Abstract
Objectives
To investigate the efficacy of insulin sensitizer on prostatic tissue in animal model with benign prostatic hyperplasia (BPH) secondary to metabolic syndrome (MetS).
Methods
Models were established by providing Sprague–Dawley rats with high fat diet (HFD) combined with metformin or not. All objects were killed 40 days later with prostatic tissue being removed, weighed before stained, as well as the expression level of insulin-like growth factor I (IGF-1) and receptor (IGF-1R) being measured, and the level of insulin resistance (IR) has also been evaluated.
Results
Model has been successfully established. Level of prostatic hyperplasia and IR as well as IGF-1 and IGF-1R expressions in the blank and saline control subunits of HFD group was higher than that of normal diet group (P < 0.05). In the subunit of metformin, along with the suppression of IR, the level of prostatic hyperplasia and the expression of IGF-1 pathway have both decreased (P < 0.05).
Conclusion
MetS can promote the growth of prostate during the formation of central obesity and IR. IGF-1 pathway may have an important role in the induction of BPH following IR. The application of metformin can suppress the expression of IGF-1 and IGF-1R, thus preventing the promotive effect of IR on prostate tissue in animal model of MetS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the development of aging society, benign prostatic hyperplasia (BPH) is extremely common and can cause significant harm [1]. Metabolic syndrome (MetS), closely related to obesity and high fat diet, refers to a cluster of cardiovascular risk factors including insulin resistance (IR)/hyperinsulinemia, obesity, primary hypertension, and other chronic diseases [2]. IR associating with the compensatory rise of insulin, which is known to have growth-promoting effects in plasma, is the core component of MetS. Results from multiple preclinical and clinical studies have found MetS and its comorbidities including sex steroid alterations and low-grade inflammation being related to the progression of BPH. Furthermore, with the proper treatment and recommended lifestyle changes, many individuals with MetS might be able to prevent or delay the onset of MetS-related complications including BPH [3]. Insulin sensitizer, which has been used to ameliorate the level of IR, has also been found to have therapeutic effects on BPH.
In this research, rat model of MetS and BPH has been established by high fat diet (HFD). Metformin was applied on the model, in order to find whether the blockage of IR had the efficacy against insulin on the induction of BPH. The expression level of insulin-like growth factor-1 (IGF-1) and receptor (IGF-1R) was also measured to find the potential mechanism.
Methods
Grouping design and model establishment
A total of 40 male specific pathogen-free Sprague–Dawley (SD) rats (4 weeks old, 70 g) purchased from animal experimental center of Anhui provincial hospital were randomly allocated to normal diet (ND) group (n = 10) and HFD group (n = 30). HFD group was randomly divided into subgroups of blank control (HFD only, n = 10), saline control (HFD + saline 10 ml gavage every day, n = 10), and metformin (HFD + metformin 20 mg/kg gavage every day, n = 10). Environmental conditions were set as room temperature of 22 ± 2 °C, humidity of 50 ± 10%, as well as day cycle with 12 h of light (06:00–18:00) and remaining dark. After 3 days of adaptive feeding, animals in HFD group were given fodder consisted of 10% lard oil, 7% white sugar, 10% whole milk powder, 6% eggs, and 2% potato starch being mixed with normal diet.
Specimen testing
After 40 days, all rats were weighed before being given 12 h fasting. Then, blood sample was collected at the tip of the tail for the measurement of fasting blood glucose (FBG). After intraperitoneal injection anesthesia using 10% chloral hydrate (3 ml/kg), abdominal cavity was opened with fasting blood insulin (FINS) being measured by cardiac blood sampling. After that, prostate tissue was carefully isolated and weighed before being fixed. Prostate index was calculated as “prostate weight (mg)/body weight (100 g).” Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as “FBG (mmol/L) × FINS (μIU/L)/22.5” [4] in order to evaluate the level of IR. All objects were killed via cervicales dislocation after trial.
