Abstract
Purpose
Chronic kidney disease (CKD) is an inflammatory process. In addition to increased morbidity and mortality, inflammation also contributes to the progression of CKD. Neutrophil/lymphocyte ratio (NLR) is a marker of inflammation. Some recent data suggest that NLR may predict the progression of CKD.
Methods
In this study, 5-year data of 740 patients with stage 2–4 CKD were reviewed retrospectively. Demographic data, NLR, CRP, albumin, the amount of proteinuria were recorded. At the beginning and the end of follow-up the glomerular filtration rate (GFR) and the annual GFR decline rate were calculated. Patients were divided to high and low NLR group according to median value of their baseline NLR. Reaching stage 5 CKD or initiation of renal replacement therapy was determined as end-point for follow-up.
Results
The mean age was 62.8 ± 0.57 years, eGFR 40 ml/min/1.73 m2, median NLR was 2.76. NLR increased as the CKD-stage increased. Mean follow-up time was 51.2 ± 30 months and 21.4% of patients reached the end-point. NLR was significantly increased at follow-up (from 3.22 to 5.68, p < 0.001). Annual GFR loss and baseline CRP were higher but baseline albumin and GFR were lower of patients with high NLR. The percent of patients reaching the end-point was not different between the groups with high and low baseline NLR. Kaplan Meier analysis showed that patients with high NLR had significantly lower mean renal survival (86.5 months) than patients with low NLR (105 months) (p < 0.001). In the Cox-regression analysis NLR was not an independent predictor in reaching the end-point but presence of diabetes mellitus, younger age and low baseline eGFR were found effective.
Conclusions
NLR is an indicator of inflammation in chronic kidney disease. It may not be an independent predictor of CKD progression except that the CKD is in a more advanced stage and reflects the associated inflammation. Classical risk factors such as DM and lower GFR are more powerful predictors of progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is a worldwide health problem with an increasing prevalence of chronic diseases such as aging of the population, diabetes mellitus (DM), and hypertension (HT). Whatever the underlying etiologic factor, CKD is a progressive disease. Even if the causative factor for kidney damage is removed, nephron loss leads to adaptive hypertrophy and hyper-filtration in the surviving intact nephron. Glomerular sclerosis progresses through proteinuria, hypertension, and other complex pathophysiological mechanisms [1,2,3]. It is well known that continuous chronic inflammation at a low level is present in the course of CKD [4]. Studies have shown that patients with CKD have higher levels of pro-inflammatory cytokines and lower levels of antioxidants and anti-inflammatory cytokines [5]. Inflammation in CKD is associated with cardiovascular events, all-cause mortality and malnutrition, as well as progression of disease [4]. Nowadays, it is still unclear which marker is the best indicator of inflammation in CKD. CRP is still the most frequently used indicator of inflammation, despite the use of many indicators such as erythrocyte sedimentation, IL-6, IL-8, IL, 12, TNF-alpha, IL-33 [4,5,6]. Recently, the neutrophil/lymphocyte ratio (NLR), obtained by dividing the absolute number of neutrophils to the lymphocyte count, has gathered interest as a new marker in evaluation of inflammation. There are substantial data that indicate NLR as a possible predictor of morbidity and mortality in patients with malignancy and cardiovascular disease [7,8,9,10]. Since CKD is accepted as a chronic inflammatory condition, studies with relatively small-scale have recently been conducted on whether NLR may be predictor of CKD progression. In this study, we aimed to investigate whether NLR is associated with CKD progression and reach to end-stage renal disease (ESRD) in a relatively large population of patients with CKD.
