Abstract
Background
The aim of this study was to assess the effects of smoking on albuminuria risk in adults with type 2 diabetes mellitus (T2DM).
Methods
A literature search was conducted using MEDLINE, EMBASE, and China National Knowledge Infrastructure from the established date to October 2017. Summary relative risks (SRR) and 95% confidence intervals (CI) were computed utilizing a random effect inverse variance method.
Results
This meta-analysis included a total of 19 relevant observational studies (four prospective cohort, seven case–control, and eight cross-sectional studies), reporting 105,031 participants and 23,366 albuminuria events. Compared with never-smokers with T2DM, the SRRs of albuminuria were 1.43 (95% CIs 1.27–1.61) for ever-smokers, 2.61 (95% CIs 1.86–3.64) for current smokers, and 1.86 (95% CIs 1.37–2.52) for former smokers. Considerable heterogeneity was observed among these studies, and study design was a significant modifier for this association. There were significantly elevated risk associations for microalbuminuria (SRRs = 1.24, 95% CIs 1.05–1.46) and for macroalbuminuria (SRRs = 1.65, 95% CIs 1.03–2.66), respectively.
Conclusions
Our systematic review and meta-analysis indicates that cigarette smoking might be a potential factor for the development of albuminuria in adults with T2DM. Future studies are required to investigate the association between smoking cessation and intensity and incident albuminuria in adults with T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since Keen et al. [1] first described microalbuminuria (MA) in patients with diabetes mellitus (DM), prospective studies [2, 3] have demonstrated that MA is an independent risk factor for developing diabetic nephropathy (DN) in type 2 diabetes mellitus (T2DM). It is also reported that MA is an important predictor for cardiovascular disease (CVD) morbidity or mortality in T2DM [4, 5]. Despite the knowledge gained in relation to early identification and intervention in T2DM patients, DN is still the main cause of end-stage renal disease (ESRD), leading to renal replacement therapy [6]. In 2011, about 50,000 Americans began treatment for kidney failure due to diabetes [7].
The acknowledged risk factors for the development of albuminuria in T2DM included older age, male sex, genetic susceptibility, poor glycemic control, long duration of diabetes, and an unfavorable lipid profile [8, 9]. The prevalence of smoking among patients with T2DM is high. Researchers have identified the deleterious effects of cigarette smoking on glycemic control and blood pressure in T2DM [10, 11]. In addition, cigarette smoking can trigger pathophysiological pathways mediating albuminuria, including activation of oxidative, proinflammation, and greater production of advanced glycation end products (AGEPs). The evidence regarding the relationships between cigarette smoking and albuminuria in patients with T2DM has been published with inconsistent results [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. When we prepared this paper, two analogous meta-analyses have recently been published about smoking as a risk factor for DN [31, 32]. One [32] was specifically for type 1 and type 2 diabetes, and the other [31] for type 1 and type 2 diabetes combined. In the present manuscript, we focused on the outcome as the risk of albuminuria in T2DM. Recent studies provide evidences that albuminuria was absent in more than 30% of DN in T2DM [33, 34]. We also systematically reviewed a dose–response relationship. In addition, we included five more studies [26,27,28,29,30], which were not included in Jiang’s paper [32]. This meta-analysis followed the guideline on meta-analysis of observational studies in epidemiology (MOOSE) [35].
Methods
Literature search
Two of us (XHL and LJ) conducted an electronic search for the relevant articles published in the following databases: EMBASE (http://www.embase.com/) and MEDLINE (http://www.ncbi.nlm.nih.gov/pubmed/) from the established date to October 2017. Chinese articles were screened through Database of Chinese Scientific and Technical Periodicals,China National Knowledge Infrastructure (CNKI), and China biology medical literature databases, which were searched from 1979, 1989, 1970, respectively, through October 2017. The search terms were as the following key words: (1) smoking OR nicotine OR cigarette OR tobacco; (2) proteinuria OR albuminuria OR macroalbuminuria OR microalbuminuria; (3) T2DM OR diabetes OR NIDDM. Manual searches of bibliographies of all relevant studies and review articles were performed. Our searches were limited to human studies and publish in English and Chinese.
