Abstract
Purpose
To identify preoperative factors correlated with postoperative early renal function in patients who had undergone radical cystectomy (RC) and intestinal urinary diversion.
Methods
We retrospectively identified 201 consecutive bladder cancer patients without distant metastasis who had undergone RC at our institution between 2003 and 2012. The estimated glomerular filtration rate (eGFR) was calculated using the modified Chronic Kidney Disease Epidemiology equation before RC and 3 months following RC. Univariate and stepwise multiple linear regression analyses were applied to estimate postoperative renal function and to identify significant preoperative predictors of postoperative renal function.
Results
Patients who had undergone intestinal urinary diversion and were available for the collection of follow-up data (n = 164) were eligible for the present study. Median preoperative and postoperative eGFRs were 69.7 (interquartile range [IQR] 56.3–78.0) and 70.7 (IQR 57.3–78.1), respectively. In univariate analyses, age, preoperative proteinuria, thickness of abdominal subcutaneous fat tissue (TSF), preoperative serum creatinine level, preoperative eGFR, and urinary diversion type were significantly associated with postoperative eGFR. In a stepwise multiple linear regression analysis, preoperative eGFR, age, and TSF were significant factors for predicting postoperative eGFR (p < 0.001, p = 0.02, and p = 0.046, respectively). The estimated postoperative eGFRs correlated well with the actual postoperative eGFRs (r = 0.65, p < 0.001).
Conclusions
Preoperative eGFR, age, and TSF were independent preoperative factors for determining postoperative renal function in patients who had undergone RC and intestinal urinary diversion. These results may be used for patient counseling before surgery, including the planning of perioperative chemotherapy administration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radical cystectomy (RC) and pelvic lymph node dissection remain the gold standard of care for muscle-invasive or recurrent high-grade non-muscle-invasive bladder cancer and provide excellent local control and survival benefits [1]. However, despite radical treatment, the prognosis for patients with non-organ-confined or lymph node-positive bladder cancer remains insufficient, with a 5-year recurrence-free survival rate <50% [2]. Growing evidence supports a greater survival benefit associated with cisplatin-based systemic chemotherapy, including that in the neoadjuvant and adjuvant setting [3,4,5,6], and several clinical guidelines recommend the consideration of perioperative systemic chemotherapy for patients with clinical muscle-invasive or pathological non-organ-confined and/or lymph node-positive bladder cancer [1, 7]. However, recent data suggest limited use of perioperative systemic chemotherapy [8], and the persistence of variation in the use of perioperative chemotherapy between centers or physicians [9, 10]. Although a survival benefit has been demonstrated, especially for neoadjuvant chemotherapy (NAC), some favor the use of adjuvant chemotherapy (AC). This may be because of the potential advantage of knowing the exact postoperative pathological staging or lymph node status while considering AC, as NAC may represent a potential overtreatment for organ-confined disease, concerns that NAC may delay definitive surgical treatment and may increase perioperative complications, and patients’ preferences. However, as a proportion of patients experience compromised renal function during the early perioperative periods following RC [11, 12], some may miss the chance to receive AC when needed. The ability to predict postoperative renal function preoperatively would allow for the identification of those whose renal function is likely to decrease or increase following RC, facilitating the discussion with patients regarding the best timing for perioperative systemic chemotherapy administration.
Post-cystectomy early renal function is determined by several patient-related and exogenous factors, including patient age, inherent comorbidities (diabetes mellitus [DM], hypertension [HT], preoperative kidney disease, etc.), presence of preoperative hydronephrosis, and perioperative blood loss or transfusion. However, limited data exist regarding potential preoperative factors that may be predictive of postoperative renal function following RC. Moreover, previous researches have failed to include possible preoperative factors such as hematological and metabolic parameters that may be associated with postoperative renal function [11, 12].
In the current study, several potential preoperative factors associated with early postoperative renal function in patients who had undergone RC and intestinal urinary diversion were investigated. Additionally, the development of a nomogram using preoperative factors to predict postoperative renal function was attempted, because to our knowledge, no such predictive nomogram for postoperative renal function following RC in patients with bladder cancer has been described.
Materials and methods
Patient selection
The institutional review board approved this retrospective study and issued a waiver of informed consent. Consecutive bladder cancer patients who had undergone RC at our university hospital from May 2003 to May 2012 (n = 201) were reviewed. The indications for RC included muscle-invasive or high-grade T1 bladder cancer without evidence of distant metastasis. We excluded patients with performed ureterocutaneous urinary diversion (n = 6), without urinary diversion due to dialysis (n = 4), performed preoperative intervention (percutaneous nephrostomy) for ureteral obstruction (n = 2), patients whose urine analysis data were unavailable (n = 3), or patients with unavailable preoperative computed tomography (CT) or magnetic resonance imaging (MRI) scans due to procedures conducted outside our hospital (n = 6). We also excluded patients for whom postoperative follow-up, including blood examination at 3 months after surgery, was not conducted at our institution (n = 16). The final patient population consisted of 164 patients, none of whom received NAC during the study period.