Methods
Study group selection
This retrospective study was conducted at Sutcu Imam University Faculty of Medicine, Department of Nephrology in Kahramanmaras. Adult patients who were admitted to the nephrology outpatient clinic within a 5 year period before October 2017 were screened for ICD codes of CKD (N18, N18.8, N18.9, N19) by a team of 11 participants. Totally, 2607 patient records were obtained. Then they were selected by considering the file numbers to avoid repeated selection. Patients’ examination notes were reviewed to ensure they were undergoing CKD diagnosis and patients’ all available serum creatinine (Cr) levels were examined during follow-up period. Although the ICD code was CKD, patients who were compatible with acute renal failure (eg patients whose creatinine levels were initially high and returned to normal on follow-up) were removed from the study. Demographics and serum creatinine noted on the first visit to the outpatient clinic of each patient were used in the MDRD formula (Modification of Diet in Renal Disease) to attain their baseline estimated glomerular filtration rate (eGFR). Then patients with a follow-up time less than 1 year in the outpatient clinic and patients with eGFR > 90 ml/min/1.73 m2 (stage-1 CKD) and < 15 ml/min/1.73 m2 (stage-5 CKD) at baseline were excluded from the study. In addition; patients with hematological diseases that may affect white blood cell (WBC) subgroups; neutrophils (N) and lymphocytes (L), patients with active infections, positive culture results, those with high WBC or CRP levels (WBC count > 15,000/mm3 and/or CRP level > 50 mg/dl), patients with malignancy and/or history of chemotherapy, patients receiving immunosuppressive drugs and patients with chronic inflammatory processes such as rheumatoid arthritis, familial Mediterranean fever, ankylosing spondylitis, systemic vasculitis, and gout were also excluded. After all these exclusion criteria were implemented, 740 patients with stage 2–4 CKD were included in the study. Along with demographics, CKD etiology, additional comorbidies (presence of DM and HT), antihypertensive drugs used and follow-up duration, systolic (SBP) and diastolic (DBP) blood pressure readings, serum creatinine, eGFR, calcium (Ca), phosphorus (P), uric acid, CRP, proteinuria, WBC, N, L and platelet (PLT) counts, hemoglobin (Hb) value at the first and last admission of outpatient visit were recorded. Proteinuria was either measured by protein amount in the 24-h urine or urine protein/creatinine ratio (UPCR) in patients whose 24-h urinary protein result was not present. The NLR was obtained by dividing the number of neutrophils by the number of lymphocytes. The time elapsed between the beginning and end of the follow-up (months) and the annual losses in eGFR were calculated. The formula “(initial eGFR − last eGFR) × 12/time” was used to calculate the annual GFR loss. Accordingly, patients whose annual loss of eGFR was higher than 5 ml/min/1.73 m2 were defined as patients with faster GFR decline as indicated in the Kidney Disease Improving Global Outcomes (KDIGO) guidelines for evaluation and management of CKD in 2012. CKD-stages were determined with eGFR according to the same guidelines [11]. Initiation of renal replacement therapy (RRT) or reaching to the stage-5 CKD was determined as the end-point of the study. Patients were also divided into two groups; (high and low NLR) according to the median baseline NLR value of the study population and the two groups were compared for clinical features. Due to the retrospective design of the study, no ethics committee approval was received.
Statistical analysis
The categorical data were expressed as percentage and ratio. The continuous variables that were obtained were expressed as mean ± standard deviation (SD). Normality of distribution in continuous variables was checked by Kolmogorow Smirnov test. For comparison of two groups; Chi square and Fishers Exact Tests were used for categorical variables. Student’s t test was used for continuous variables if the data distribution was normal and the Mann–Whitney U test if the data distribution was not normal. In comparison of more than two groups; ANOVA was used when the distribution of data was normal and Kruskal Wallis variance analysis was used when the distribution of data was not normal. Correlation analysis of continuous variables was performed with Pearson and/or Spearman correlation analyzes. Renal survival analysis in patients with low or high NLR values was done by Kaplan Meier survival analysis. Cox-regression analysis was used to determine the factors that were effective on reaching to ESRD. Any p value was less than 0.05 was considered as significant. All data were analyzed using SPSS 16.0 (Released 2007. SPSS for Windows, Version 16.0, Chicago, SPSS Inc.) software program.