Outcome measures
Microalbuminuria is generally defined as a urine albumin-to-creatinine ratio (UACR) of 2.5–25 g/mmol (30–300 mg/g) or a urinary albumin excretion rate (UAER) of 20–200 μg/min (30–299 mg/day). Macroalbuminuria is defined as a UACR of > 25 g/mmol (> 300 mg/g) or a UAER of > 200 μg/min (> 300 mg/day) [36].
Study selection and data extraction
Studies were included according to the following criteria: (1) used a cohort, case–control or cross-sectional design; (2) evaluated the association between smoking and risk of proteinuria in patients with T2DM; and (3) reported quantitative estimates of the multivariate-adjusted (at least for age and hypertension) relative risk (RR) and their confidence intervals (CI), or provided necessary data to calculate them. If more than two studies came from the same population, the most informative report was included. Studies that used slightly varying definitions were included if they were otherwise comparable.
Studies were excluded if they were animal experiments, chemistry, cell-line studies, editorial, commentaries, review articles, or case reports. We also excluded data on other forms of tobacco use (e.g., cigar and pipe). We did not consider the gray literature.
All data from eligible studies were abstracted independently by two investigators (XHL and LJ), and disagreement was resolved by discussion between the investigators and by referencing the original report. When studies provided several risk estimates that reflected different degree of control for potential confounders, we selected the one with the greatest degree of control for potential confounders.
Statistical analysis
Data analysis used multivariate-adjusted outcome data (expressed as RRs and 95% CIs), which were converted by using their natural logarithms. The study-specific log RRs and their 95% CIs were pooled based on a random effects model, which accounts for heterogeneity among studies [37]. Because most of the included articles did not present results specifically on smoking status (i.e., current or former smoking), we used ever-smoking as the exposure. Some articles [16, 19, 23] reported results on both former and current cigarette smoking use. We computed results on ever use by pooling the results for former and current users based on a fixed-effects model. We also used a fixed-effects model to obtain overall risk estimates for albuminuria when studies reported results separately for different smoking dose [16, 22, 25], different genotype [13], and smoking before or after DM diagnosis [12].
Homogeneity of effects across studies was assessed using the χ2 and quantified by I2 statistics, which represents the percentage of total variation across studies that is attributable to heterogeneity rather than chance. Results were defined as heterogeneous for P values < 0.10 or I2 was > 50% [38]. To explore the origin of heterogeneity, we performed subgroup and random effects meta-regression analysis. To examine the robustness of our results, a further sensitivity analysis were performed by excluding each study in turn and obtaining the pooled estimates from the remaining studies.
Publication bias was assessed by using funnel plots and the further Begg’s adjusted rank correlation and Egger’ regression asymmetry tests [39, 40]. P < 0.10 for Egger’s or Begg’s tests was considered to be representative of a significantly statistical publication bias. We also performed the Duval and Tweedie nonparametric “trim-and-fill” procedure to further assess the possible effect of publication bias. All statistical analyses were performed using STATA, version 11.0 (STATA, College Station, TX, USA). All reported probability values were two sided with significance set < 0.05.
Results
Search results and study characteristics
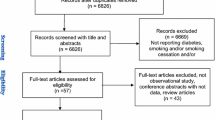
The literature review identified 2473 articles, of which 64 had potential value and were available as full-text articles (Fig. 1). Additional three articles were included from the reference reviews. Among these 61 articles for detailed assessment, a total of 48 articles were excluded: 31 did not evaluate this association, three reported the same population, two reported other forms of tobacco use, eight reported outcome as ESRD or estimated glomerular filtration rate (eGFR) or renal function decline, and four did not adjust for blood pressure or hypertension. Our final analysis included 19 observational studies: four prospective cohort, seven case–control, and eight cross-sectional studies. There was a 100% concordance between reviewers with respect to final inclusion and exclusion of studies based on the predefined inclusion and exclusion criteria.
The study characteristics are given in Table 1. The studies were conducted were: from 1995 to 2016. There were a total 105,031 participants (from 212 [18] to 54,670 [26]) and 23,366 albuminuria events in the current meta-analysis. The majority of studies reported smoking status as ever-smokers. Five studies [16, 19, 22, 23, 25] reported specifically for former and current smokers, among which three studies [16, 22, 25] reported for current smokers as cumulative doses of pack-year. Six of the 14 studies used a UACR for albuminuria measurement [12, 14, 16, 17, 21, 22], whereas other eight studies used a UAER [13, 15, 18,19,20, 23,24,25].