Data analyzed and statistical methods
The preoperative factors analyzed were age at RC, gender, smoking history, concomitant comorbidity (DM and HT), preoperative proteinuria, body mass index (BMI), thickness of abdominal subcutaneous fat tissue (TSF), presence of hydronephrosis, level of hemoglobin, preoperative renal function, and type of urinary diversion (ileal conduit or ileal neobladder). The estimated glomerular filtration rate (eGFR), a proxy for renal function, was calculated using the modified Chronic Kidney Disease Epidemiology (CKD-EPI) equation as follows: eGFR = 141 × min(SCr/κ, 1)α × max(SCr/κ, 1)−1.209 × 0.993Age × 1.018 [if female] × 0.813 [if Japanese], where SCr is serum creatinine, κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates the minimum of SCr/κ or 1, and max indicates the maximum of SCr/κ or 1 [13, 14]. Preoperative and postoperative eGFRs were calculated just before RC and 3 months after surgery. If AC was performed within 3 months following RC, renal function just before AC was used. All patients underwent urine analysis at the time of admission for RC at our institution, and preoperative proteinuria was determined as urine containing >30 mg/dL of protein. TSF was evaluated preoperatively using CT or MRI at a location just below the umbilicus, as described in a previous report [15].
The interquartile ranges (IQRs) for each continuous variable were summarized, as were the percentages for categorical variables. Correlation between variables was analyzed using the Mann–Whitney rank sum test or the Fisher’s exact test. Simple regression analysis was used to evaluate the correlation between the continuous variables and to investigate the relationship between preoperative variables and postoperative eGFRs 3 months after surgery. Multiple linear regression analyses with a forward stepwise selection procedure were performed to identify significant preoperative predictors of postoperative eGFRs and to estimate postoperative eGFRs. All probabilities were two-sided, and a p value of <0.05 was considered significant. All analyses were performed using STATA 12 (Stat Corp., College Station, TX, USA).
Results
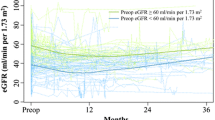
Patient demographic information and clinical characteristics are presented in Table 1. Overall, RC and intestinal urinary diversion had a minimal effect on the change between the absolute values of the preoperative and postoperative eGFRs, which were 69.7 (IQR 56.3–78.0) and 70.7 (IQR 57.3–78.1), respectively. The preoperative and postoperative eGFRs correlated significantly (p < 0.001). Of the 164 patients, postoperative eGFRs decreased in 66 (40%: median change; 10.65 mL/min/1.73 m2, range 0.48–56.39), increased in 92 (56%: median change; 8.77 mL/min/1.73 m2, range 0.46–61.72), and remained stable in 6 (4%) patients in comparison with that for preoperative eGFRs. In univariate regression analyses, age, preoperative proteinuria, TSF, preoperative serum creatinine level, preoperative eGFR, and urinary diversion types were significantly associated with postoperative eGFRs (Table 2). Age correlated significantly with smoking history (p = 0.001), HT (p = 0.041), hemoglobin level (p = 0.001), preoperative eGFR (p < 0.001), and urinary diversion type (p < 0.001). Preoperative eGFR correlated significantly with presence of hydronephrosis (p = 0.01), preoperative creatinine level (p < 0.001), and urinary diversion type (p < 0.001). In a stepwise multiple linear regression analysis, preoperative eGFR, age, and TSF were significant factors in predicting postoperative eGFRs (p < 0.001, p = 0.02, and p = 0.046, respectively; Table 3). The predictive equation for postoperative renal function was as follows: predicted postoperative eGFR = a1 + a2 × preoperative eGFR − a3 × Age − a4 × TSF. The values of a1, a2, a3, and a4 were 52.7, 0.57, 0.27, and 0.27, respectively. The adjusted R-square for this multivariate model was 0.43. The predicted postoperative eGFRs were well correlated with the actual postoperative eGFRs (Fig. 1, r = 0.65, p < 0.001). We also created a nomogram to predict postoperative eGFR using three independent factors on multivariate regression analysis (Fig. 2). To use the nomogram, the patient’s value must first be located on each predictor variable scale. Each value has corresponding “Points” (top axis). For example, a preoperative eGFR of 70 mL/min/1.73 m2 is equivalent to approximately 40 points. This is determined by comparing the location of 70 mL/min/1.73 m2 value on the “Preoperative eGFR” axis to the corresponding “Points” scale above and then drawing a vertical line between the two axes. The point values for all predictor variables were determined in a similar manner and summed to arrive at a “Total Points” value. This value is then plotted on the “Total Points” axis, which is a vertical line drawn from the “Total Points” axis to the “Postoperative eGFR” axis and indicates the patient’s predictive postoperative eGFR value.
