]. At therapeutic doses, the p-aminophenol is conjugated with GSH, similar to NAPQI metabolite in the liver, and is excreted as inactive GSH conjugates [6]. APAP increases lipid peroxidation and causes intracellular GSH depletion which is reported to increase oxidative damage in the kidneys [7, 8].
N-acetyl cysteine (NAC) is used as an antidote for APAP toxicity. NAC is a pioneer compound for cysteine and protects the liver by filling the depleted glutathione pool in the liver. NAC is thought to increase the glutathione amount and ensure detoxification of NAPQI responsible for hepatotoxic effects [9, 10]. Within the kidney, NAPQI is basically produced via the CYP450 pathway and prostaglandin synthase in the medulla [11]. However, experimental and clinical research has reported NAC is effective on APAP nephrotoxicity [12].
Hydrogen sulfide (H2S) is a gas-structure mediator like nitric oxide and carbon monoxide and is endogenously produced in mammalian tissue [13]. Until endogenous H2S was identified in the rat brain, H2S was accepted as toxic. H2S is synthesized from two pyridoxal 5-phosphate-linked enzymes of l-cysteine, in other words cystathionine β synthase (CBS) and cystathionine γlyase (CSE), and an enzyme independent of phosphate of 3-mercaptopyruvate sulfur transferase (3-MST). In recent years, a new synthesis route for H2S from d-cysteine was discovered, and it was shown that especially in kidneys and cerebellum d-cysteine is more dominant that l-cysteine [14, 15]. Here, the effective enzyme is known to be d-amino acid oxidase. H2S prevents inflammation and oxidative stress in ischemia/reperfusion injury and is shown by many studies to possibly have therapeutic effect in many organs and metabolic diseases [16, 17]. H2S, additionally, is reported to have effects in a variety of renal pathologies including diabetic and hypertensive nephropathies [18, 19], medication-linked nephrotoxicity, and hypothermia-linked acute kidney injury (AKI) [20].
In light of all this information, in our study, we aimed to investigate the possible renoprotective effect of H2S on APAP-induced acute nephrotoxicity and compare this effect with the standard treatment of NAC.
Materials and methods
Experimental animals and groups
In this study, 42 Wistar albino male rats weighing 240–280 g were used. Animals were kept at room temperature (24 ± 2 °C) and 55 ± 15% relative humidity with 12 h light/dark cyclus with fixed limits. Water and standard rat feed were given ad libitum. All procedures related to animals were completed in accordance with national and international regulations about animal experiments. The study received permission from Dumlupınar University (DPU) Animal Experiments Local Ethics Committee. The study was completed in DPU Experimental Animal Breeding Research and Application Center, DPU Advanced Technology Center and DPU Faculty of Medicine Pharmacology Laboratory.
Experimental study groups and medication doses:
The experimental study groups and medication doses were arranged as follows (n = 7):
Group 1 (control): physiologic serum (PS) oral (2 mL) + 0.2 mL PS intraperitoneal (ip).
Group 2 (APAP): 2 g/kg APAP [21]. in 2 mL PS oral + 0.2 mL PS ip.
Group 3 (APAP + NAC): 2 g/kg APAP oral + 140 mg/kg NAC ip [21].
Group 4 (APAP + 25 µmol/kg NaHS): 2 g/kg APAP oral + 25 µmol/kg sodium hydrosulfide (NaHS) ip.
Group 5 (APAP + 50 µmol/kg NaHS): 2 g/kg APAP oral + 50 µmol/kg NaHS ip.
Group 6 (APAP + 100 µmol/kg NaHS): 2 g/kg APAP oral + 100 µmol/kg NaHS ip [22].
Animals were fasted for 24 h and then administered acetaminophen (N-acetyl-p-aminophenol, APAP, paracetamol) (Sandoz, Turkey) at 2 g/kg dose in suspension in distilled water via oral gavage in a single dose. Four hours after APAP administration, animals were allowed to have food and water.