Meta-analysis
Ever-smoking was associated with the risk of albuminuria, with a SRR of 1.43 (95% CI 1.27–1.61). Tests for homogeneity of the SRR across the 19 studies gave a χ2 value of 50.44 (p < 0.001, I2 = 72.6%; Fig. 2a); that is, the homogeneity assumption was rejected. Summarizing the three studies [16, 19, 23] that presented results specifically on former/current smoking led to the SRRs of 1.86 (95% CIs 1.37–2.52; Pheterogeneity = 0.661, I2 = 0) for former smoking and of 2.61 (95% CIs 1.86–3.64; Pheterogeneity = 0.316, I2 = 13.2%) for current smoking (Fig. 2b).
Subgroup, sensitivity, and meta-regression analyses
Table 2 shows the results of subgroup analyses for the association between ever-smoking and albuminuria risk in T2DM. Stratified analyses by study locations led to statistically significant SRRs (95% CIs) of 1.24 (1.04–1.47) for studies from the USA, 1.73 (1.17–2.56) for studies from Europe, and of 1.47 (1.22–1.77) for studies from Asia. The SRRs (95% CIs) were significantly higher for cross-sectional/case–control studies (SRR = 1.53; 95% CI 1.32–1.77 than those for prospective cohort studies (SRR = 1.16; 95% CI 1.02–1.33; P for difference = 0.06). Eight studies [12, 16, 20, 22, 26, 28,29,30] represented results for MA, with the SRRs (95% CIs) of 1.24 (1.05–1.46). There were five studies [12, 16, 26, 28, 29] representing the risk associations for macroalbuminuria, with the SRR of 1.65 (1.03–2.66). Restricting studies with adjustments for diabetic retinopathy (DR), dyslipidemia, DM duration, and BMI resulted in significant associations between ever-smoking and incident albuminuria.
In sensitivity analyses, we recalculated the overall homogeneity and effect size by excluding one study at a time. The SRRs ranged from a low of 1.47 (95% CI 1.31–1.63) to a high of 1.60 (95% CI 1.38–1.82) when the study by Pijls et al. [23] and Parving et al. [20] were omitted, respectively (Supplementary Figure. 1). Meta-regression analysis showed that only study design was a significant variable for the association of ever-smoking–albuminuria, which might account for 26.3% of the heterogeneity.
Dose–response relationship
We further examined the dose–response relationship of smoking and risk of albuminuria in patients with T2DM, which was shown in three studies [16, 22, 25]. In a case–control study of Taiwanese men with T2DM, Hsu et al. [16] demonstrated that compared with non-smokers, those who had smoked 15–30 or more than 30 pack-years were, respectively, 2.78 (95% CI 1.34–5.76) and 3.20 (95% CI 1.74–5.86) times more likely to develop proteinuria. Another case–control study in African-Americans with T2DM [22] showed that each increase of 10 pack-years of smoking corresponded to a 14% (95% CI 3–26%) increase in microalbuminuria risk. Similarly, the study [25] of 5431 of older-onset diabetic individuals revealed an elevated risk of albuminuria as the cumulative amount of smoking increased. Together, these evidences indicated a dose–response relationship, with microalbuminuria risk increasing as pack-years increased.
Publication bias
Egger’s (P = 0.305) tests did not reveal evidence of publication bias, but visual inspection of the funnel plots and further Begg’s (P = 0.059) tests revealed significant asymmetry. The trim-and-fill method suggested that nine additional risk estimates were needed to balance the funnel plot, and the summary risk estimates became weaker, but still statistically significant (SRR = 1.17; 95% CI 1.03–1.32; Fig. 3).
Discussion
Based on the data extracted from 19 observational studies, we found that smoking status (ever, former and current smoking) was associated with the increased risk of albuminuria in patients with T2DM. The increased risk associations were consistent across diverse study locations (i.e., Asia, Europe and the USA) and design (i.e., prospective cohort and case–control/cross-sectional studies). Furthermore, there were elevated risk associations for both microalbuminuria and macroalbuminuria in ever-smokers with T2DM.
