Abstract
Purpose
To evaluate the effect of cranberry extract (PAC-A ~ proanthocyanidin-A) on the in vitro bacterial properties of uropathogenic (E. coli) and its efficacy/tolerability in patients with subclinical or uncomplicated recurrent UTI (r-UTI).
Materials and methods
After obtaining clearance from the ethics committee and administering a written informed consent, 72 patients with r-UTI were enrolled as per protocol (November 2011 to March 2013) in this prospective study, to randomly receive (PAC-A: group I, 36) or (placebo: group II, 36), for 12 weeks. Any change/reduction in the incidence of r-UTI at 12 weeks was construed to be the primary endpoint of this study.
Results
After 12 weeks, bacterial adhesion scoring decreased (0.28)/(2.14) in group I/II (p < 0.001); 32/36 (88.8 %) and 2/36 (5.5 %) in groups I and II, respectively, turned MRHA negative (p < 0.001); biofilm (p < 0.01) and bacterial growth (p < 0.001) decreased in group I; microscopic pyuria score was 0.36/2.0 in group I/II (p < 0.001); r-UTI decreased to 33.33 versus 88.89 % in group I/II (p < 0.001); mean subjective dysuria score was 0.19 versus 1.47 in group I/II (p < 0.001), while mean urine pH was 5.88 versus 6.30 in group I/II (p < 0.001). No in vitro antibacterial activity of cranberry could be demonstrated, and no adverse events were noted.
Conclusions
The overall efficacy and tolerability of standardized cranberry extract containing (PAC-A) as a food supplement were superior to placebo in terms of reduced bacterial adhesion; bacterial MRHA negativity; urine pH reduction; and in preventing r-UTI (dysuria, bacteriuria and pyuria). Larger randomized controlled trials are needed to elucidate the precise role, exact dose and optimal duration of PAC-A therapy in patients at risk of r-UTI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recurrent urinary tract infection (r-UTI~ three episodes of UTI in the last 12 months or two episodes in the last 6 months) is common with more than 25 % of them recurring within 6 months [1]. The adhesin protein (pili/fimbriae) helps in adhesion of E. coli to uroepithelial cells causing UTI, and these proteins may be mannose resistant (MR)/mannose sensitive (MS) [2]. E. coli with MS type-1 pili that facilitate bacterial adhesion (inhibited by fructose in cranberries) are non-pathogenic, while r-UTI (p-fimbriae or pyelonephritis fimbriae ~MR) is due to more virulent strains of E. coli in which proanthocyanidins-A (PACs-A) help prophylactically by reducing E. coli adherence [2, 3] and r-UTI [4]. Prolonged antimicrobial prophylaxis in r-UTI may be associated with resistance/superinfection by bacteria like clostridium difficile [4]. Other non-pharmacological alternatives include immunotherapy, probiotics, urinary acidification and cranberry therapy though no direct comparative studies have been reported [1]. There appears to be lack of clarity in the published English literature on the efficacy of prophylactic cranberry therapy in patients with r-UTI, and this study was undertaken to explore the effect of prophylactic cranberry as an alternative to antimicrobials in patients prone to r-UTI; this formed the primary rationale for the current study.
Materials and methods
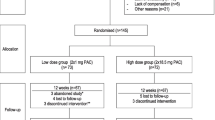
Patients with subclinical asymptomatic bacteriuria and/or r-UTI, not responding to antimicrobials, and patients prone to r-UTI with entry/exit criteria as per registered protocol CTRI/2014/08/004864 were enrolled in this study. The working definition of r-UTI was as described earlier in the EUA guidelines [1]. After approval by IEC-HR dt-03.12.2011 and administering a written informed consent, eligible patients were screened for inclusion and randomized as per www.radomization.com (seed 18151, created on september 17, 2011, 10:14:1). Group I patients received a standardized oral preparation of cranberry as Cranpac™ (containing PAC-A 60 mg per capsule), one capsule twice a day as nutritional supplement for 12 weeks, while group II patients received lactobacillus (containing 400 million lactobacillus acidophilus per capsule), 1 capsule twice a day as placebo for 12 weeks. Follow-up was done at three (2nd visit), six (3rd visit) and 12 weeks (4th visit) with compliance visits scheduled once a week until the completion of therapy, at which patients were contacted to produce the consumed empty strips. Patients were assessed for efficacy, side/adverse events and compliance. Urine routine microscopy, pH (dipstick) and culture, patient’s subjective well-being scoring (excellent to poor) and objective scoring were done by adding points given to evaluated efficacy parameters at the primary endpoint; however, a primary change in the occurrence of r-UTI at 12 weeks after therapy with interventional/comparator agent was treated as the final primary endpoint of the current study. The study flow diagram of the protocol for this study is depicted in Fig. 1.