One hour before APAP administration, 140 mg/kg NAC (Bilim Ilac, Turkey), 25, 50 and 100 µmol/kg doses of NaHS (Sigma, Turkey) [22] and 0.2 mL PS ip were administered based on the group. NaHS and NAC were administered via the ip route within 0.2 mL physiologic serum. NaHS is a H2S donor and transforms to H2S in the body [22]. NAC, NaHS and PS administration continued as single doses for 7 days. In the control group, 1 h after PS ip was administered, 2 mL PS was administered orally in a single dose and then physiological serum was administered via ip for 7 days.
On the 7th day of the study, 2 h after medication administration, animals were anesthetized with ketamine (70 mg/kg ip, Ketalar, Pfizer, Turkey) and xylazine (10 mg/kg ip, Alfazyne, Ata-fen, Turkey). Intracardiac blood was taken and animals were euthanized with both kidneys removed for histopathologic and biochemical investigations.
Biochemical examination
Immediately after the left kidney of animals were removed, the kidneys were homogenized in 50 mmol/L phosphate buffer (pH 7.40) in a mechanical homogenizer (Analytic Jena SpeedMill PLUS, Jena, Germany). Homogenates were centrifuges at 4 °C at 10,000 x g for 15 min and supernatants were stored at − 80 °C.
KIM-1 and NGAL measurements
KIM-1 (kidney injury molecule-1) and NGAL (neutrophil gelatinase-associated lipocalin) were measured with the ELISA technique to identify AKI. Rat kits (Elabscience rat KIM-1 and NGAL ELISA Kit) were used on renal homogenates (BMG Labtech Spectrostar Nano, GmbH, Ortenberg, Germany).
TNFα and TGFβ measurements
For assessment of the inflammatory process, kidney TNF-α (tumor necrotizing factor-α) and TGF-β (transforming growth factor-β) levels were measured with the ELISA method using rat kits (Elabscience rat TNF-α and TGF-β ELISA Kit) (BMG Labtech Spectrostar Nano, GmbH, Ortenberg, Germany).
TAS and TOS measurements
For oxidative stress levels, TOS (total oxidant capacity) and TAS (total antioxidant capacity) levels in kidneys were measured with rat kits (Rel Assay Diagnostic) with a Beckman Coulter AU680 analyzer. TAS values are given as troloxEq/mg protein and TOS values are given as H2O2Eq/mg. The Oxidative Stress Index (OSI) is accepted as the % ratio of TAS and TOS values. TAS values were translated to mmol/L. The OSI value was calculated using the formula OSI (arbitrary unit) = TOS (mmol H2O2Equiv/L)/TAS (mmol TroloxEquiv/L).
Protein amount in homogenates
These were measured using a maestrogen-maestronano spectrophotometer.
Histopathologic examination
The removed kidney tissue was fixed in 4% neutral formalin solution. Later, it was submerged in paraffin and 4 µm thickness sections were obtained. Kidneys were stained with hematoxylin and eosin staining and investigated with a light microscope. Additionally, tubulointerstitial fibrosis was assessed with Masson trichrome and collagen staining. Control staining of all experimental groups were performed with secondary antibody alone simultaneously with immünohistochemical staining. For assessment of apoptosis, samples taken from kidneys were immunohistochemically stained with caspase. Areas in the glomeruli, tubules and interstitial area marked positive with caspase had apoptotic nuclei counted to assess results.
Kidney injury in the external medulla was determined by investigating ten sections chosen at random. Tubular injury, interstitial inflammatory cell infiltration, tubular dilatation, atrophy, increased connective tissue, hemorrhage, and collagen accumulation were assessed. The degree of injury was scored as follows [23]:
- 0:
-
Normal
- 1:
-
Mild, less than 25% involvement of injury in the cortex
- 2:
-
Moderate, 25–50% injury in the cortex
- 3:
-
Severe, 50–75% injury in the cortex
- 4:
-
Widespread injury involving 75–100% of the cortex
Statistical analysis
Results are given as mean ± SEM. Data were assessed in the SPSS program using the one-way analysis of variance (ANOVA) test (post hoc Dunnett test). For comparison of histopathologic data, the Kruskal–Wallis (post hoc Dunn method) test was used. P < 0.05 was accepted as significant.