Histological examination
Sections (4 μm) were prepared from the paraffin blocks and stained with hematoxylin and eosin to examine the cellular morphology. After being deparaffinized with xylene and followed by antigen retrieval via heating in the citrate buffer (10 mM), the prostatic sections were incubated with IGF-1 or IGF-1R polyclonal primary antibody for 24 h. Polyvalent biotinylated goat anti-rabbit secondary antibody and streptavidin peroxidase (STV-HRP) system was used to amplify the signals, followed by detection with diaminobenzidine (DAB) as a chromogen. Slides were counterstained with hematoxylin, dehydrated with graded alcohols, and cleared by xylene. Histological images were captured by charged coupled device (CCD) camera attached with a light microscope (MIAS 2000, Olympus, Tokyo, Japan) connected to digital photomicrograph software (Olysia Bioreport, Olympus, Tokyo, Japan).
Western blot analysis
Prostate tissues were homogenized by a tissue homogenizer in the protein lysis buffer. Protein extraction was performed using total extraction kit (Best Bio, Shanghai, China). Proteins from the samples were obtained at appropriate working concentrations by mixing with an equal volume of Laemmli buffer with mercaptoethanol and heated at 90 °C for 5 min, loaded into each lane, and electrophoresed in a 10% SDS–polyacrylamide gel in 1 × Tris/glycine/SDS buffer at 150 V for 1.5 h at room temperature. Separated proteins were electrophoretically transferred to a PVDF membrane using 1 × Tris/glycine buffer for 30 min at 100 V and 4 °C. The membrane was washed in Tris-buffered saline + Tween (TBS-t) and blocked in 5% milk for 1 h at room temperature.
Primary antibody for IGF-1 (Abcam, MA, USA) in a 1:200 dilution, and IGF-1R (Abcam, MA, USA) in a 1:200 dilution, incubated for overnight. Then, 1:5000 HRP-conjugated secondary antibody (Pierce Chemical, TX, US) incubated for 1 h at room temperature and membrane was developed with enhanced chemiluminescence (ECL) Western blotting detection reagents.
ImageJ (National Institutes of Health, MD, US) was used for quantitative analysis of the bands. To account for any differences in loading, target band densitometries were divided by actin densitometries obtained from the same lane. These corrected densitometries were normalized to controls in each experiment.
Instruments, medicaments, and reagents
Metformin hydrochloride extended-release tablets (GLUMETZA®, Bristol-Myers Squibb, NY, US) were purchased from pharmacy. 10% chloral hydrate solution was provided by animal experimental center of Anhui provincial hospital. Fasting blood glucose was measured by automatic tester (Accu-chek®, Roche). All the primary and secondary antibodies were procured from Abcam (MA, US). IGF-1 antibody (bs-4588R) and IGF-1R antibody (bs-4985R) were purchased from Bioss Biotechnology corporation (Beijing, China). General kit rabbit/mouse (PV-6000) and DAB staining agent (ZLI-9018) were purchased from ZSGB-BIO corporation (Beijing, China).
Statistical methods
The Kolmogorov–Smirnov goodness-of-fit test was used to determine whether the distribution of a variable was normal. One-way ANOVA and Student’s t test were applied between different groups/subunits of independent continuous variables. All analyses were carried out using the routines of SPSS version 19.0 (SPSS, Chicago, IL, USA); statistical significance was defined as the P value < 0.05.
Results
Metabolic and prostatic feature
Significant increase in body weight, prostate weight as well as prostate index was observed in all subunits of the HFD group as compared to the ND group (P < 0.05, Table 1), while metformin has abated the promotive effect of HFD on prostate weight and prostate index (P < 0.05, Table 1).
IR feature
No significant difference in serum FBG level was found between groups and subunits. FINS levels of blank and saline control were higher than ND group (P < 0.05, Table 1). However, FINS and HOMA-IR level of metformin subunit was much lower compared with that of saline control (P < 0.05, Table 1, Fig. 1).