Results
Clinical data and NLR
The mean age was 62.8 ± 0.57 years and 59.6% of the patients were male. At baseline, 22 patients were stage 2, 561 patients were stage 3 and 157 patients were stage 4 CKD according to their eGFR. DM frequency was 46%, the mean amount of proteinuria was 1592 mg/day and the mean eGFR was 40.5 ml/min/1.73 m2. The mean SBP and DBP were 139 and 82 mmHg respectively. The mean follow-up period was 51.2 months during which 21.4% of patients had reached the designated end-point. Demographic data, BP readings, antihypertensive medication and laboratory values of 740 patients are presented in Table 1, which includes the data at the beginning and the end of their follow-up.
Mean NLR at baseline was 3.22 which increased to 5.68 at the subsequent follow-up data (p < 0.001). This change was predominantly related to the significant decrease in the lymphocyte count between the two evaluations (from 3.22 to 5.68, p < 0.001). Mean eGFR at the end of follow-up was also decreased to 27.2 ml/min/1.73 m2 (p < 0.001).
In simple correlation analysis; NLR at the beginning of follow-up was positively correlated with the variables; annual loss of eGFR (p = 0.005, r = 0.1), CRP (p < 0.001, r = 0.24) and CKD-stage (p = 0.001, r = 0.13). Baseline NLR was also inversely correlated with baseline eGFR (p = 0.03, r = − 0.08). When the patients at stage 2, 3 and 4 CKD were compared among each other for baseline NLR, it was seen that NLR increased along with the CKD stage. Mean baseline NLR of stage 4 patients was significantly higher than those with stage 2 and stage 3 CKD (mean NLR for stage 2 = 2.74, mean NLR for stage 3 = 3.10 and NLR of stage 4 = 3.78, p = 0.006) (Fig. 1).
Comparison of patients with high and low baseline NLR
The patient groups with high (NLR > 2.76, n = 328) and low baseline NLR (NLR < 2.76, n = 337) were not different in terms of age, gender, history of DM, HT and CAD, proteinuria, SBP, DBP, and the number of patients reaching the study end-point. Patients in the high NLR group had lower mean eGFR (39.4 ± 12.0 vs. 41.6 ± 12.0, p = 0.03), lower mean albumin (3.7 ± 0.6 vs. 3.9 ± 0.6, p = 0.002), higher mean CRP (16.1 ± 19.2 vs. 7.7 ± 8.4, p < 0.001), and higher rate of annual GFR decline (4.96 ± 8.13 vs. 3.59 ± 6.15, p = 0.04) compared to patients in the low NLR group at the beginning of their follow-up (Table 2).
The mean baseline NLR of the patients who reached the end-point was not different from those who did not (3.54 ± 2.85 vs. 3.14 ± 1.99 respectively; p = 0.09). These patients had higher annual GFR decline (9.2 ± 9.3 vs. 2.8 ± 5.7, p < 0.001) and proteinuria, also lower initial eGFR and serum albumin levels (Table 3).
The frequency of DM was higher in patients with faster GFR decline (> 5 ml/min/1.73 m2/year), than those without (54% vs. 42%, p = 0.002). These patients with a faster GFR decline had higher mean SBP and DBP readings, lower serum albumin levels and higher proteinuria (Table 4). Mean NLR of patients with faster GFR decline was also significantly higher than the patients without faster GFR decline (3.41 ± 1.82 vs. 3.13 ± 2.34, p = 0.01).
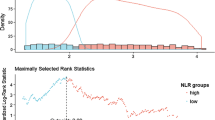
Kaplan–Meier analysis showed that patients with high baseline NLR had significantly lower mean renal survival (86.5 vs. 105 months, p < 0.001) (Fig. 2). When the diabetic and non-diabetic patients were compared, renal survival was lower in patients with DM. In the Cox-regression analysis, when baseline NLR was examined alone, it showed a significant effect on reaching the study end-point. However, in a mix model, including NLR and other variables (patient age, DM, baseline eGFR, SBP, and uric acid), baseline NLR was not found to be effective in reaching the end-point of the study (p = 0.764). In this analysis, the factors; patient age (p = 0.02), history of DM (p = 0.022), and lower baseline eGFR (p < 0.001) were effective in reaching the end-point (Table 5).