Sample size computation
‘PASS 2008’ software was used to calculate the sample size. Considering a power factor of 80 and 5 % level of significance and considering 30 % change in mean, a minimum sample size of 60 (30 in each group) was considered necessary. After compensating for 20 % as a follow-up loss, 36 subjects per group were required for this study.
Method of studying bacterial adhesion
Centrifuged urine/cells were washed in phosphate-buffered saline pH 7.1 and air-dried with Gram-stained slides analyzed under oil immersion for cytoadherence. The mean number of bacteria adherent per uroepithelial cell was expressed as number of bacteria/cell in the manner as described by Reid et al. [5] after randomly viewing at least 50 cells in high-power field.
Method of studying mannose-resistant hemagglutination assay (MRHA) [6]
Bacterial antiadhesion activity was evaluated with MRHA by direct bacterial hemagglutination test (DBHT) by noting clumping of erythrocytes by E. coli fimbriae in the presence of d-mannose, and E. coli strains were inoculated in 1 % nutrient broth and incubated at 37 °C for 48 h for full fimbriation. A panel of red blood cells (RBC) was selected by obtaining human blood group ‘O.’ The RBCs were washed thrice in normal saline and prepared as a stock 3 % suspension in fresh saline to be used immediately within a week stored at 3–5 °C. DBHT was done on multiple concavity slides by adding a drop of RBC suspension to a drop of broth culture, and slide was rocked to and fro at room temperature for 5 min; presence of clumping was taken as positive for hemagglutination. Mannose-sensitive hemagglutination was detected by the absence of hemagglutination in a parallel test in which a drop of 2 % w/v d-mannose was added to RBC and a drop of broth culture. MRHA was detected by the presence of hemagglutination of 3 % ‘O’ group human RBC in the presence of 2 % d-mannose as described by Vagarali et al. [6].
Demonstration of antibacterial activity of proanthocyanidin-A
Cranpac™ extract was too viscous to allow disk diffusion; hence, to demonstrate bactericidal activity of PAC-A, broth microdilution method was used as per ‘Clinical Laboratory Standards Institute (CLSI)’ guidelines [7]. Since CLSI does not define breakpoints for PAC-A, a stock solution was made in dimethylsulfoxide (DMSO) [an organic solvent non-toxic for bacteria used to dissolve oil-based drugs]. Serial doubling dilutions were made ranging from 2048 to 16 µg/ml in microdilution plates to test in vitro efficacy of PAC-A against wild strains obtained from PAC group in their initial visit. Control strains from American Type Culture Collection [E. coli (ATCC 25922) and S. aureus (ATCC 29213)] were used; due to its opaque nature the visual minimum inhibitory concentration (MIC) of the bacterial isolates could not be read; therefore, subcultures made from wells containing mixture of bacteria/PAC-A on Mueller–Hinton Agar to determine the minimum bactericidal concentration [8].
Method of detecting biofilm formation on urinary catheters
Biofilm on catheter tips was detected by serial roll-plate technique; proximal 2 inches of the catheter tip was cut with a sterile blade and was rolled over a blood agar plate one and half times to check for bacterial growth and biofilm formation. The plates were incubated in ambient air for 24 h at 35 °C for bacterial colony count [9].