Results
KIM-1 and NGAL measurements results
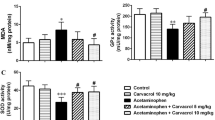
KIM-1 was identified as one of the most induced proteins in kidneys following AKI in animal models. KIM-1 is a trans-membrane glycoprotein increasing in proximal tubule cells after ischemic or nephrotoxic AKI. In our study, in animals with kidney injury induced with APAP, KIM-1 levels were significantly increased compared to the control group (P < 0.001) (Fig. 1a). This shows that the AKI was successfully induced. The NAC group appeared to have significant improvement compared to the APAP group. However, the most noteworthy finding appears to be the NaHS dose. NaHS at the 50 µmol/kg dose was observed to lower KIM-1 to control levels. At 100 µmol/kg dose, the group administered NaHS had significantly higher KIM-1 levels compared to the control group (P < 0.05), and this was significantly low compared to the APAP group (P < 0.05) (Fig. 1a). In this situation, the 50 µmol/kg dose of H2S was effective on APAP-induced AKI but as the dose increased, this effect can be said to reduce. Compared with NAC, 50 µmol/kg of NaHS dose appeared to bring KIM-1 values closer to the control group (Fig. 1).
Effects of NaHS treatment on biochemical parameters in rats with APAP nephrotoxicity. a KIM-1, b NGAL, c TGFβ and d TNFα. Data are given as mean ± SEM. *P < 0.05,**P < 0,01, ***P < 0.001 versus control group, #P < 0,05, ###P < 0,001 versus APAP group ANOVA (n = 7). APAPAcetaminofen, NaHS sodium hydrosulfide, NAC N-acetyl cysteine, KIM-1 kidney injury molecule-1, NGAL neutrophil gelatinase-associated lipocalin, TGFβ transforming growth factor-β, TNFα tumor necrotizing factor-α
For NGAL levels, results similar to KIM-1 were obtained. Again at 50 µmol/kg dose NaHS, NGAL levels were almost the same as in the control group (Fig. 1b). However, at 100 µmol/kg dose NaHS the results were significantly high NGAL values compared to the control group (P < 0.05) but significantly low compared to the APAP group (P < 0.05) (Fig. 1b). According to this result, NGAL values, considered to be a specific and early marker of AKI and increasing with AKI, increased compared to control values at 100 µmol/kg dose NaHS. It was concluded that at this dose, the curative properties of H2S were lost and did not even exist. When compared with NAC used as an antidote to APAP toxicity, treatment with 50 µmol/kg dose NaHS appeared to bring NGAL levels close to control values. According to this result, 50 µmol/kg NaHS may be said to be more effective for treatment of AKI than NAC.
TNFα and TGFβ measurement results
When TNFα values are examined, again 50 µmol/kg NaHS dose appeared to bring TNFα value close to control and NAC group values (Fig. 1d). At 100 µmol/kg NaHS, these values were significantly high compared to control levels (P < 0.05). When compared with APAP values, they appeared to be significantly low (P < 0.05) (Fig. 1d). According to this result, 50 µmol/kg NaHS dose was effective on the inflammatory process in AKI and can be said to be as effective as NAC used as antidote for APAP toxicity.
For TGFβ measurements, similar results were obtained as those for TNFα values (Fig. 1c). At 50 µmol/kg dose of NaHS, renal TGFβ levels elevated by APAP were at control and NAC group levels. However, again 100 µmol/kg of NaHS dose was significantly increased compared to controls (P < 0.05) (Fig. 1c).
TAS and TOS measurement results
TAS and TOS measurements were performed on homogenates to assess the oxidant and antioxidant capacity in the kidneys linked to APAP toxicity and the Oxidative Stress Index was calculated. APAP lowered TAS values and increased TOS values (P < 0.001) (Table 1). This result showed that oxidative stress increased in the kidneys linked to APAP. At 50 µmol/kg dose of NaHS, the TAS values increased to control and NAC group values (Table 1). Similar results were observed for TOS and OSI values. In the 100 µmol/kg dose of NaHS group, TAS values were significantly low compared to the control group (P < 0.05), while TOS values were observed to be high (P < 0.05) (Table 1).
