Abstract
Purpose
Despite encouraging results in other cancers, in renal cell cancer, no consensus exists regarding the diagnostic and prognostic relevance of MMP-7. The aim of this study was to assess the diagnostic and prognostic potential of serum MMP-7 levels in renal cell cancer. Furthermore, parallel to the widely used ELISA method, we tested a new, fluid-phase, fluorescent immunoassay (B.R.A.H.M.S KRYPTOR®) for the quantitation of MMP-7.
Methods
We analyzed the serum samples of 174 individuals (77 patients and 97 age-matched healthy controls) by a commercially available sandwich ELISA and by a novel, automated, fluid-phase immunofluorescent assay (B.R.A.H.M.S KRYPTOR®). Results were correlated with the clinicopathological and follow-up data.
Results
MMP-7 concentrations showed a high concordance level (R 2 = 0.979) between the two methods (p < 0.001). Serum MMP-7 concentrations were significantly higher in patients compared to controls. At a cutoff value of 3.15 ng/ml, a specificity and a sensitivity of 70 and 82 % for the detection of RCC was found. Patients with metastasis had significantly higher MMP-7 levels as those without metastasis (p = 0.038 by KRYPTOR, p = 0.011 by ELISA). High MMP-7 levels proved to be independently associated with shorter overall, disease-specific and metastasis-free survival, regardless of the analytical method.
Conclusions
Based on these results, serum MMP-7 levels have both diagnostic and prognostic potential. The KRYPTOR method provided comparable results to the standard ELISA analysis, with a high concordance level and can therefore be considered as a surrogate method. Its flexibility and automated operation make the KRYPTOR MMP-7 assay suitable for routine laboratory use in the daily practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal cell cancer (RCC) accounts for approximately 90 % of all kidney malignancies [1]. Its worldwide annual incidence is 209.000 and continues to rise at ~2 % each year [2]. Many RCCs remain asymptomatic even in advanced tumor stages. As a result, more than 30 % of cases are diagnosed at an advanced stage with lymphonodular or distant metastasis or local invasion to the inferior vena cava [2, 3]. In addition, up to 30 % of patients treated by radical nephrectomy for localized RCC will relapse [4]. Current prognostication is mainly based on histological variables and provides only a limited value. Therefore, molecular markers for the improvement of preoperative prognostication are needed. Currently, there are no recommended biomarkers available for the prediction of RCC.
Matrix metalloproteinases-7 (MMP-7) has been reported as a promising tissue and serum marker for the detection of metastasis and prediction of patients’ outcome in different malignancies including colorectal, gastric and ovarian cancer [5–8]. In accordance, we formerly identified high serum MMP-7 level as a metastasis marker and an independent risk factor in urinary bladder and prostate cancer [9–11]. In RCC, MMP-7 tissue protein expression was found to be correlated with invasive tumor growth, enhanced angiogenesis and poor survival [12, 13]. To date, only two studies have been published concerning the serum levels of MMP-7 in RCC with conflicting results [14, 15]. Sarkissian et al. by combining proteomics technology with serology, identified pro-MMP-7 as a potential diagnostic serum marker of RCC. They found a promising diagnostic value for circulating MMP-7 levels, while the prognostic value of MMP-7 was not investigated [13]. In contrast, Ramankulov et al. using a commercially available particle-based flow cytometric (Luminex) immunoassay observed similar MMP-7 blood concentrations in controls and patients with non-metastatic RCCs. Furthermore, they found MMP-7 levels to be associated with the presence of metastasis and poor prognosis [15]. Despite the clinical importance of these data, no further research on the diagnostic and prognostic relevance of circulating MMP-7 analysis in RCC has been published.
Therefore, we assessed the potential of MMP-7 as (1) a diagnostic RCC marker, (2) a preoperative marker for metastasis and (3) an independent risk factor for postoperative metastatic progression and/or survival in patients with RCC. For this purpose, we measured MMP-7 concentrations by two different immunoassay methods. Results were correlated with clinical and follow-up data by using both univariable and multivariable analyses.