From a pathophysiological perspective, the development of albuminuria in patients with T2DM involves the interplay of endothelial dysfunction (diminished nitric oxide availability and intimal cell hyperplasia), oxidative stress, AGEPs, and the abnormal production of cytokines and growth factors [41]. It is reported that cigarette smoking can elevate the levels of carboxyhemoglobin, platelet activation, and prothrombotic factors [42], resulting in oxidative stress, inflammation, and endothelial cell dysfunction in the kidney [43,44,45]. As a result, cigarette smoking may increase susceptibility to renal complications in type 2 diabetic patients [46]. Furthermore, tobacco smoke induces albuminuria and abnormalities in renal function through AGEPs, which are responsible for enhanced vascular permeability [47, 48].
Although no quantitative review was available, our systematic review based on three studies indicated a dose–response relationship, with albuminuria risk increasing as pack-years increased. Furthermore, our meta-analysis found a stronger risk of albuminuria in current smokers than in former smokers (RR: 2.61 vs. 1.86, P for difference < 0.001), suggesting that cessation of smoking may significantly reduce the risk of incident albuminuria in patients with T2DM. Some prospective studies have reported that smoking cessation slowed the progression of diabetic nephropathy [49, 50]. Results from Chuahirun et al. [49] showed that cigarette smoking exacerbated renal injury in type 2 diabetes when adjustments for control of blood pressure and/or angiotension converting enzyme (ACE) inhibitors use, but its cessation in those with microalbuminuria ameliorates the progressive renal injury caused by continued smoking. Similarly, another report indicated that continued cigarette smoking exacerbates and its cessation ameliorates progression of the early nephropathy of T2DM from microalbuminuria to macroalbuminuria [50]. Our meta-analysis also showed that an elevated risk of albuminuria in former smokers would persist for many years. The mechanisms underlying the persistence of smoking-associated albuminuria or renal damage after smoking cessation remain unclear, but it may be related to smoking induced changes in the epigenetics of blood platelets, which can persist for more than 10 years after smoking cessation [51, 52].
Strengths of the study included as follows: (1) Studies were included after a comprehensive, systematic search of the literature and by using a broad search strategy to capture all relevant information. (2) This meta-analysis included a large sample, which is a potentially powerful approach to assess the effects of smoking on albuminuria risk in patients with T2DM. (3) All of the studies included in the meta-analysis evaluated multiple confounders including hypertension, DM duration, history of DR, BMI, and dyslipidemia. (4) We performed subgroup analysis and meta-regression to explore the source of heterogeneity. We found that study design might be the source of heterogeneity. The sensitivity analysis also indicated that the results were stable and reliable.
However, our study has some limitations, which should be taken into account. First, our meta-analysis, based on observational studies, cannot prove causality. Fifteen of 19 studies were according to a case–control or cross-sectional design. When restricting to prospective cohort studies, a significant, albeit weaker, association was found between cigarette smoking and the development of albuminuria in patients with T2DM.
Second, there is statistical heterogeneity across studies. The difference in the definition of albuminuria and smoking status may be the main sources of this heterogeneity. For example, some studies [13, 15, 18,19,20, 23,24,25] take UAER 20–200 μg/min as microalbuminuria, UAER > 200 μg/min as overt nephropathy, while other studies [12, 14, 16, 17, 21, 22] define albuminuria using UACR > 30 mg g−1 in a spot urine specimen. However, the high heterogeneity remained when we performed subgroup analysis according to the methods of albuminuria assessment. In addition, most studies collected the smoking history through self-reports. Nevertheless, the reliability of self-report information on smoking behavior has been validated in the literatures [53].
Furthermore, study design may also be a source of heterogeneity. A total of 11 of 14 studies used a cross-sectional or case–control design, a design that does not allow for causal inference and can overestimate relative risks given its reliance on prevalence ratios. When restricted to three prospective studies, a significant, albeit weaker, relationship was found between smoking status and the risk of albuminuria. There was much less heterogeneity in the prospective cohort studies (P heterogeneity = 0.766, I2 = 0) than case–control/cross-sectional studies (Pheterogeneity < 0.001, I2 = 79.4%). When performing meta-regression analyses, we found that study design has modified effects on this association between smoking status and the risk of albuminuria, which might partially (26.3%) account for the high heterogeneity among studies.