Discussion
In this study, we evaluated the data of 740 patients diagnosed with CKD for a possible relation between the progression of the disease and NLR, which is an indicator of inflammation. We found that patients with higher NLR had higher annual loss of eGFR, lower albumin values, and higher CRP levels. We showed that renal survival of patients with high NLR levels were significantly lower than patients with low NLR levels. Patients who also had more annual GFR decline had higher initial NLR levels, higher proteinuria, higher frequency of DM, and lower albumin values. In the Cox-regression analysis, we showed that DM, young age and low baseline eGFR were independent risk factors for reaching renal end-points such as advancing to ESRD or initiation of RRT instead of baseline NLR. When these results are interpreted extensively, we think that NLR is not an independent indicator for predicting renal end-points, but it may reflect the profile of a patient with a more inflammatory status who possibly has more co-morbidities within a more advanced stage of CKD.
It is known that chronic inflammation is involved in the course of CKD. Inflammation contributes to progression of CKD, malnutrition and cardiovascular disease all together decreasing the life expectancy of the patient. This ongoing inflammatory condition is linked to the progression of CKD by the contribution of various situations such as; oxidative and carbonyl stress, inadequate intake of antioxidants, increased blood levels of pro-inflammatory cytokines, infections, atherosclerosis, unused arteriovenous fistulas, and malnutrition caused by anorexia, absorptive defects, and dietary restrictions.
It is possible that inflammatory markers may provide information about the disease progression and the prognosis in patients with CKD. However, we do not yet know which are the ideal inflammatory markers that may be linked to the progression of CKD. Many studies showed that NLR is an important indicator of inflammation in the CKD population, including patients on chronic peritoneal dialysis (PD) and maintenance hemodialysis (HD). An et al. showed that NLR was higher in PD patients than in healthy controls and found that cardiovascular and all-cause mortality was higher in PD patients with higher NLR and CRP levels [12]. Okyay et al. showed that the NLR of patients on PD, HD, and those with pre-dialytic CKD were higher compared to healthy controls. They also showed that NLR was positively correlated with high sensitive CRP and IL-6 levels and a negatively correlated with serum albumin levels [13]. Another study revealed higher TNF-alpha levels in ESRD patient with high NLR values [14].
Recently there has been an increase in the amount of data that may suggest that NLR is not only an indicator of inflammation, but also a predictor of CKD progression. Kocyigit et al. showed that patients with high NLR had higher baseline CRP levels and faster GFR decline to advance to ESRD in their study of 105 patients with stage-4 CKD [15]. However, these patients with high NLR and rapid progression to ESRD had lower baseline GFRs. In Cox-regression analysis, the baseline renal function was one of the two factors that were effective in reaching the end-point of their study. We also showed that renal survival was lower in the Kaplan Meier analysis of patients with high NLR in our study. Patients with high NLR have lower albumin values, higher CRP values, but lower initial GFR values. More than that, our study had a quite larger sample size also including stage-2 and stage-3 CKD patients in the analysis. Tatar et al. [16] conducted a study with 165 patients over 65 years of age and found that the rates of mortality and initiation of RRT were higher in patients who had increasing NLR values over time. Also, they found that patients with GFR below 29 ml/min/1.73 m2 had high NLR values in addition to higher rates of mortality and initiation of RRT. In fact, an increase in mortality, RRT requirement and higher NLR due to more inflammation is not unexpected as GFR declines. In our study, the frequency of dialysis initiation was not different between the groups with high and low baseline NLR. Also, the baseline NLRs at the beginning of follow-up of the patients who reached the renal end-point were not higher. When these patients who reached the end-point of study were examined, the baseline eGFR and serum albumin were lower, and baseline proteinuria and annual loss of eGFR were higher. When these results are interpreted collectively, it can be concluded that the prognosis of patients with high NLR could be worse. However, we think that high NLR values may indicate those patients in higher stages of CKD with their higher inflammatory status rather than being an independent predictor for progression of CKD. This is because our Cox-regression analysis showed that diabetes mellitus, younger age and low baseline eGFR were effective in reaching the end-point rather than baseline NLR. The fact that patients who reached the study end-point had lower baseline eGFR and NLR being positively correlated with eGFR and negatively correlated with serum CRP levels also supported our suggestion.