Histopathologic results
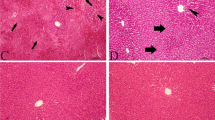
After hematoxylin and eosin staining in kidneys and light microscope investigation, scoring identified normal histological findings in the control group. In the group with APAP toxicity developed, moderate and severe levels of tubular damage were identified to develop (P < 0.001) (Figs. 2a, 3). In the APAP group, kidney injury characterized by interstitial inflammatory cell infiltration, tubular dilatation and atrophy were observed in kidney tissue. This injury was identified to fall below 25% levels in the group-administered NAC. However, in the group-administered 50 µmol/kg NaHS, this injury was identified to reach levels close to the control group (Fig. 3). Counts of apoptotic nuclei after caspase staining to identify apoptotic cells found the number of apoptotic nuclei significantly increased in the APAP group (P < 0.001) (Figs. 2b, 4). In the NAC and 25 µmol/kg NaHS groups, there were significant falls in the number of apoptotic nuclei (P < 0.001) (Figs. 2b, 4). However, for these parameters, as with other parameters, the most significant fall was observed in the 50 µmol/kg NaHS group. Again, in the 100 µmol/kg NaHS group, the number of apoptotic cells were identified to be significantly increased compared to other treatment groups (P < 0.001) (Figs. 2b, 4). Masson trichrome and collagen staining observed normal histological findings in the control group. In the treatment groups, especially the 50 µmol/kg NaHS group, reduced tubulointerstitial fibrosis was identified (Figs. 5, 6).
Effects of NaHS treatment on histological parameters in rats with APAP nephrotoxicity. a Tubuler ınjury score. b Caspase-3 nüclei count. Data are given as mean ± SEM. ***P < 0.001 versus control group, ###P < 0.001 versus APAP group ANOVA (n = 7). APAP Acetaminofen, NaHS sodium hydrosulfide, NAC N-acetyl cysteine
Discussion
In our study, we aimed to research the renoprotective effects of H2S on acute nephrotoxicity developing linked to APAP toxicity and to compare these effects with NAC used against APAP toxicity. With the aim of determining the effective dose of H2S and researching whether a dose-linked effect was present or not, we used increasing doses of the H2S donor NaHS. The most important results of this study are i- 50 µmol/kg dose of NaHS had renoprotective effect against acute nephrotoxicity and that lower or higher doses did not have this effect and ii- the 50 µmol/kg dose of NaHS was as effective as NAC.
Though the analgesic effect of APAP is not fully understood, the hepatotoxic effect is well known [24]. In vivo data proposed that another metabolite of APAP called AM404 may be a key factor in the analgesic effect [25], but some researchers indicate the cyclooxygenase-3 enzyme is the target enzyme for APAP [26, 27]. There are some reports that APAP causes AKI [28]. After APAP overdose, there are reviews that AKI occurs in 1–2% of patients [29]. A cohort study in Taiwan identified that the risk of developing nephrotoxicity was two times higher in patients with APAP intoxication [30]. This study discussed whether NAPQI may cause AKI. In our study, we induced AKI in rat kidneys with APAP. Our results comply with results from previous studies. We obtained findings that AKI was induced based on the parameters assessed with high doses of APAP.
In our study, we aimed to treat the nephrotoxicity induced with APAP using 25, 50 and 100 µmol/kg doses of NaHS. A noteworthy finding in our study is that 50 µmol/kg NaHS was effective on all parameters. In our study, we investigated the NGAL and KIM-1 levels to identify AKI. NGAL is a marker of injury to Kwon tubules in AKI. In recent times, it was found that NGAL is upregulated in AKI. NGAL is a newly defined member of the lipocalin family significantly expressed in injured epithelial cells. Injury of epithelial cells causes glomeruli to secrete significant amounts of NGAL. As a result, NGAL levels in urine increase [31]. KIM-1 is a trans-membrane protein not expressed in normal kidneys. However, KIM-1 is defined as the protein that increases most in renal proximal tubules after kidney injury. This protein occurs in many species including humans and animals and occurs in kidneys exposed to acute and chronic injury [32]. In our study, were identified NGAL and KIM-1 levels significantly increased compared to control levels after AKI due to APAP. At 50 µmol/kg dose of NaHS, both parameters successfully reduced to control levels. In fact, Zhang et al. [33] researched the effects of H2S on AKI developing after amputation and observed renal KIM-1 levels reduced after administration of H2S. A study by Lobb et al. [34] investigated whether or not H2S reduced the ischemia/reperfusion injury occurring after renal transplantation in Lewis rats and identified KIM-1 levels reduced necrosis and apoptosis. Nußbaum et al. [35] investigated the metabolic, cardiac and renal effects of a molecule called GYY4137 which is a slow-release H2S secretory molecule during resuscitation septic shock in pigs with coronary artery disease. They identified that this molecule reduced elevated NGAL levels. All these studies support the results of our study.