Third, residual confounding likely exists as full information on various confounders has not been given in all studies retrieved. As an example, data on smoking intensity, alcohol use, and second-hand smoke which are important potential confounders were not available in most of the studies retrieved. However, most of the known confounders (e.g., history of hypertension, DM duration, DR, dyslipidemia, and BMI) were considered in the studies, and whether or not adjustments for these variables did not modify the risk association. Residual confounding and the contribution of other unexamined factors were not negated. However, given the strength of the associations observed, it is unlikely that residual confounding would negate our results.
Forth, while the number of albuminuria events is large, the number of MA and macroalbuminuria is relatively small. Thus, the statistically significant results for albuminuria types should be interpreted with caution. When we carried out the dose–response analysis, there were only three studies. So, we cannot derive a dose–response association between smoking intensity and albuminuria risk. Data on the use of antihypertensive medications were incomplete, such as ACE inhibitors use, because ACE inhibitors are known to reverse the nephrotoxic effects of smoking [54].
Finally, despite the extensive search we made in three databases, we could not completely deny the potential publication bias. In fact, Begg’s test (P = 0.059) provided evidence for such bias. Therefore, we used the trim-and-fill method to solve the question and also found that nine additional risk estimates were needed to balance the funnel plot. However, the statistically significant association, albeit weaker (SRR = 1.17), remained.
In conclusion, results from our meta-analysis of observational studies demonstrate an adverse impact of smoking on the development of albuminuria in patients with T2DM. Further studies are warranted to investigate whether smoking cessation can decrease incident albuminuria in the T2DM population.
References
Keen H, Chlouverakis C, Fuller J et al (1969) The consomitants of raised blood sugar: studies in newly-detected hyperglycaemics. II. Urinary albumin excretion, blood pressure and their relation to blood sugar levels. Guys Hosp Rep 118(2):247–254
Viberti GC, Hill RD, Jarrett RJ et al (1982) Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1(8287):1430–1432
Mogensen CE (1984) Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med 310(6):356–360
Bakris GL, Molitch M (2014) Microalbuminuria as a risk predictor in diabetes: the continuing saga. Diabetes Care 37(3):867–875
Tebbe U, Bramlage P, Thoenes M et al (2009) Prevalence of microalbuminuria and its associated cardiovascular risk: German and Swiss results of the recent global i-SEARCH survey. Swiss Med Wkly 139(33–34):473–480
Ahn JH, Yu JH, Ko SH et al (2014) Prevalence and determinants of diabetic nephropathy in Korea: Korea national health and nutrition examination survey. Diabetes Metab J 38(2):109–119
Thompson JL, Allen P, Cunningham-Sabo L et al (2002) Environmental, policy, and cultural factors related to physical activity in sedentary American Indian women. Women Health 36(2):59–74
Radcliffe NJ, Seah JM, Clarke M et al (2017) Clinical predictive factors in diabetic kidney disease progression. J Diabetes Investig 8(1):6–18
Xue R, Gui D, Zheng L et al (2017) Mechanistic insight and management of diabetic nephropathy: recent progress and future perspective. J Diabetes Res 2017:1839809
Linneberg A, Jacobsen RK, Skaaby T et al (2015) Effect of smoking on blood pressure and resting heart rate: a mendelian randomization meta-analysis in the CARTA consortium. Circ Cardiovasc Genet 8(6):832–841
Li WH, Wang MP, Lam TH et al (2017) Brief intervention to promote smoking cessation and improve glycemic control in smokers with type 2 diabetes: a randomized controlled trial. Sci Rep 7:45902