Efficacy (outcome) parameters
The efficacy outcome measures included improved clinical symptoms, decreased pyuria/bacterial count in urine culture and/or conversion of positive urine culture to sterile, microscopic decreased bacterial adherence to uroepithelial cells, bactericidal activity and MRHA. The endpoints (objective outcome measures) at 3, 6 and 12 weeks (final endpoint) were scored, and change/reduction in the incidence of r-UTI at 12 weeks was construed to be the primary endpoint in this study. Tolerability (outcome) parameters: Adverse events/side effects/compliance to therapy was recorded. Statistical analysis: Data were statistically analyzed for efficacy/tolerability of cranberry extract over the placebo using Chi-square test, repeated-measure ANOVA test and Fisher’s exact t test.
Observations and results
The salient demographic characteristics of the two patient groups are described in Table 1. The baseline mean [age, urinary pH, dysuria score, pyuria score, bacterial growth, bacterial adhesion score, biofilm and MRHA(+)] was 41.6/35.8 years, 6.28/6.19, 1.42/1.08, 2.28/1.5, 33/36, 2.11/1.81, 8/6 and 33/35 in groups I/II, respectively (see Table 1). Group I/II were compared and found to be similar. With regard to our patient types, in group I of the 8 catheterized patients, 4 were on intermittent catheterization, 2 had BPH, 1 had neurogenic bladder and 1 had supra-pubic cystostomy (SPC), while in group II of the 6 catheterized patients 3 were on intermittent catheterization, 1 had SPC and 2 had BPH. No patient had diabetes mellitus in either of the groups. A significant reduction in the mean bacterial adhesion score, significant increase in MRHA negativity and a significant reduction in biofilm were observed in patients of group I. In group I, there was a significant decrease (18, 72 and 75 %) in the number of patients with bacterial growth at 3, 6 and 12 weeks of therapy, respectively. Microscopic pyuria score (MPS) decreased by 41, 70 and 84 % (group I) and increased by 8, 20 and 25 % (group II) after 3, 6 and 12 weeks of therapy, respectively; thus, PAC-A was significantly effective in lowering the MPS (p < 0.001). There was a significant decrease in mean burning micturition score in group 1 (p < 0.001), and it appeared that cranberry therapy was associated with a more acidic urine (p < 0.001) (see Table 1).
A total of 12/36 (33 %) and 32/36 (89 %) patients developed (r-UTI) over 12 weeks in group I/II, respectively; thus, PAC-A was significantly effective in lowering the episodes of UTI (p < 0.001). Cranberry failed to demonstrate any in vitro antibacterial activity in any patient. There were no adverse events noted apart from one patient in each group reporting self-limiting constipation. There was no withdrawal from therapy in any patient.
Discussion
PAC-A was probably effective in decreasing the mean bacterial adhesion scoring versus placebo (see Table 1). Various studies comparing the effect of cranberry/different PAC concentrations on bacterial adhesions in patients of UTI are depicted in Table 2a.
In a study by Risco et al. [10], the mean bacterial adhesion (MBA) was 36 at PAC level of 0 mg/ml and 23 at PAC level of 75 mg/ml showing 34.5 % decrease (p < 0.05); the authors concluded that PAC demonstrated significant inhibition of E. coli adherence to uroepithelial cells. In a study by Reid et al. [5], the MBA was 5.40 at day 0 and 1.18 after 15 days (78 % decrease, p < 0.05). Di Martino et al. [11] demonstrated a MBA of 76 and 3 in the placebo versus cranberry group (62 % decrease, p < 0.05) and concluded that cranberry had significant antiadherence activity against E. coli. Gupta et al. [12] demonstrated that the MBA was 2.5 × 105 at PAC level of 0 mg/ml and 0.9 × 105 at PAC level of 50 mg/ml (70 % decrease, p < 0.05), concluding thereby that PAC may be considered as an inhibitory agent for drug-resistant strains of E. coli. Howel et al. [7] also showed a 56 % decrease in MBA (22.3 vs. 9.9 in placebo versus cranberry, p < 0.05) and concluded that PAC may be protective against bacterial adhesion in the urinary tract. Gupta et al. [13] in an experimental study demonstrated a MBA of 6.9 and 1.6/cell (PAC level of 0 and 75 mg/ml) with a 77 % decrease (p < 0.001) in E. coli adherence.