The TNF-α and TGF-β values were investigated with the aim of assessing the inflammatory process in AKI. At 50 µmol/kg dose NaHS, both values were close to control and NAC levels; in other words, the inflammatory process was suppressed. A study by Chen et al. [15] researched the protective effects of H2S on sepsis-related AKI. The researchers created a model with lipopolysaccharide (LPS) i.p. injection. In conclusion, the researchers revealed that 50 µmol/kg dose of exogenous H2S showed protective effect against AKI by inhibiting inflammation and oxidative stress at the TLR4/NLRP3 pathway. This study complies with our study results and in terms of the effective dose. This study by Chen et al. used mice. However, they did not try different doses of exogenous H2S. Sekijima et al. [36] in a study of CLAWN miniature pigs showed that H2S had cytoprotective effects on renal ischemia–reperfusion damage. In this study, the levels of inflammatory cytokines showed similar results to our study.
When oxidative stress is assessed, we see the TOS values increased and TAS values decreased in the APAP group. The noteworthy finding is that the TAS, TOS and OSI values in the NAC and 50 µmol/kg NaHS groups were very similar to each other. We see NaHS, especially at 50 µmol/kg dose, clearly compensated for the gap in antioxidant levels. Ibrahim et al. [37] in a study of renal ischemia/reperfusion in a rat model assessed the role of nitric oxide (NO) as a possible mediator of NaHS effects. Similar to our study, they identified that NaHS lowered oxidant levels and improved the gap in antioxidant levels developing linked to ischemia/reperfusion injury. The researchers identified the effects of NaHS at 100 µmol/kg dose in this study. We observed the same effect of NaHS at lower doses. At 100 µmol/kg dose, we found a deviation in the therapeutic effects for all parameters. The researchers did not try lower doses in their study. A study by Ali et al. [38] induced acute pulmonary inflammation with lipopolysaccharides in male rats and concluded that H2S reduced this inflammation and increased TAS levels. This study by the researchers supports the results of our study.
H&E staining of tissues is one of the most commonly used staining varieties for pathology diagnosis. In our study, results show increased tubular injury linked to APAP toxicity. This injury was reduced by NAC and 25 and 50 µmol/kg doses of NaHS. However, the most effective improvement was observed with 50 µmol/kg NaHS dose. A study by Wu et al. [39] was observed to obtain similar results. The researchers studied the effects of H2S on the kidneys of obese mice and they identified that 50 µmol/kg H2S improved tubular injury. Immunohistochemical analysis with caspase staining observed 25 and 50 µmol/kg NaHS reduced the number of apoptotic nuclei; however, 100 µmol/kg dose increased them. This result is similar to a study by Wu et al. [40]. In this study, Wu et al. proposed that H2S inhibited apoptosis and inflammation in rats improving chronic renal failure and the results confirmed their hypothesis. This study by Wu et al. investigated kidney tissue with H&E and Masson trichrome staining and obtained similar results to our study. A study by Yang et al. [41] investigated the protective effect of H2S in kidneys of rats with type 1 diabetes and concluded that H2S reduced caspase 3 activity and suppressed cell apoptosis.
Another important result of our study is that in the group administered 100 µmol/kg dose NaHS, all parameters deviated from control levels. We interpret this result as being due to H2S being toxic at high doses. However, the results of our literature scan observed that some researchers used this dose and even higher doses like 200 µmol/kg and observed effects [22, 37, 42]. Some researchers observed effects with 50 µmol/kg dose [15]. In our study, we observed that at 50 µmol/kg dose, all parameters for acute renal nephrotoxicity reached control levels.
Conclusion
The results of our study showed that H2S improved AKI induced with APAP, the most effective dose intervals are between 25 and 100 µmol/kg, that doses above 100 µmol/kg may begin to have toxic effects and that it may be as effective as NAC used as antidote to APAP toxicity in the kidneys. The antiapoptotic, anti-inflammatory and antioxidant effects of H2S may play a role in preventing AKI induced by APAP. However, there is a need for more studies to determine the dose interval.