Yeom H, Lee JH, Kim HC et al (2016) The association between smoking tobacco after a diagnosis of diabetes and the prevalence of diabetic nephropathy in the Korean male population. J Prev Med Public Health 49(2):108–117
Zhang W, Yang Z, Li X et al (2015) The functional Q84R polymorphism of TRIB3 gene is associated with diabetic nephropathy in Chinese type 2 diabetic patients. Gene 555(2):357–361
Furukawa S, Yamamoto S, Todo Y et al (2014) Association between subclinical hypothyroidism and diabetic nephropathy in patients with type 2 diabetes mellitus. Endocr J 61(10):1011–1018
Wolf G, Busch M, Muller N et al (2011) Association between socioeconomic status and renal function in a population of German patients with diabetic nephropathy treated at a tertiary centre. Nephrol Dial Transplant 26(12):4017–4023
Hsu CC, Hwang SJ, Tai TY et al (2010) Cigarette smoking and proteinuria in Taiwanese men with Type 2 diabetes mellitus. Diabet Med 27(3):295–302
Parving HH, Lewis JB, Ravid M et al (2006) Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int 69(11):2057–2063
Hou XH, Wang JH, Feng P et al (2005) A case control study on the risk factors of proteinuria in patients with type 2 diabetes. Zhonghua Liu Xing Bing Xue Za Zhi 26(1):39–43
Herrera-Pombo JL, Aguilar-Diosdado M, Hawkins F et al (2005) Is increasing urinary albumin a better marker for microvascular than for macrovascular complication of type 2 diabetes mellitus? Nephron Clin Pract 101(3):c116–c121
Cederholm J, Eliasson B, Nilsson PM et al (2005) Microalbuminuria and risk factors in type 1 and type 2 diabetic patients. Diabetes Res Clin Pract 67(3):258–266
Tam TK, Cheng LP, Lau DM et al (2004) The prevalence of microalbuminuria among patients with type II diabetes mellitus in a primary care setting: cross-sectional study. Hong Kong Med J 10(5):307–311
Kohler KA, McClellan WM, Ziemer DC et al (2002) Smoking and microalbuminuria: a case-control study in African-Americans with type 2 diabetes. Diabetes Care 25(1):243–245
Pijls LT, de Vries H, Kriegsman DM et al (2001) Determinants of albuminuria in people with Type 2 diabetes mellitus. Diabetes Res Clin Pract 52(2):133–143
Yokoyama H, Okudaira M, Otani T et al (1998) High incidence of diabetic nephropathy in early-onset Japanese NIDDM patients. Risk analysis. Diabetes Care. 21(7):1080–1085
Klein R, Klein BE, Moss SE et al (1995) Ten-year incidence of gross proteinuria in people with diabetes. Diabetes 44(8):916–923
Al-Rubeaan K, Youssef AM, Subhani SN et al (2014) Diabetic nephropathy and its risk factors in a society with a type 2 diabetes epidemic: a Saudi National Diabetes Registry-based study. PLoS ONE 9(2):e88956. https://doi.org/10.1371/journal.pone.0088956
Liu L, Zheng T, Wang F et al (2010) Pro12Ala polymorphism in the PPARG gene contributes to the development of diabetic nephropathy in Chinese type 2 diabetic patients. Diabetes Care 33(1):144–149. https://doi.org/10.2337/dc09-1258
Aekplakorn W, Srivanichakorn S, Sangwatanaroj S (2009) Microalbuminuria and metabolic risk factors in patients with type 2 diabetes in primary care setting in Thailand. Diabetes Res Clin Pract 84(1):92–98. https://doi.org/10.1016/j.diabres.2008.12.020
Unnikrishnan RI, Rema M, Pradeepa R et al (2007) Prevalence and risk factors of diabetic nephropathy in an urban South Indian population: the Chennai Urban Rural Epidemiology Study (CURES 45). Diabetes Care 30(8):2019–2024. https://doi.org/10.2337/dc06-2554
Amini M, Safaei H, Aminorroaya A (2007) The incidence of microalbuminuria and its associated risk factors in type 2 diabetic patients in Isfahan, Iran. Rev Diabet Stud 4(4):242–248. https://doi.org/10.1900/RDS.2007.4.242
Su S, Wang W, Sun T et al (2017) Smoking as a risk factor for diabetic nephropathy: a meta-analysis. Int Urol Nephrol 49(10):1801–1807. https://doi.org/10.1007/s11255-017-1638-3
Jiang N, Huang F, Zhang X (2017) Smoking and the risk of diabetic nephropathy in patients with type 1 and type 2 diabetes: a meta-analysis of observational studies. Oncotarget 8(54):93209–93218. https://doi.org/10.18632/oncotarget.21478