Kim et al. showed that the relative lymphocyte count (RLC) was related to progression to ESRD in patients with CKD [17]. RLC is the ratio of the number of lymphocytes to the total number of WBC, which has recently been investigated as a possible marker of inflammation like the NLR. In fact, when GFR decreases due to the progression of CKD, both NLR and RLC reflect a decrease in the number of lymphocytes over against an increase in the number of neutrophils. Tonyali et al. showed that NLR increased along with decreasing GFR and disease progression in CKD patients with nephrectomy [18]. In our study, NLR and eGFR were inversely correlated. Yılmaz et al. also showed that NLR increased along with decreasing GFR and increasing proteinuria in CKD patients [19]. In support of this, a study by Agarval et al. revealed that the number of lymphocytes may decrease over time in CKD patients and also healthy populations [20]. Lymphocyte counts have been shown to decrease in the elderly population, which was explained by immunological aging [21, 22]. The decreased number of lymphocytes in the elderly was also found to be associated with anemia and increased IL-6 and CRP levels [23]. The fact that CKD may be referred as a geriatric syndrome and these features are more prominent in CKD patients may explain the decrease in lymphocyte counts.
One interesting result from the data we obtained was that younger age being associated with CKD progression. The patients with faster GFR decline and those who reached the study end-point were younger. Regression analysis also revealed young age to be effective in reaching the end-point. Kim et al. also found in their study that young age can be associated with CKD progression. Similar results have been shown in some other studies [24].
This is study with the largest sample size that investigates 740 cases under follow-up for a possible relationship between NLR and CKD progression. Similar to the results of previous studies, NLR could indicate the inflammatory status in CKD, which is more prominent in the advanced stages of the disease. Yet, there are no powerful data to assert that NLR may predict the progression of CKD. Although it has been used in many studies, the prediction of CKD progression with only one baseline biochemical measurement may not be scientifically sound approach. The most important limitation of our study is its retrospective nature. There were incomplete data or difficulties of obtaining them. Besides, unrecognized interventions that accelerate the natural progression of CKD such as acute kidney injury episodes and drug toxicities had to be ignored. Another limitation is that the study contained patients with mild chronic kidney disease and CKD EPI formula may be preferred to the MDRD in the evaluation of GFRs in these patients. Fortunately only 22 patients had mild CKD and this limitation may not be expected to affect the results.
Conclusion
Higher NLR may be related to increased inflammation and malnutrition, lower GFR and a worse clinical profile, all together reflecting an advanced stage of CKD instead of indicating the progression of the disease. We think that the prediction of CKD progression with only one baseline biochemical measurement may not be scientifically sound approach. As a result, high NLR marks the higher level of inflammation in advanced CKD and indirectly implicates that the progression could also be faster. Rather than baseline NLR, classical risk factors such as DM and lower GFR are still more powerful predictors of progression.