References
Abdel-Hafez SMN, Rifaai RA, Abd Elzaher WY (2017) Mechanism of grape seeds extract protection against paracetamol renal cortical damage in male Albino rats. Bratisl Lek Listy 118(4):233–242. https://doi.org/10.4149/BLL_2017_046
Kennon-McGill S, McGill MR (2018) Extrahepatic toxicity of acetaminophen: critical evaluation of the evidence and proposed mechanisms. J Clin Transl Res. https://doi.org/10.18053/jctres.03.201703.005
Jones AF, Vale JA (1993) Paracetamol poisoning and the kidney. J Clin Pharm Ther 18(1):5–8
Sarumathy KA (2011) Protective effect of Caesalpinia sappan on acetaminophen phen induced nephrotoxicity and oxidative stress in male albino rats. J Pharmacol Toxicol 15(2):598–605
Bessems JG, Vermeulen NP (2001) Paracetamol (acetaminophen)-induced toxicity: molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol 31:55–138. https://doi.org/10.1080/20014091111677
Mugford CA, Tarloff JB (1997) The contribution of oxidation and deacetylation to acetaminophen nephrotoxicity in female Sprague-Dawley rats. Toxicol Lett 93:15–22
Li C, Liu J, Saavedra JE, Keefer LK, Waalkes MP (2003) The nitric oxide donor, V-PYRRO/NO, protects against acetaminophen-induced nephrotoxicity in mice. Toxicology 189:173–180
Das J, Ghosh J, Manna P, Sil PC (2010) Taurine protects acetaminophen-induced oxidative damage in mice kidney through APAP urinary excretion and CYP2E1 inactivation. Toxicology 269(1):24–34. https://doi.org/10.1016/j.tox.2010.01.003
Atkuri KR, Mantovani JJ, Herzenberg LA (2007) N-Acetylcysteine, a safe antidote forcysteine/glutathione deficiency. Curr Opin Pharmacol 7:355–359. https://doi.org/10.1016/j.coph.2007.04.005
Heard KJ (2008) Acetylcysteine for acetaminophen poisoning. N Engl J Med 359:285–292. https://doi.org/10.1056/NEJMct0708278
Murad HA, Habib H, Kamel Y, Alsayed S, Shakweer M, Elshal M (2016) Thearubigins protect against acetaminophen-induced hepatic and renal injury in mice: biochemical, histopathological, immunohistochemical, and flow cytometry study. Drug Chem Toxicol 39(2):190–198. https://doi.org/10.3109/01480545.2015.1070170
Hanly LN, Chen N, Aleksa K, Cutler M, Bajcetic M, Palassery R, Regueira O, Turner C, Baw B, Malkin B, Freeman D, Rieder MJ, Vasylyeva TL, Koren G (2012) N-acetylcysteine as a novel prophylactic treatment for ifosfamide-induced nephrotoxicity in children: translational pharmacokinetics. J Clin Pharmacol 52:55–64. https://doi.org/10.1177/0091270010391790
Sen U, Pushpakumar SB, Amin MA, Tyagi SC (2014) Homocysteine in renovascular complications: hydrogen sulfide is a modulator and plausible anaerobic ATP generator. Nitric Oxide 15;41:27–37. https://doi.org/10.1016/j.niox.2014.06.006
Shibuya N, Koike S, Tanaka M, Ishigami-Yuasa M, Kimura Y, Ogasawara Y (2013) A novel pathway for the production of hydrogen sulfide from d-cysteine in mammalian cells. Nat Commun 4:1366. https://doi.org/10.1038/ncomms2371
Chen Y, Jin S, Teng X, Hu Z, Zhang Z, Qiu X, Tian D, Wu Y(2018). Hydrogen sulfide attenuates LPS-induced acute kidney injury by inhibiting inflammation and oxidative stress. Oxid Med Cell Longev 2018: 6717212. https://doi.org/10.1155/2018/6717212
Huang Z, Zhuang X, Xie C, Hu X, Dong X, Guo Y, Li S, Liao X (2016) Exogenous hydrogen sulfide attenuates high glucose-induced cardiotoxicity by inhibiting NLRP3 inflammasome activation by suppressing TLR4/NF-κB pathway in H9C2 cells. Cell Physiol Biochem 40:6;1578–90. https://doi.org/10.1159/000453208