Pathological changes in prostatic tissue
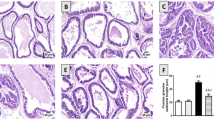
The level of prostatic hyperplasia is not remarkable with any epithelial hyperplasia and enlargement of lumen being found in ND group. Transformation of glandular epithelium into single layer of column or cube with unapparent enlargement of lumen, as well as pink dyeing of secretion, has been observed. Prominent hyperplasia has been found in subunits of blank and saline control, including significantly increased volume of prostate, epithelial hyperplasia and enlargement of lumen, as well as hyperactive secretion with formation of scarlet coagulum. However, slight hyperplasia has been found in subunit of metformin, with mild enlargement of lumen and pink secretion obviously lower than other two subunits (Fig. 2).
Differential expression of IGF pathway along with prostatic hyperplasia
IGF-1 and IGF-1R both expressed mainly in cytoplasm of glandular epithelial cell, while sporadic expression of IGF-1R has also been found in intercellular substance (Fig. 3). The protein expressions of IGF-1 and IGF-1R in the subunits of blank and saline control were increased significantly than those of the ND group (P < 0.05, Fig. 4), while significant decrease in the expressions of IGF-1 and IGF-1R in the subunits of metformin was observed as compared to the other two subunits (P < 0.05, Fig. 4).
IHC of prostatic tissue. 1: ND group; 2: blank control subunit; 3: saline control subunit; 4: metformin subunit. Immunohistochemical localization of IGF-1 and IGF-1R in prostatic epithelial cells was marked with red circle. IHC immunohistochemistry, ND normal diet, IGF-1 insulin-like growth factor 1, IGF-1R IGF-1 receptor
Discussion
MetS is a widespread epidemic disease including certain metabolic alteration with high medical recourse consumption, due to its association with increased morbidity and mortality. Many epidemiological evidences have underlined an emerging link between MetS and BPH [5,6,7,8]. Therefore, BPH is not only viewed as a mere hydraulic problem, to be solved by a surgical intervention, but also as a metabolic problem, to be solved with a multidisciplinary approach, which includes also the endocrinologist.
Definite evidences of a possible role of MetS and its individual components have been provided in the development of BPH, prostate growth, and worsening of lower urinary tract symptom (LUTS) [9]. Central obesity, lipidic disorder as well as hyperinsulinemia secondary to insulin resistance represent the dominant causes of MetS and related pathological conditions, while the critical pathological pathways between MetS and BPH are mainly attributable to hyperinsulinemia, increased sympathetic nervous system activity, and smooth muscle tone of the prostate [10, 11]. Central obesity is considered the core of the pathophysiology of MetS [12] and was suggested to induce prostatic enlargement either by promoting insulin resistance and secondary hyperinsulinemia or through an increment in the estrogen-to-androgen ratio [13]. Several studies suggested that insulin resistance with secondary hyperinsulinemia is associated with prostatic enlargement [14, 15], proving that hyperinsulinemia is a final and common path of several components of MetS on the pathogenesis of BPH. It has been hypothesized that MetS could influence BPH at intra-prostatic level via hyperinsulinemia-related chronic inflammation-driven prostate overgrowth, associating not only with increased prostate volume, but also with severe intra-prostatic inflammation [16]. In this research, body weight of HFD group was higher than ND group. Along with the increase in body weight, the level of prostatic hyperplasia has also risen, proving that MetS can induce the progression of prostatic hyperplasia at the meantime of the formation of central obesity.