Methods
Clinical samples
MMP-7 concentrations were analyzed in serum samples of 77 RCC patients and 97 age-matched healthy controls. Preoperative samples were obtained between 1990 and 1994 at the Department of Urology, University Hospital of Essen. Samples were centrifuged at 1500 rpm for 15 min, immediately aliquoted and kept at −80 °C until analyzed. The criteria for study enrollment were histopathological diagnosis of RCC, no history of other tumor. The study was performed in accordance with the ethical standards of the Helsinki Declaration and was approved by the ethical board of the University of Duisburg-Essen. Each case with available formalin-fixed paraffin-embedded tumor sample was stained with hematoxylin and eosin and reclassified by a pathologist according to the 2004 WHO classification [16].
We calculated survival time as the period between surgical treatment/sample collection and prognostic endpoint. The endpoints of this study were overall survival, cancer-specific survival and metastasis-free survival. Cause of death was obtained from death certificates. The median MMP-7 concentrations were used as a cutoff (5.0 ng/ml for KRYPTOR and 4.8 ng/ml for ELISA).
MMP-7 analysis by the ELISA method
Serum MMP-7 levels were quantified in single measurements by a commercially available sandwich-type ELISA Kit (R&D Systems, Abingdon, UK), according to the manufacturer’s instructions. The intra-assay variability for duplicate measurement was 4.3 % while the inter-assay variability was 7.7 %.
MMP-7 analysis by the KRYPTOR method
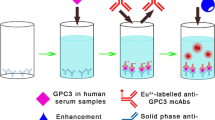
MMP-7 levels were measured on the fully automated B.R.A.H.M.S KRYPTOR® instrument (Thermo Scientific B.R.A.H.M.S GmbH, Hennigsdorf/Berlin, Germany) using an advanced homogeneous sandwich prototype fluoroimmuno-assay (not yet commercialized).
The system is based on the Time Resolved Amplified Cryptate Emission (TRACE®) technology using a non-radiative energy transfer between two fluorophores and detecting the subsequent emission of a specific wavelength energy [17].
The B.R.A.H.M.S MMP-7 KRYPTOR® immunoassay is composed of a goat polyclonal and a mouse monoclonal antihuman MMP-7 antibody, labeled with Europium Cryptate-EuC (Cis Bio International, Bagnols/Cèze, France) and Alexa Fluor 647 (Molecular Probes—Life Technologies, Eugene, USA) as a donor, respectively. Recombinant MMP-7 (R&D Systems Europe) diluted in calf serum (Trina Bioreactives AG, Nänikon, Switzerland) was used as calibrator. Pools of serum samples collected from healthy individuals were used as technical Quality Control samples (QCs).
Measurements were automatically performed by incubating 50 µl of serum sample with 50 µl of each conjugated antibody solution at 37 °C for 29 min. The assay was calibrated with recombinant human MMP-7 protein (R&D Systems, Abingdon, UK). Both ELISA and KRYPTOR assays were performed blinded to the study endpoint. The intra-assay variability for duplicate measurement was 4.4 %, while the inter-assay variability was 5.3 %.
Statistical analysis
The nonparametric two-sided Wilcoxon rank sum test (Mann–Whitney test) was applied for paired group comparisons. Univariable overall survival, disease-specific survival and metastasis-free survival analyses were done using both Kaplan–Meier log-rank test and univariable Cox analysis. For multivariable analysis, the Cox proportional hazards regression model was used. Spearman’s analysis was performed to determine the correlation between the serum MMP-7 levels measured by the two assay methods. To determine the optimal cutoff value with the highest sensitivity and specificity for the detection of RCC and metastasis, we used the nonparametric receiver operating characteristics (ROC) curves. In all tests, a p value of at least 0.05 was considered to be significant. All statistical analyses were done with SPSS software package, version 21.0 (Chicago, IL, USA).
Results
Follow-up characteristics
The results are presented in accordance with the reporting recommendations for tumor marker prognostic studies (REMARK) [18]. Forty-two (57 %) of the 77 patients died during the follow-up period, while 24 (31 %) of them die due to RCC. The median survival time was 110 months for all patients and 185 months for survivors. In nine patients, lymph node or distant metastasis was detected at diagnosis. In 10 further cases, metastatic progression was detected during follow-up. Patients with metastasis at the time of surgery were excluded from the metastasis-free survival analysis.
Correlation between MMP-7 levels generated by the KRYPTOR versus ELISA method
Spearman’s correlation analysis comparing MMP-7 levels measured by the ELISA versus KRYPTOR method revealed a highly significant, linear correlation (R 2 = 0.956, p < 0.001) (Supporting figure 1).