Han SJ, Kim JI, Park JW, Park KM (2015) Hydrogen sulfide accelerates the recovery of kidney tubules after renal ischemia/reperfusion injury. Nephrol Dial Transpl. 30:1497–1506. https://doi.org/10.1093/ndt/gfv226
Dugbartey GJ (2016) Diabetic nephropathy: A potential savior with ‘rotten-egg’ smell. Pharmacol Rep 69(2):331–9. https://doi.org/10.1016/j.pharep.2016.11.004
Dugbartey GJ (2017) H2S as a possible therapeutic alternative for the treatment of hypertensive kidney injury. Nitric Oxide 64:52–60. https://doi.org/10.1016/j.niox.2017.01.002
Dugbartey (2018) The smell of renal protection against chronic kidney disease: hydrogen sulfide offers a potential stinky remedy. Pharmacol Rep 70(2):196–205. https://doi.org/10.1016/j.pharep.2017.10.007
Canayakin D, Bayir Y, Kilic Baygutalp N, Sezen Karaoglan E, Atmaca HT, Kocak Ozgeris FB, Keles MS, Halici Z (2016) Paracetamol-induced nephrotoxicity and oxidative stress in rats: the protective role of Nigella sativa. Pharm Biol 54(10):2082–2091. https://doi.org/10.3109/13880209.2016.1145701
Zeng O, Li F, Li Y, Li L, Xiao T, Chu C, Yang J (2016) Effect of novel gasotransmitter hydrogen sulfide on renal fibrosis and connexins expression in diabetic rats. Bioengineered 7(5):314–320. https://doi.org/10.1080/21655979.2016.1197743
Zhang W, Sha Y, Wei K, Wu C, Ding D, Yang Y, Zhu C, Zhang Y, Ding G, Zhang A, Jia Z, Huang S (2018) Rotenone ameliorates chronic renal injury caused by acute ischemia/reperfusion. Oncotarget 9(36):24199–24208. https://doi.org/10.18632/oncotarget.24733
Hiragi S, Yamada H, Tsukamoto T, Yoshida K, Kondo N, Matsubara T, Yanagita M, Tamura H, Kuroda T (2018) Acetaminophen administration and the risk of acute kidney injury: a self-controlled case series study. Clin Epidemiol 10:265–276. https://doi.org/10.2147/CLEP.S158110
Högestätt ED, Jönsson BA, Ermund A, Andersson DA, Björk H, Alexander JP, Cravatt BF, Basbaum AI, Zygmunt PM (2005) Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. J Biol Chem 280(36):31405–31412. https://doi.org/10.1074/jbc.M501489200
RM (2000) Botting. Mechanism of action of acetaminophen: is there a cyclooxygenase 3? Clin Infect Dis 31(suppl 5):S202–S210. https://doi.org/10.1086/317520
Botting R, Ayoub SS (2005) COX-3 and the mechanism of action of paracetamol/acetaminophen. Prostaglandins Leukot Essent Fatty Acids 72(2):85–87. https://doi.org/10.1016/j.plefa.2004.10.005
Mazer M, Perrone J (2008) Acetaminophen-induced nephrotoxicity: pathophysiology, clinical manifestations, and management. J Med Toxicol 4(1):2–6
Prescott LF (1983) Paracetamol overdosage. Pharmacological considerations and clinical management. Drugs 25(3):290–314
Chen YG, Lin CL, Dai MS, Chang PY, Chen JH, Huang TC, Wu YY, Kao CH (2015) Risk of acute kidney injury and long-term outcome in patients with acetaminophen intoxication: a nationwide population-based retrospective cohort study. Medicine 94(46):e2040. https://doi.org/10.1097/MD.0000000000002040
Lei L, Li LP, Zeng Z, Mu JX, Yang X, Zhou C, Wang ZL, Zhang H (2018) Value of urinary KIM-1 and NGAL combined with serum Cys C for predicting acute kidney injury secondary to decompensated cirrhosis. Sci Rep 21(1):7962. https://doi.org/10.1038/s41598-018-26226-6
Bonventre JV (2014) Kidney injury molecule-1: a translational journey. Trans Am Clin Climatol Assoc 125:293–299