Discussion
In the present study, determinants of postoperative renal function were found to be multifactorial. Although a host of potential confounding factors may have correlated with renal function, study results confirmed that preoperative eGFR, age, and TSF were significant preoperative determinants of postoperative renal function 3 months after RC and intestinal urinary diversion. It should be noted that changes in renal function were not uniform across patients. For example, renal function decreased in 40%, increased in 56%, and showed highly variable change ranges across patients.
The significance of age on perioperative renal function changes after RC has been reported in prior studies [11, 12]. Thompson et al. [12] investigated the factors correlated with GFR changes in patients with bladder cancer who were treated with RC without NAC. In multivariable analyses, they found that older age (p < 0.001), higher preoperative GFR (p < 0.001), and continent urinary diversion (p = 0.011) significantly associated with a negative change in GFR after RC [12]. Canter et al. [11] found that 33–41% of patients with bladder cancer before RC and 29–40% of patients after RC were not eligible for cisplatin-based chemotherapy based on their GFR values. They reported that age at surgery and preoperative renal function were significant preoperative factors associated with postoperative renal function on multivariate regression analysis. The results from the current study are in good agreement with those of these previous studies, in that age and preoperative eGFRs associated significantly with postoperative renal function on both univariate and multivariate analyses. In addition, we found that age significantly correlated with smoking history, HT, hemoglobin level, preoperative eGFR, and urinary diversion type in our cohort. Furthermore, TSF was also confirmed as a significant determinant of postoperative renal function in multivariate analyses. These results suggest that a more comprehensive assessment of renal function may assist in clarifying the pathophysiology underlying the change between pre- and post-RC renal function.
We have thought that preoperative hydronephrosis could be one of the potential factors to affect postoperative renal function after radical cystectomy (in terms of renal function recovery) because of release of the ureteral obstruction by surgery; however, it was not an independent factor in the present study. Indeed, we observed that the renal function in patients with preoperative hydronephrosis did not necessarily improve postoperatively. In the 34 patients with preoperative hydronephrosis, renal function improved in 24 patients and did not improve in 10. If we categorize the hydronephrosis as unilateral or bilateral, 20 of 29 patients with unilateral hydronephrosis and 4 of 5 with bilateral hydronephrosis showed increased eGFR postoperatively, but this difference was not statistically significant (two-sided Fisher’s exact p value of >0.05). We think that if hydronephrosis develops suddenly and the degree of ureteral obstruction is severe, postoperative renal function may improve dramatically because damage to the renal parenchyma in such cases might be minimal. However, in most cases with hydronephrosis due to bladder cancer, ureteral obstruction progresses slowly (over several months before diagnosis) and irreversible renal damage may have occurred in a proportion of the patients. Furthermore, especially in patients with preoperative unilateral hydronephrosis, if the contralateral normal kidney functions well and can compensate for the renal function, the overall renal function may not be much affected even if the hydronephrosis is released by surgery.
Recent evidence supports correlations between renal dysfunction and BMI [16] and central obesity [17]. Oh et al. [18] suggested that central obesity was associated with a more rapid decline in renal function in an Asian population. A mechanism relating obesity to renal damage may include inflammation, oxidative stress, insulin resistance, and pathophysiological alterations. Adipose tissue may affect the incidence and progression of chronic kidney disease (CKD) through the release of inflammatory cytokines such as interleukin-6 or tumor necrosis factor-α [19]. In the present study, TSF was a significant predictor of postoperative eGFR, as opposed to BMI, on multivariate analysis. This finding may suggest that patients with thicker TSF may have a decreased renal functional reserve (de novo kidney disease) preoperatively and their renal function would likely decrease during the early post-RC periods. These findings are supported by the results of a previous study that demonstrated waist-to-hip ratio, but not BMI, was associated with incident CKD [17]. As central obesity is reported to be a risk factor for DM [20] and HT [21], and these diseases could increase the risk of renal dysfunction [16], a higher susceptibility to DM or HT among those with thicker TSF may explain the significant correlation between TSF and perioperative renal function in the present study. However, the associations between TSF and HT or DM were marginal (p = 0.069 and 0.083, respectively), suggesting that adiposity may have direct effects on the development of renal damage independent of DM or HT, as suggested by Oh et al. [18].