MMP-7 serum levels and clinicopathological parameters
The main characteristics of study population and their correlations with MMP-7 serum levels are shown in Table 1. MMP-7 concentrations were higher in RCC patients than in controls and in high-stage (T2–T4) compared to low-stage tumors (p = 0.034). MMP-7 levels tended to be higher in high-grade (G3) tumors. Also, MMP-7 serum levels were higher in elderly patients both in the RCC and control group. Finally, MMP-7 concentrations were significantly higher in metastatic compared to non-metastatic tumors (table 1, Supporting figure 2).
ROC analysis revealed an optimal cutoff value of 3.15 ng/ml at which the diagnostic specificity was 82 % while the sensitivity was calculated as 70 %. The AUC (area under the curve) value of 0.85 was significantly higher than the reference of 0.50 (p < 0.001) (Supporting figure 3a).
A further ROC analysis was performed to calculate the metastasis detection accuracy of MMP-7. At an optimal cutoff value of 5.05 and 5.50 ng/ml for the KRYPTOR and ELISA method, respectively, the AUCs of 0.76 and 0.71 were significantly higher compared to the reference AUC of 0.50 (p = 0.039 and p = 0.011, respectively). At this cutoff, the sensitivity and specificity values were of 59 and 89 % for KRYPTOR and 62 and 89 % for ELISA (Supporting figure 3b and 3c).
Univariable and multivariable survival analyses
Results of univariable and multivariable analyses and prognostic endpoints (overall survival, disease-specific survival and metastasis-free survival) are listed in Table 2. Patients’ age and gender did not significantly influence overall survival, disease-specific survival or metastasis-free survival (Table 2). Advanced tumor stage (T3–T4) was significantly associated with disease-specific survival but not overall or metastasis-free survival. Tumor grade 3 was associated with overall survival but not disease-specific survival or metastasis-free survival. The presence of metastasis was correlated with poor disease-specific and overall survival (p < 0.001 both). MMP-7 levels above the median (5.0 ng/ml for KRYPTOR and 4.8 ng/ml for ELISA) or as continuous variables proved to be strongly associated with overall survival, disease-specific survival and metastasis-free survival (Table 2; Fig. 1). These correlations remained significant also in multivariable models (Table 2) where MMP-7 proved to be an even stronger prognostic factor as tumor stage, grade or the presence of metastasis.
Kaplan–Meier curves of overall survival (OS), disease-specific survival (DSS) and metastasis-free survival (MFS) stratified by serum MMP-7 concentrations determined by the KRYPTOR and ELISA method. The median values were used as a cutoff (5.0 ng/ml for the KRYPTOR and 4.8 ng/ml for the ELISA analysis). For the analysis of MFS, cases with present metastasis at surgery were excluded
Discussion
The present study confirms the diagnostic and independent prognostic value of MMP-7 in RCC. In addition, we tested a novel, rapid automated immunofluorescent assay for the detection of MMP-7, which was developed to meet the needs of the everyday clinical routine. While the prognostic value of MMP-7 in other urological malignancies has been clearly demonstrated, the two available studies in RCC provided conflicting results. Sarkissian et al. analyzing MMP-7 serum levels in 30 healthy donors, 40 control patients and 30 RCC patients found MMP-7 to be significantly elevated in RCC patients compared to both healthy controls and control patients. In addition, no significant difference in the MMP-7 levels was found between RCC patients with and without metastasis. Based on these, MMP-7 has been suggested as a potential diagnostic marker for RCC [14]. Ramankulov et al. using a commercially available particle-based flow cytometric (Luminex) immunoassay analyzed the circulating MMP-7 concentrations in 97 RCC patients and 50 healthy controls. They found similar MMP-7 levels in controls and patients with non-metastatic RCCs, while MMP-7 serum concentrations were significantly elevated in metastatic (N1M0 or M1) RCCs. Furthermore, high MMP-7 levels were independently associated with shorter disease-specific survival. Based on these, the authors suggested MMP-7 as a marker of metastasis and as an independent prognostic but not as a diagnostic marker [15]. The conflicting data between the two studies were suggested to be related to different blood sampling methods, differences in epitope recognition sites between the two assays and different study design regarding sample/patient selection [19]. Our data are in line with those of Sarkissian et al. regarding the diagnostic relevance of MMP-7 in RCC. However, this finding has to be handled with caution since the sample collection time for patients and controls were largely different and MMP-7 levels are not specific for RCC.