References
Silverstein DM (2009) Inflammation in chronic kidney disease: role in the progression of renal and cardiovascular disease. Pediatr Nephrol 24:1445–1452
Yu HT (2003) Progression of chronic renal failure. Arch Intern Med 163:1417–1429
Jacobson HR (1991) Chronic renal failure: pathophysiology. Lancet 338:419–423
Akchurin OM, Kaskel F (2015) Update on inflammation in chronic kidney disease. Blood Purif 39:84–92
Kalantar-Zadeh K (2007) Inflammatory marker mania in chronic kidney disease: pentraxins at the crossroad of universal soldiers of inflammation. Clin J Am Soc Nephrol 2:872
Gungor O, Unal HU, Guclu A, Gezer M, Eyileten T, Guzel FB, Altunoren O, Erken E, Oguz Y, Kocyigit I, Yilmaz MI (2017) IL-33 and ST2 levels in chronic kidney disease: associations with inflammation, vascular abnormalities, cardiovascular events, and survival. PLoS ONE 14(6):e0178939
Xiao WK, Chen D, Li SQ, Fu SJ, Peng BG, Liang LJ (2014) Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. BMC Cancer 14:1–10
Wei Y, Jiang YZ, Qian WH (2014) Prognostic role of NLR in urinary cancers: a meta-analysis. PLoS ONE 9:e92079
Azab B, Zaher M, Weiserbs KF, Torbey E, Lacossiere K, Gaddam S, Gobunsuy R, Jadonath S, Baldari D, McCord D, Lafferty J (2010) Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol 106:470–476
Chua W, Charles KA, Baracos VE, Clarke SJ (2011) Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer 104:1288–1295
Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group (2013) KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Inter Suppl 3:1–150
An X, Mao HP, Wei X et al (2012) Elevated neutrophil to lymphocyte ratio predicts overall and cardiovascular mortality in maintenance peritoneal dialysis patients. Int Urol Nephrol 44:1521–1528
Okyay GU, Inal S, Oneç K, Er RE, Paşaoğlu O, Paşaoğlu H, Derici U, Erten Y (2013) Neutrophil to lymphocyte ratio in evaluation of inflammation in patients with chronic kidney disease. Ren Fail 35:29–36
Turkmen K, Guney I, Yerlikaya FH, Tonbul HZ (2012) The relationship between neutrophil-to-lymphocyte ratio and inflammation in end-stage renal disease patients. Ren Fail 34:155–159
Kocyigit I, Eroglu E, Unal A, Sipahioglu MH, Tokgoz B, Oymak O, Utas C (2013) Role of neutrophil/lymphocyte ratio in prediction of disease progression in patients with stage-4 chronic kidney disease. J Nephrol 26:358–365
Tatar E, Mirili C, Isikyakar T, Yaprak M, Guvercin G, Ozay E, Asci G (2016) The association of neutrophil/lymphocyte ratio and platelet/lymphocyte ratio with clinical outcomes in geriatric patients with stage 3–5 chronic kidney disease. Acta Clin Belg 71:221–226
Kim SM, Kim HW (2014) Relative lymphocyte count as a marker of progression of chronic kidney disease. Int Urol Nephrol 46:1395–1401
Tonyali S, Ceylan C, Yahsi S, Karakan MS (2018) Does neutrophil to lymphocyte ratio demonstrate deterioration in renal function? Ren Fail 40:209–212
Yilmaz G, Sevinc C, Ustundag S, Yavuz YC, Hacıbekiroglu T, Hatipoglu E, Baysal M (2017) The relationship between mean platelet volume and neutrophil/lymphocyte ratio with inflammation and proteinuria in chronic kidney disease. Saudi J Kidney Dis Transpl 28:90–94
Agarwal R, Light RP (2011) Patterns and prognostic value of total and differential leukocyte count in chronic kidney disease. Clin J Am Soc Nephrol 6:1393–1399
Hulstaert F, Hannet I, Deneys V, Munhyeshuli V, Reichert T, De BM, Strauss K (1994) Age-related changes in human blood lymphocyte subpopulations. II. Varying kinetics of percentage and absolute count measurements. Clin Immunol Immunopathol 70:152–158
MacKinney AA Jr (1978) Effect of aging on the peripheral blood lymphocyte count. J Gerontol 33:213–216
Ferrucci L, Guralnik JM, Bandinelli S, Semba RD, Lauretani F, Corsi A, Ruggiero C, Ershler WB, Longo DL (2007) Unexplained anaemia in older persons is characterised by low erythropoietin and low levels of pro-inflammatory markers. Br J Haematol 136:849–855
O’Hare AM, Choi AI, Bertenthal D, Bacchetti P, Garg AX, Kaufman JS, Walter LC, Mehta KM, Steinman MA, Allon M, McClellan WM, Landefeld CS (2007) Age affects outcomes in chronic kidney disease. J Am Soc Nephrol 18:2758–2765
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Altunoren, O., Akkus, G., Sezal, D.T. et al. Does neutrophyl to lymphocyte ratio really predict chronic kidney disease progression?. Int Urol Nephrol 51, 129–137 (2019). https://doi.org/10.1007/s11255-018-1994-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-018-1994-7