To our knowledge, this is the first study to demonstrate that TSF, which may represent obesity, is a significant factor associated with postoperative renal function following RC and intestinal urinary diversion. Although prior studies have shown the clinical significance of age and preoperative renal function as factors correlated with postoperative renal function [11, 12], those studies did not assess correlations between metabolic factors and renal function in their analyses. Findings from the current study provide a more detailed profile of perioperative renal function changes in patients with bladder cancer who undergo RC and urinary diversion, and may facilitate more comprehensive discussions between patients and doctors regarding perioperative chemotherapy administration prior to RC. However, our study had several limitations. The retrospective, single institution study design may be associated with unknown biases. In addition, the study cohort included Asian patients only. Because ethnic differences have been observed in the amount and distribution of adipose tissues [22], validation studies will be necessary in order to determine whether the results of the current study are generalizable to those of other races and from different countries.
Conclusion
TSF, as well as preoperative eGFR and age, correlated significantly with early postoperative renal function in patients with bladder cancer who had undergone RC and intestinal urinary diversion. The nomogram developed using these variables may be applied preoperatively to predict the postoperative eGFR value with good accuracy. These findings may be beneficial when counseling patients about potential changes in renal function following RC and decision regarding perioperative chemotherapy administration.
References
Clark PE, Agarwal N, Biagioli MC, Eisenberger MA, Greenberg RE, Herr HW et al (2013) Bladder cancer. J Natl Compr Cancer Netw 11:446–475
Stein JP, Lieskovsky G, Cote R et al (2001) Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1054 patients. J Clin Oncol 19:666–675
Advanced Bladder Cancer Meta-analysis Collaboration (2005) Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol 48:202–205
Advanced Bladder Cancer Meta-analysis Collaboration (2005) Adjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis of individual patient data Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Eur Urol 48:189–199
Grossman HB, Natale RB, Tangen CM et al (2003) Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 349:859–866
Griffiths G, Hall R, Sylvester R, Raghavan D et al (2011) International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol 29:2171–2177
Witjes JA, Compérat E, Cowan NC et al (2014) EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol 65:778–792
David KA, Milowsky MI, Ritchey J et al (2007) Low incidence of perioperative chemotherapy for stage III bladder cancer 1998–2003: a report from the National Cancer Data Base. J Urol 178:451–454
Zaid HB, Patel SG, Stimson CJ et al (2014) Trends in the utilization of neoadjuvant chemotherapy in muscle-invasive bladder cancer: results from the National Cancer Database. Urology 83:75–80
Rehman S, Crane A, Din R et al (2013) Understanding avoidance, refusal, and abandonment of chemotherapy before and after cystectomy for bladder cancer. Urology 82:1370–1375
Canter D, Viterbo R, Kutikov A et al (2011) Baseline renal function status limits patient eligibility to receive perioperative chemotherapy for invasive bladder cancer and is minimally affected by radical cystectomy. Urology 77:160–165
Thompson RH, Boorjian SA, Kim SP et al (2014) Eligibility for neoadjuvant/adjuvant cisplatin-based chemotherapy among radical cystectomy patients. BJU Int 113:17–21
Horio M, Imai E, Yasuda Y et al (2010) Modification of the CKD epidemiology collaboration (CKD-EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis 56:32–38
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
Fujii T, Tsutsumi S, Matsumoto A et al (2010) Thickness of subcutaneous fat as a strong risk factor for wound infections in elective colorectal surgery: impact of prediction using preoperative CT. Dig Surg 27:331–335
Foster MC, Hwang SJ, Larson MG et al (2008) Overweight, obesity, and the development of stage 3 CKD: the Framingham Heart Study. Am J Kidney Dis 52:39–48
Elsayed EF, Sarnak MJ, Tighiouart H et al (2008) Waist-to-hip ratio, body mass index, and subsequent kidney disease and death. Am J Kidney Dis 52:29–38
Oh H, Quan SA, Jeong JY et al (2013) Waist circumference, not body mass index, is associated with renal function decline in korean population: Hallym Aging Study. PLoS ONE 8:e59071
Bavbek N, Isik B, Kargili A et al (2008) Association of obesity with inflammation in occult chronic kidney disease. J Nephrol 21:761–767
Carey VJ, Walters EE, Colditz GA et al (1997) Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses’ Health Study. Am J Epidemiol 145:614–619
Janssen I, Katzmarzyk PT, Ross R (2004) Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr 79:379–384
Tanaka S, Horimai C, Katsukawa F (2003) Ethnic differences in abdominal visceral fat accumulation between Japanese, African-Americans, and Caucasians: a meta-analysis. Acta Diabetol 40:S302–S304
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest with any institution or product.
Informed consent
The institutional review board approved this retrospective study and issued a waiver of informed consent. IRB approved protocol number is 1621.
Rights and permissions
About this article
Cite this article
Gondo, T., Ohno, Y., Nakashima, J. et al. Preoperative determinant of early postoperative renal function following radical cystectomy and intestinal urinary diversion. Int Urol Nephrol 49, 233–238 (2017). https://doi.org/10.1007/s11255-016-1462-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-016-1462-1