In the present study, most of our MRHA(+) strains turned MRHA(−) with PAC-A therapy in group I. Comparison with other studies on MRHA is summarized in Table 2b. Gupta et al. [12] in a study comparing the effect of PAC (150-750 µg/µl) on MRHA demonstrated that PAC exhibited significant antiadhesion activity on E. coli by rendering them MRHA (−). Howel et al. [7] and Gupta et al. [13] independently showed a dose-dependent effect of PAC-A therapy on MRHA activity propounding its protective efficacy against bacterial adhesion. In our study, it appeared that PAC-A therapy reduced biofilm formation significantly in our catheterized patients, and other similar studies depicting the effect of cranberry are compared in Table 2c. Reid et al. [5] and LaPlente et al. [14] demonstrated major reduction in biofilm-producing E. coli after consuming cranberry. PAC-A did not demonstrate any in vitro antibacterial activity in this study. Monroy-Torres et al. [15] showed that cranberry was not associated with any bacteriostatic properties, while Lee Y et al. [16] in a pilot study showed that 7/20 (35 %) patients on cranberry had antibacterial activity against E. coli. In this study, PAC-A therapy was associated with decreased bacterial growth (see Table 1), and other studies depicting the effect of cranberry on bacterial growth are summarized in Table 2d. Other researchers like Reid et al. [5] and Bailey et al17 independently demonstrated no bacterial growth, while some like McMurdo et al. [17] demonstrated fewer E. coli infections, after cranberry therapy, with both concluding that PAC may prevent r-UTIs. However, Barbosa-Cesnik et al. [18], Beerepoot et al. [19], Waites et al. [20] and Linsenmeyer et al. [21] in four independent studies failed to show any beneficial effect of cranberry on r-UTI.
As depicted in Table 3, in our study UTI had recurred in 12/36 (33 %) versus 32/36 (89 %) in groups I and II, respectively, and PAC-A had decreased r-UTI by about 55 % at the end of the 12-week observation period. Other studies on the effect of cranberry in r-UTI are also summarized in Table 3. Kontiokari et al. [22] demonstrated 22 % reduction in r-UTI episodes with cranberry. Bailey et al. [23] found no patient developed UTI within the 12 weeks with cranberry therapy concluding that cranberry may prevent r-UTI in women. McMurdo et al. [17] also concluded that cranberry was acceptable in reducing symptomatic UTIs in their older patients. Barbosa-Cesnik et al. [18] showed that 31/155 patients (20 %) in the cranberry group versus 23/164 patients (14 %) who developed UTI in the placebo group concluded that cranberry did not lower the incidence of second UTI. McMurdo et al. [24] concluded that trimethoprim had a little advantage over cranberry in the prevention of r-UTIs in older women with 25/69 (36.23 %) and 14/68 (20.59 %) developing UTI (p = 0.084) in cranberry versus trimethoprim group.
Beerepoot et al. [19] concluded that trimethoprim-sulfamethoxazole was more effective than cranberry (500 mg daily) in preventing r-UTIs. Waites et al. [20] and Linsemeyer et al. [21] in separate studies found no difference in the episodes of UTIs in the patients with neurogenic bladder and concluded that cranberry was ineffective in reducing pyuria/UTIs in their patients. Hess et al. [25] demonstrated a reduction in UTI in the cranberry versus placebo group in 47 spinal cord-injured patients with neurogenic bladder after 6-month cranberry therapy, and they concluded that cranberry should be considered for preventing UTIs in the patients with neurogenic bladder. In a meta-analysis by Beerepoot et al. [26], the authors found that cranberries decreased r-UTI.