Zhang Y, Liu N, Ren Q, Zhang H, Xie X (2013) Sodium hydrosulfide for prevention of kidney damage in rats after amputation. Nan Fang Yi Ke Da Xue Xue Bao 33(8):1146–1150. https://doi.org/10.3969/j.issn.1673-4254.2013.08.10
Lobb I, Davison M, Carter D, Liu W, Haig A, Gunaratnam L, Sener A (2015) Hydrogen sulfide treatment mitigates renal allograft ischemia-reperfusion injury during cold storage and improves early transplant kidney function and survival following allogeneic renal transplantation. J Urol 194(6):1806–1815. https://doi.org/10.1016/j.juro.2015.07.096
Nußbaum BL, Vogt J, Wachter U, McCook O, Wepler M, Matallo J, Calzia E, Gröger M, Georgieff M, Wood ME, Whiteman M, Radermacher P, Hafner S (2017) Metabolic, cardiac, and renal effects of the slow hydrogen sulfide-releasing molecule GYY4137 during resuscitated septic shock in swine with pre-existing coronary artery disease. Shock 48(2):175–184. https://doi.org/10.1097/SHK.0000000000000834
Sekijima M, Sahara H, Miki K, Villani V, Ariyoshi Y, Iwanaga T, Tomita Y, Yamada K (2017) Hydrogen sulfide prevents renal ischemia-reperfusion injury in CLAWN miniature swine. J Surg Res 219:165–172. https://doi.org/10.1016/j.jss.2017.05.123
Ibrahim MY, Aziz NM, Kamel MY, Rifaai RA (2015) Sodium hydrosulphide against renal ischemia/reperfusion and the possible contribution of nitric oxide in adult male Albino rats. Bratisl Lek Listy. 116(11):681–688. https://doi.org/10.4149/BLL_2015_133
Ali FF, Abdel-Hamid HA, Toni ND (2018) H2S attenuates acute lung inflammation induced by administration of lipopolysaccharide in adult male rats. Gen Physiol Biophys. https://doi.org/10.4149/gpb_2018002
Wu D, Gao B, Li M, Yao L, Wang S, Chen M, Li H, Ma C, Ji A, Li Y (2016) Hydrogen sulfide mitigates kidney injury in high fat diet-induced obese mice. Oxid Med Cell Longev 2016:2715718 https://doi.org/10.1155/2016/2715718
Wu D, Luo N, Wang L, Zhao Z, Bu H, Xu G, Yan Y, Che X, Jiao Z, Zhao T, Chen J, Ji A, Li Y, Lee GD (2017) Hydrogen sulfide ameliorates chronic renal failure in rats by inhibiting apoptosis and inflammation through ROS/MAPK and NF-κB signaling pathways. Sci Rep 28;7(1):455. https://doi.org/10.1038/s41598-017-00557-2
Yang R, Liu XF, Ma SF, Gao Q, Li ZH, Jia Q (2016) Protective effect of hydrogen sulfide on kidneys of type 1 diabetic rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi 8(2):181–184. https://doi.org/10.13459/j.cnki.cjap.2016.02.023.
Karimi A, Absalan F, Khorsandi L, Valizadeh A, Mansouri E (2017) Sodium hydrogen sulfide (NaHS) ameliorates alterations caused by cisplatin in filtration slit diaphragm and podocyte cytoskeletal in rat kidney. J Nephropathol 6(3):150–156. https://doi.org/10.15171/jnp.2017.26
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the Dumlupinar University, Animal Experiments Local Ethics Committee at which the studies were conducted (Decision no: 2017.07.01).
Additional information
This Study was carried out in Dumlupinar University, Experimental Animal Laboratory.
Rights and permissions
About this article
Cite this article
Ozatik, F.Y., Teksen, Y., Kadioglu, E. et al. Effects of hydrogen sulfide on acetaminophen-induced acute renal toxicity in rats. Int Urol Nephrol 51, 745–754 (2019). https://doi.org/10.1007/s11255-018-2053-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-018-2053-0