Hyperinsulinemia is associated with increasing level of IGF-1, and the IGF pathway may also contribute to the association between insulin resistance and BPH. Insulin presents a structural similarity to IGF-1 and can bind its receptor, which may activate a complex pathway influencing prostate cell growth and proliferation. Alternatively, as insulin increases, IGF-1 binding protein-1 decreases, thus increasing IGF bioavailability. Also, the insulin receptor exhibits a high degree of homology with the IGF receptor, and the related ligands are well known to cross-activate their receptors. Several studies reported that the increase in the level of IGF-1 predisposes patients to a higher risk of BPH [17, 18]. Luo et al. [19] found IGF-I and -II, and a restricted set of growth factors and their binding proteins to be upregulated in BPH. Many analyses have localized IGF family including IGF-I, IGF-IR, IGF-II, and IGFBPs on prostate epithelial cells [20, 21]. In addition, the prostate stroma has been shown to be a major source of IGFs, which can function via paracrine action to promote prostate epithelial cell proliferation [22]. Oppositely, IGF-I deficiency has been found to be the proximate cause of impaired prostate development in IGF-I null mice and wild-type littermates [23]. This suggests that insulin may cause prostatic growth during type 2 diabetes by activating the IGF pathway, which again emphasizes the need for a comprehensive analysis of BPH tissues to define the true mechanistic action. In this research, along with the increase of HOMA-IR, both IGF1 and IGF1R were found to have higher expression level in the prostate of hyperplasia than normal, thus enhancing the possible role of IGF pathway in the linkage of IR and BPH.
In the meantime, both insulin resistance and pathological change of prostate were found to have arisen before the occurrence of hyperglycemia, suggesting that MetS might induce the hyperplasia of prostate before diabetes mellitus being diagnosed.
Metformin is an oral anti-hyperglycemic agent of biguanide class and insulin sensitizer, which inhibits hepatic gluconeogenesis and enhances peripheral glucose uptake [24]. It also possesses anticancer activity against several tumors through induction of apoptotic signaling and cell cycle arrest [25]. Several studies have found a beneficial effect of metformin in reducing prostate cancer incidence and improving overall survival [26,27,28,29,30,31]. Zhang et al. [32] have found that metformin can reduce the secretion of IGF-1 in endometrial carcinoma cell lines and their expression of IGF-1R to inhibit endometrial carcinoma cell growth.
The similar effects of metformin have also been found in BPH. Wang et al. [33] has found that metformin inhibits the proliferation of benign prostatic epithelial cells by suppressing the expression of IGF-1R and IGF-1 secretion in stromal cells. Mosli et al. [34] have found the attenuation effect of metformin on the SD rat model of testosterone-induced BPH, with significant protection against the elevation of mRNA expression of IGF-1, IGF-1R being revealed.
In this research, the application of metformin has suppressed FINS and HOMA-IR level in HFD feeding rats, as well as reduced the hyperplasia level of prostate. In the meantime, metformin has also reduced the expression of IGF-1 and IGF-1R, suggesting that metformin may meliorate the level of prostatic hyperplasia via the influence of IGF pathway in patients with MetS or IR. However, the period of model establishment is only 40 days in this research. A more chronic model with high similarity to human should be established in the future to find the proper method of metformin application.
Conclusion
This research has following conclusions: (1) MetS can promote the growth of prostate during the formation of central obesity and IR; (2) IGF-1 pathway may have an important role in the induction of BPH following IR; (3) BPH may happen earlier than the change of glycaemia in patients with MetS;(4) The application of metformin can suppress the expression of IGF-1 and IGF-1R, thus preventing the effect of IR on prostate in rats model of MetS.