Micali et al. [27] and Burleigh et al. [28] in a review of clinical studies on the evaluation of cranberry efficacy in the prevention of UTIs approved the use of cranberry in prophylaxis of r-UTI in women. Takahashi [29] in a double-blind placebo-controlled RCT demonstrated a 20 % reduction in r-UTI in women after 5 months of cranberry therapy in 118 women at risk of r-UTI. In a major review by Vasileiou et al. [30], the authors concluded that the beneficial effects of cranberry against UTIs were probably prophylactic, though their effectiveness in those at higher risk of r-UTIs was low. While cranberries (PAC-A) may inhibit adherence of p-fimbriated E. coli to the uroepithelial cells and also decrease r-UTI in about 30–40 % in premenopausal women with r-UTIs, its precise efficacy and optimal dosage remain unresolved [31].
Foxamn et al. [32] in a double-blind, placebo-controlled RCT explored the therapeutic efficacy of administering cranberry juice capsules in preventing UTI in 160 patients undergoing elective benign gynecological surgery entailing urinary catheterization in which the authors demonstrated a significant reduction in the rate of UTI by half (p < 0.008) without any significant difference in the incidence of adverse events.
Ledda et al. [33] in a pilot field-practice setting study independently demonstrated a negative urine in 20/22 (90.9 %) and 11/22 (50.0 %) in the cranberry versus control (p < 0.005) group without any adverse events, reiterating the effectiveness and safety of oral nutritional supplementation with a well-standardized cranberry preparation in the prevention of r-UTI.
There were no major side effects/adverse events/reactions (apart from one patient in each group with self-limiting constipation) in this study. Beerepoot et al. [19] in a randomized double-blind trial of cranberry versus trimethoprim-sulfamethoxazole on 221 patients demonstrated a 48.6 % (53/109 patients—cranberry group) incidence of adverse drug reactions consisting of rash, urticaria, nausea, vomiting, diarrhea, constipation (10 %) vaginal complaints and other adverse drug reactions. Lee et al. [16], Kontiokari et al. [22] and Bailey et al. [23] also did not observe any adverse drug reaction with the use of cranberry; however, Murdo et al. [17, 24] noted some adverse drug reactions which were probably related to trimethoprim and not due to cranberry.
Limitations of the present study
The 1st author was blinded to the data at the time of analysis, the second author collected relevant patient data and randomized the administered therapy, this blinding was not ideal, and a larger head-to-head trial for a longer duration could have increased the strength of the present study. Randomized comparison with antimicrobials and use of urinary bladder epithelial cell lines were omitted due to protocol limitations. The placebo (lactobacillus) used here may not have been ideal, as some reports [31] suggest that some lactobacilli strains may affect the adherence and growth of uropathogenic bacteria. Though the two patient groups were comparable, it is admitted that these patients were not entirely homogenous due to the coexisting urological disorders. Lifestyle advice measures like personal hygiene and fluid intake which could have partly contributed in amelioration of r-UTI could not be entirely excluded. This study attempted to explore the prophylactic benefit of PAC on those at risk of uncomplicated r-UTI only and not on the actual therapy of UTI due to E. coli. Further, we did not evaluate the effect of PAC-A on UTI due to organisms other than E. coli.
Conclusions
The overall efficacy and tolerability of standardized cranberry extract containing (PAC-A) were superior to placebo in terms of reduced bacterial adhesion; bacterial MRHA negativity; urine pH reduction; and in preventing recurrent UTI (dysuria, bacteriuria and pyuria). Larger randomized controlled trials are needed to elucidate the precise role, exact dose and duration of PAC-A therapy in patients at risk of recurrent UTI. Despite these limitations, the results of the present study appear to suggest a beneficial effect of cranberry when used prophylactically as a food supplement in selected patients with recurrent uncomplicated UTI.