Kramer HJ, Nguyen QD, Curhan G et al (2003) Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 289(24):3273–3277. https://doi.org/10.1001/jama.289.24.3273
Yokoyama H, Sone H, Oishi M et al (2009) Prevalence of albuminuria and renal insufficiency and associated clinical factors in type 2 diabetes: the Japan Diabetes Clinical Data Management study (JDDM15). Nephrol Dial Transplant 24(4):1212–1219. https://doi.org/10.1093/ndt/gfn603
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283(15):2008–2012
Ovbiagele B (2008) Microalbuminuria: risk factor and potential therapeutic target for stroke? J Neurol Sci 271(1–2):21–28
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Higgins JP, Thompson SG, Deeks JJ et al (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Egger M, Davey Smith G, Schneider M et al (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Chen Y, Zhi Y, Li C et al (2016) HDL cholesterol and risk of diabetic nephropathy in patient with type 1 diabetes: a meta-analysis of cohort studies. Diabetes Res Clin Pract 122:84–91
Barua RS, Ambrose JA (2013) Mechanisms of coronary thrombosis in cigarette smoke exposure. Arterioscler Thromb Vasc Biol 33(7):1460–1467
Caimi G, Hopps E, Montana M et al (2014) Nitric oxide metabolites (nitrite and nitrate) in several clinical condition. Clin Hemorheol Microcirc. 56(4):359–369
Salvatore SP, Troxell ML, Hecox D et al (2015) Smoking-related glomerulopathy: expanding the morphologic spectrum. Am J Nephrol 41(1):66–72
Baggio B, Budakovic A, Dalla Vestra M et al (2002) Effects of cigarette smoking on glomerular structure and function in type 2 diabetic patients. J Am Soc Nephrol 13(11):2730–2736
Jose MJ, Varkey V, Chandni R et al (2016) The Role of Smoking as a Modifiable Risk Factor in Diabetic Nephropathy. J Assoc Physicians India 64(7):34–38
Lan L, Han Y, Ren W et al (2015) Advanced glycation endproducts affect the cytoskeletal structure of rat glomerular endothelial cells via the Rasrelated C3 botulinum toxin substrate 1 signaling pathway. Mol Med Rep. 11(6):4321–4326
Pala L, Cresci B, Manuelli C et al (2005) Vascular endothelial growth factor receptor-2 and low affinity VEGF binding sites on human glomerular endothelial cells: biological effects and advanced glycosilation end products modulation. Microvasc Res 70(3):179–188
Chuahirun T, Simoni J, Hudson C et al (2004) Cigarette smoking exacerbates and its cessation ameliorates renal injury in type 2 diabetes. Am J Med Sci 327(2):57–67
Phisitkul K, Hegazy K, Chuahirun T et al (2008) Continued smoking exacerbates but cessation ameliorates progression of early type 2 diabetic nephropathy. Am J Med Sci 335(4):284–291. https://doi.org/10.1097/MAJ.0b013e318156b799
Launay JM, Del Pino M, Chironi G et al (2009) Smoking induces long-lasting effects through a monoamine-oxidase epigenetic regulation. PLoS ONE 4(11):e7959
Hellemons ME, Agarwal PK, van der Bij W et al (2011) Former smoking is a risk factor for chronic kidney disease after lung transplantation. Am J Transplant 11(11):2490–2498
Bowlin SJ, Morrill BD, Nafziger AN et al (1996) Reliability and changes in validity of self-reported cardiovascular disease risk factors using dual response: the behavioral risk factor survey. J Clin Epidemiol 49(5):511–517
Orth SR, Stockmann A, Conradt C et al (1998) Smoking as a risk factor for end-stage renal failure in men with primary renal disease. Kidney Int 54(3):926–931. https://doi.org/10.1046/j.1523-1755.1998.00067.x
Authors’ contribution
Xu Haili and Lian jing participated in the design of this manuscript. Xu Haili, Jinliu Suo, and Lian jing participated in abstracting the data and performing statistical analysis. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, H., Suo, J. & Lian, J. Cigarette smoking and risk of albuminuria in patients with type 2 diabetes: a systematic review and meta-analysis of observational studies. Int Urol Nephrol 50, 911–922 (2018). https://doi.org/10.1007/s11255-018-1825-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-018-1825-x