References
Parsons JK (2010) Benign prostatic hyperplasia and male lower urinary tract symptoms: epidemiology and risk factors. Curr Bladder Dysfunct Rep 5(4):212–218
Kasturi S, Russell S, McVary KT (2006) Metabolic syndrome and lower urinary tract symptoms secondary to benign prostatic hyperplasia. Curr Urol Rep 7(4):288–292
Vignozzi L, Gacci M, Maggi M (2016) Lower urinary tract symptoms, benign prostatic hyperplasia and metabolic syndrome. Nat Rev Urol 13(2):108–119
Antunes LC, Elkfury JL, Jornada MN, Foletto KC, Bertoluci MC (2016) Validation of HOMA-IR in a model of insulin-resistance induced by a high-fat diet in Wistar rats. Arch Endocrinol Metab 60(2):138–142
Gacci M, Corona G, Vignozzi L, Salvi M, Serni S, De Nunzio C, Tubaro A, Oelke M, Carini M, Maggi M (2015) Metabolic syndrome and benign prostatic enlargement: a systematic review and meta- analysis. BJU Int 115(1):24–31
Gacci M, Sebastianelli A, Salvi M, De Nunzio C, Tubaro A, Vignozzi L, Corona G, McVary KT, Kaplan SA, Maggi M, Carini M, Serni S (2015) Central obesity is predictive of persistent storage LUTS after surgery for benign prostatic enlargement: results of a multicenter prospective study. BJU Int 116(2):271–277
Lee RK, Chung D, Chughtai B, Te AE, Kaplan SA (2012) Central obesity as measured by waist circumference is predictive of severity of lower urinary tract symptoms. BJU Int 110(4):540–545
Russo GI, Castelli T, Privitera S, Fragalà E, Favilla V, Reale G, Urzì D, La Vignera S, Condorelli RA, Calogero AE, Cimino S, Morgia G (2015) Increase of Framingham cardiovascular disease risk score is associated with severity of lower urinary tract symptoms. BJU Int 116(5):791–796
De Nunzio C, Aronson W, Freedland SJ, Giovannucci E, Parsons JK (2012) The correlation between metabolic syndrome and prostatic diseases. Eur Urol 61(3):560–570
Minutoli L, Altavilla D, Marini H, Rinaldi M, Irrera N, Pizzino G, Bitto A, Arena S, Cimino S, Squadrito F, Russo GI, Morgia G (2014) Inhibitors of apoptosis proteins in experimental benign prostatic hyperplasia: effects of serenoa repens, selenium and lycopene. J Biomed Sci 21:19
Kassi E, Pervanidou P, Kaltsas G, Chrousos G (2011) Metabolic syndrome: definitions and controversies. BMC Med 9:48
Zou C, Gong D, Fang N, Fan Y (2016) Meta-analysis of metabolic syndrome and benign prostatic hyperplasia in Chinese patients. World J Urol 34(2):281–289
De Nunzio C, Kramer G, Marberger M, Montironi R, Nelson W, Schröder F, Sciarra A, Tubaro A (2011) The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. Eur Urol 60(1):106–117
Wang Z, Olumi AF (2011) Diabetes, growth hormone-insulin-like growth factor pathways and association to benign prostatic hyperplasia. Differentiation 82(4–5):261–271
Gacci M, Vignozzi L, Sebastianelli A, Salvi M, Giannessi C, De Nunzio C, Tubaro A, Corona G, Rastrelli G, Santi R, Nesi G, Serni S, Carini M, Maggi M (2013) Metabolic syndrome and lower urinary tract symptoms: the role of inflammation. Prostate Cancer Prostatic Dis 16(1):101–116
Neuhouser ML, Schenk J, Song YJ, Tangen CM, Goodman PJ, Pollak M, Penson DF, Thompson IM, Kristal AR (2008) Insulin-like growth factor-I, insulin-like growth factor binding protein-3 and risk of benign prostate hyperplasia in the prostate cancer prevention trial. Prostate 68(13):1477–1486
Rohrmann S, Giovannucci E, Smit E, Platz EA (2007) Association of IGF-1 and IGFBP-3 with lower urinary tract symptoms in the third national health and nutrition examination survey. Prostate 67(15):1693–1698
Luo J, Dunn T, Ewing C, Sauvageot J, Chen Y, Trent J, Isaacs W (2002) Gene expression signature of benign prostatic hyperplasia revealed by cDNA microarray analysis. Prostate 51(3):189–200