Abbreviations
- PAC-A:
-
Proanthocyanidin-A
- r-UTI:
-
Recurrent urinary tract infection
- MS:
-
Mannose sensitive
- MR:
-
Mannose resistant
- MRHA:
-
Mannose-resistant hemagglutination assay
- MPS:
-
Microscopic pyuria score
- MBA:
-
Mean bacterial adhesion
References
Grabe M, Bjerklund-Johansen TE, Botto H et al (2012) Guidelines on urological infections. Eur Assoc Urol. http://www.uroweb.org/fileadmin/tx_eauguidelines/2009/Full/Urological_Infections.pdf. Accessed on 1st Jan 2013
Zafriri D, Ofek I, Adar R, Pocino M, Sharon N (1989) Inhibitory activity of cranberry juice on adherence of type 1 and type P fimbriated Escherichia coli to eucaryotic cells. Antimicrob Agents Chemother 33:92–98
Ofek I, Mirelman D, Sharon N (1977) Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature 265:623–625
Gupta K, Scholes D, Stamm WE (1999) Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA 281:736–738
Reid G, Hsiehl J, Potter P et al (2001) Cranberry juice consumption may reduce biofilms on uroepithelial cells: pilot study in spinal cord injured patients. Spinal Cord 39:26–30
Vagarali MA, Karadesai SG, Patil CS et al (2008) Haemagglutination and siderophore production as the urovirulence markers of uropathogenic Escherichia coli. Indian J Med Microbiol 26(1):68–70
Howell AB, Botto H, Combescure C et al (2010) Dosage effect on uropathogenic Escherichia coli anti-adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidin content: a multicentric randomized double blind study. BMC Infect Dis 10:94
Clinical and laboratory Standards Institute. Methods for dilution Antimicrobial Susceptibility Tests for Bacteria that grow Aerobically; approved Standard- Ninth edition. CLSI Document M07-A9, Wayne, PA: Clinical and Laboratory Standards Institute; 2012
Maki DG, Weise CE, Sarafin HW (1977) A semiquantitative culture method for identifying intravenous catheter related infection. New Engl J Med 296:1305–1309
Risco E, Miguélez C, Sánchez de Badajoz E et al (2010) Effect of American cranberry (Cysticlean) on Escherichia coli adherence to bladder epithelial cells: in vitro and in vivo study. Arch Esp Urol 63(6):422–430
Di Martino P, Agniel R, David K et al (2006) Reduction of Escherichia coli adherence to uroepithelial bladder cells after consumption of cranberry juice: a double-blind randomized placebo-controlled cross-over trial. World J Urol 24:21–27
Gupta A, Dwivedi M, Mahdi AA et al (2012) Inhibition of adherence of multi-drug resistant E. coli by proanthocyanidin. Urol Res 40:143–150
Gupta K, Chou MY, Howell A, Wobbe C, Grady R, Stapleton AE (2007) Cranberry products inhibit adherence of p-fimbriated Escherichia coli to primary cultured bladder and vaginal epithelial cells. J Urol 177:2357–2360
LaPlante KL, Sarkisian SA, Woodmansee S et al (2012) Effects of cranberry extracts on growth and biofilm production of Escherichia coli and Staphylococcus species. Phytother Res 26:1371–1374
Monroy-Torres R, Macías AE (2005) Does cranberry juice have bacteriostatic activity? Rev Invest Clin 57:442–446
Lee YL, Najm WI, Owens J et al (2010) Anti-microbial activity of urine after ingestion of cranberry: a pilot study. Evid Based Complement Alternat Med 7:227–232
McMurdo ME, Bissett LY, Price RJ et al (2005) Does ingestion of cranberry juice reduce symptomatic UTI in older people in hospital? a double-blind, placebo-controlled trial. Age Ageing 34:256–261
Barbosa-Cesnik C, Brown MB, Buxton M et al (2011) Cranberry juice fails to prevent recurrent urinary tract infection: results from a randomized placebo-controlled trial. Clin Infect Dis 52:23–30
Beerepoot MA, Ter Riet G, Nys S et al (2011) Cranberries vs antibiotics to prevent UTI: a randomized double-blind non inferiority trial in premenopausal women. Arch Intern Med 171:1270–1278
Waites KB, Canupp KC, Armstrong S et al (2004) Effect of cranberry extract on bacteriuria and pyuria in persons with neurogenic bladder secondary to spinal cord injury. J Spinal Cord Med 27:35–40