Furthermore, our data in contrast to those of Sarkissian et al. but in line with those of Ramankulov et al. revealed significantly elevated serum levels in metastatic compared to non-metastatic RCC cases. However, a direct comparison of MMP-7 concentrations between our data and those of Ramankulov is not possible. The median values in metastatic RCCs found by Ramankulov et al. are lower as in the present study (3.34 vs. 6.60 ng/ml) [15]. This difference is related to different sampling methods as we recently observed consistently lower MMP-7 levels in plasma samples than in corresponding sera (unpublished data). Our data provided higher sensitivities (89 vs. 76 %) but lower specificities (60 vs. 72 %) for the detection of metastasis as those of Ramankulov. However, in our study, the specificity for metastasis detection might be substantially higher as calculated, since metastatic progression was observed in 7 of 10 RCC patients who had high preoperative MMP-7 levels but were initially diagnosed as non-metastatic disease. It can therefore be assumed that these patients already had (micro) metastases at the time of surgery. This is also underlined by the fact that the metastatic progression in these cases was observed typically within only 24 months. These patients may benefit from early administration of targeted therapies.
In the last years, several promising serum markers have been identified for the prediction of RCC including carbonic anhydrase IX, VEGF, IL-6 and CRP [20–23]. One of the major limitations of using these markers is that they are in developmental stage, since standardized methods and validation in large independent patient cohorts are missing. A further obstacle to the implementation of these markers in daily routine is the lack of automated and flexible methods for their reliable quantitation. Here, we could firmly confirm the independent prognostic relevance of preoperative MMP-7 in an independent RCC cohort. Furthermore, comparing the MMP-7 values determined by the KRYPTOR method with those measured by ELISA, we found excellent correlations. Therefore, we concluded that the KRYPTOR method provides comparable performance to the most commonly used sandwich ELISA. There are, however, two important advantages of the KRYPTOR method compared to ELISA. First, the KRYPTOR platform provides more flexibility regarding variations in sample numbers in a given period as the ELISA method. Second, the KRYPTOR method is fully automated and therefore less sensitive to intra-examiner variability. A prospective, multicentre clinical testing of this assay is proposed to assess its performance in the daily routine. Larger studies should also address the interesting question whether there is a difference in MMP-7 levels between different subtypes of RCC.
In conclusion, this study confirms both the diagnostic and independent prognostic relevance of circulating MMP-7 levels in RCC. Therefore, preoperative MMP-7 analysis may help to identify high-risk RCC patients before surgery and may influence therapy decisions. In addition, we present here, for the first time, an automated immunoassay for the quantitative determination of MMP-7 in RCC. The validation and the development of an MMP-7 assay tailored to the needs of the clinical routine is an important step toward the implementation of MMP-7 as a marker in the daily clinical routine.
References
Cho E, Adami HO, Lindblad P (2011) Epidemiology of renal cell cancer. Hematol Oncol Clin North Am 25:651–655
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127(12):2893–2917
Niedworok C, Dörrenhaus B, Vom Dorp F, Piotrowski JA, Tschirdewahn S, Szarvas T, Rübben H, Schenck M (2015) Renal cell carcinoma and tumour thrombus in the inferior vena cava: clinical outcome of 98 consecutive patients and the prognostic value of preoperative parameters. World J Urol 33(10):1541–1552
Cohen HT, McGovern FJ (2005) Renal-cell carcinoma. N Engl J Med 353(23):2477–2490
Martínez-Fernandez A, García-Albeniz X, Pineda E et al (2009) Serum matrilysin levels predict outcome in curatively resected colorectal cancer patients. Ann Surg Oncol 16:1412–1420
Acar A, Onan A, Coskun U et al (2008) Clinical significance of serum MMP-2 and MMP-7 in patients with ovarian cancer. Med Oncol 25:279–283
Sun DW, Zhang YY, Qi Y, Zhou XT, Lv GY (2015) Prognostic significance of MMP-7 expression in colorectal cancer: a meta-analysis. Cancer Epidemiol 39(2):135–142