Fiorelli G, De Bellis A, Longo A, Giannini S, Natali A, Costantini A, Vannelli GB, Serio M (1991) Insulin-like growth factor-I receptors in human hyperplastic prostate tissue: characterization, tissue localization, and their modulation by chronic treatment with a gonadotropin-releasing hormone analog. J Clin Endocrinol Metab 72(4):740–746
Monti S, Di Silverio F, Iraci R, Martini C, Lanzara S, Falasca P, Poggi M, Stigliano A, Sciarra F, Toscano V (2001) Regional ariations of insulin-like growth factor I (IGF-I), IGF-II, and receptor type I in benign prostatic hyperplasia tissue and their correlation with intraprostatic androgens. J Clin Endocrinol Metab 86(4):1700–1706
Li W, Wu CL, Febbo PG, Olumi AF (2007) Stromally expressed c-Jun regulates proliferation of prostate epithelial cells. Am J Pathol 171(4):1189–1198
Ruan W, Powell-Braxton L, Kopchick JJ, Kleinberg DL (1999) Evidence that insulin-like growth factor I and growth hormone are required for prostate gland development. Endocrinology 140(5):1984–1989
Kaplan SA, Lee JY, O’Neill EA, Meehan AG, Kusek JW (2013) Prevalence of low testosterone and its relationship to body mass index in older men with lower urinary tract symptoms associated with benign prostatic hyperplasia. Aging Male 16(4):169–172
Kahn BB, Alquier T, Carling D, Hardie DG (2005) AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1:15–25
Colquhoun AJ et al (2012) Metformin enhances the antiproliferative and apoptotic effect of bicalutamide in prostate cancer. Prostate Cancer Prostatic Dis 15:346–352
Ruiter R et al (2012) Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care 35(1):119–124
Murtola TJ et al (2008) Antidiabetic medication and prostate cancer risk: a population-based case-control study. Am J Epidemiol 168(8):925–931
He XX, Tu SM, Lee MH et al (2011) Thiazolidinediones and metformin associated with improved survival of diabetic prostate cancer patients. Ann Oncol 22(12):2640–2645
Margel D et al (2013) Metformin use and all-cause and prostate cancer- specific mortality among men with diabetes. J Clin Oncol 31(25):3069–3075
Wright JL, Stanford JL (2009) Metformin use and prostate cancer in Caucasian men: results from a population-based case-control study. Cancer Causes Control 20(9):1617–1622
Kusturica J, Kulo Ćesić A, Gušić E, Maleškić S, Rakanović-Todić M, Šečić D (2017) Metformin use associated with lower risk of cancer in patients with diabetes mellitus type 2. Med Glas (Zenica) 14(2):176–181
Zhang Y, Li MX, Wang H, Zeng Z, Li XM (2015) Metformin down-regulates endometrial carcinoma cell secretion of IGF-1 and expression of IGF-1R. Asian Pac J Cancer Prev 16(1):221–225
Wang Z, Xiao X, Ge R, Li J, Johnson CW, Rassoulian C, Olumi AF (2017) Metformin inhibits the proliferation of benign prostatic epithelial cells. PLoS ONE 12(3):e0173335
Mosli HH, Esmat A, Atawia RT, Shoieb SM, Mosli HA, Abdel-Naim AB (2015) Metformin attenuates testosterone-induced prostatic hyperplasia in rats: a pharmacological perspective. Sci Rep 23(5):15639
Acknowledgements
Relevant personnel from the department of pathology, laboratory animal center, and urology of Anhui provincial hospital have provided a great deal of help.
Funding
This study was funded by Natural Science Foundation of Anhui Province (1408085QH152).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethics committee
The use of animal in this research was approved by the ethics committee of experimental animal of Anhui medical university.
Rights and permissions
About this article
Cite this article
Xu, C., Xu, Y., Shen, Z. et al. Effects of metformin on prostatic tissue of rats with metabolic syndrome and benign prostatic hyperplasia. Int Urol Nephrol 50, 611–617 (2018). https://doi.org/10.1007/s11255-018-1826-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-018-1826-9