Linsenmeyer TA, Harrison B, Oakley A et al (2004) Evaluation of cranberry supplement for reduction of UTI in individuals with neurogenic bladders secondary to spinal cord injury: a prospective, double-blinded, placebo-controlled, crossover study. J Spinal Cord Med 27:29–34
Kontiokari T, Sundqvist K, Nuutinen M et al (2001) Randomised trial of cranberry-lingonberry juice and lactobacillus GG drink for the prevention of UTI in women. BMJ 322:1571
Bailey DT, Dalton C, Joseph Daugherty F et al (2007) Can a concentrated cranberry extract prevent recurrent UTI in women? a pilot study. Phytomedicine 14:237–241
McMurdo ME, Argo I, Phillips G et al (2009) Cranberry or trimethoprim for the prevention of recurrent UTI? a randomized controlled trial in older women. J Antimicrob Chemother 63:389–395
Hess MJ, Hess PE, Sullivan MR et al (2008) Evaluation of cranberry tablets for the prevention of UTI in spinal cord injured patients with neurogenic bladder. Spinal Cord 46:622–626
Beerepoot MA, Geerlings SE, van Haarst EP, van Charante NM, Ter Riet G (2013) Non antibiotic prophylaxis for recurrent UTI: a systematic review and meta-analysis of randomized controlled trials. J Urol 190:1981–1989
Micali S, Isgro G, Bianchi G, Miceli N, Calapai G, Navarra M (2014) Cranberry and recurrent cystitis: more than marketing? Crit Rev Food Sci Nutr 54(8):1063–1075
Burleigh AE, Benck SM, McAchran SE, Reed JD, Krueger CG, Hopkins WJ (2013) Consumption of sweetened, dried cranberries may reduce UTI incidence in susceptible women: a modified observational study. Nutr J 12(1):139
Vasileiou I, Katsargyris A, Theocharis S, Giaginis C (2013) Current clinical status on the preventive effects of cranberry consumption against UTI. Nutr Res. 33(8):595–607
Takahashi S, Hamasuna R, Yasuda M, Arakawa S, Tanaka K, Ishikawa K et al (2013) A randomized clinical trial to evaluate the preventive effect of cranberry juice (UR65) for patients with recurrent UTI. J Infect Chemother 19(1):112–117
Beerepoot M, Geerlings S (2016) Non-antibiotic prophylaxis for urinary tract infections. Pathogens 5(2):36. doi:10.3390/pathogens5020036
Foxman B, Cronenwett AE, Spino C, Berger MB, Morgan DM (2015) Cranberry juice capsules and UTI after surgery: results of a randomized trial. Am J Obstet Gynecol 213(2):194.e1-8. doi:10.1016/j.ajog.2015.04.003
Ledda A, Bottari A, Luzzi R, Belcaro G, Hu S, Dugall M et al (2015) Cranberry supplementation in the prevention of non-severe lower urinary tract infections: a pilot study. Eur Rev Med Pharmacol Sci 19(1):77–80
Funding
The authors certify that the above study was conducted entirely from within regular running expenditure available to the government institution and no extra institutional financial grant or funding was availed of in any manner whatsoever.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors further declare that they have nothing to disclose and have no direct or indirect commercial and/or financial incentive associated with this research.
Ethical statement
The authors declare that the above manuscript is in compliance with ethical standards for research in human participants and the authors have no potential sources of conflict of interest associated with its publication.
Informed consent
The authors also certify that informed consent was obtained from all the human participants in this study as per its protocol registered with the Clinical Trials Registry of India.
Rights and permissions
About this article
Cite this article
Singh, I., Gautam, L.K. & Kaur, I.R. Effect of oral cranberry extract (standardized proanthocyanidin-A) in patients with recurrent UTI by pathogenic E. coli: a randomized placebo-controlled clinical research study. Int Urol Nephrol 48, 1379–1386 (2016). https://doi.org/10.1007/s11255-016-1342-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-016-1342-8