Sentani K, Matsuda M, Oue N et al (2014) Clinicopathological significance of MMP-7, laminin γ2 and EGFR expression at the invasive front of gastric carcinoma. Gastric Cancer 17:412–422
Szarvas T, Becker M, vom Dorp F et al (2010) Matrix metalloproteinase-7 as a marker of metastasis and predictor of poor survival in bladder cancer. Cancer Sci 101:1300–1308
Szarvas T, Jäger T, Becker M et al (2011) Validation of circulating MMP-7 level as an independent prognostic marker of poor survival in urinary bladder cancer. Pathol Oncol Res 17:325–332
Szarvas T, Becker M, Vom Dorp F et al (2011) Elevated serum matrix metalloproteinase 7 levels predict poor prognosis after radical prostatectomy. Int J Cancer 128:1486–1492
Miyata Y, Iwata T, Ohba K, Kanda S, Nishikido M, Kanetake H (2006) Expression of matrix metalloproteinase-7 on cancer cells and tissue endothelial cells in renal cell carcinoma: prognostic implications and clinical significance for invasion and metastasis. Clin Cancer Res 12:6998–7003
Lu H, Yang Z, Zhang H, Gan M, Zhou T, Wang S (2013) The expression and clinical significance of matrix metalloproteinase 7 and tissue inhibitor of matrix metalloproteinases 2 in clear cell renal cell carcinoma. Exp Ther Med 5:890–896
Sarkissia G, Fergelot P, Lamy PJ et al (2008) Identification of pro-MMP-7 as a serum marker for renal cell carcinoma by use of proteomic analysis. Clin Chem 54:574–581
Ramankulov A, Lein M, Johannsen M, Schrader M, Miller K, Jung K (2008) Plasma matrix metalloproteinase-7 as a metastatic marker and survival predictor in patients with renal cell carcinomas. Cancer Sci 99:1188–1194
Reuter VE, Davis CJ, Moch H (2004) Oncocytoma. In: Eble JN, Togashi K, Pisani P, Sauter G, Epstein JI, Sesterhenn IA (eds) Pathology and genetics of tumours of the urinary system and male genital organs. World Health Organization Classification of Tumours, vol 6. IARC Press, Lyon, pp 9–87
Mathis G (1993) Rare earth cryptates and homogeneous fluoroimmunoassays with human sera. Clin Chem 39:1953–1959
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2005) Statistics subcommittee of the NCI-EORTC working group on cancer diagnostics: reporting recommendations for tumor MARKer prognostic studies (REMARK). Nat Clin Pract Urol 2:416–422
Jung K, Ramankulov A, Schrader M, Miller K, Lein M (2008) Circulating matrix metalloproteinase-7: an early or metastatic marker for renal cell carcinoma? Clin Chem 54:1927–1929
Bui MH, Seligson D, Han KR et al (2003) Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res 9:802–811
Sabatino M, Kim-Schulze S, Panelli MC et al (2009) Serum vascular endothelial growth factor and fibronectin predict clinical response to high-dose interleukin-2 therapy. J Clin Oncol 27:2645–2652
Negrier S, Perol D, Menetrier-Caux C et al (2004) Interleukin-6, interleukin-10, and vascular endothelial growth factor in metastatic renal cell carcinoma: prognostic value of interleukin-6. J Clin Oncol 22:2371–2378
Hu Q, Gou Y, Sun C et al (2014) The prognostic value of C-reactive protein in renal cell carcinoma: a systematic review and meta-analysis. Urol Oncol 32(1):50.e1–8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supporting figure 1
Cross-plots and linear regression analysis of MMP-7 values measured by the ELISA and KRYPTOR method. A significant correlation with a high Spearman’s correlation coefficient (R2 = 0.956) was found between the two analytical methods (n = 77). (TIFF 4031 kb)
Supporting figure 2
MMP-7 serum concentrations in RCC patients and controls. MMP-7 serum levels are significantly higher in patients than in controls (P < 0.001). The highest serum MMP-7 levels are found in patients with metastatic RCC. (TIFF 5707 kb)
Supporting figure 3
a) Receiver operating characteristics (ROC) analysis of MMP-7 serum concentrations for the detection of RCC (AUC = 0.851) b-c) ROC analysis of MMP-7 serum concentrations measured by the KRYPTOR (AUC = 0.762) and the ELISA method (AUC = 0.713) for the detection of metastasis in RCC. (TIFF 4059 kb)
Rights and permissions
About this article
Cite this article
Niedworok, C., vom Dorp, F., Tschirdewahn, S. et al. Validation of the diagnostic and prognostic relevance of serum MMP-7 levels in renal cell cancer by using a novel automated fluorescent immunoassay method. Int Urol Nephrol 48, 355–361 (2016). https://doi.org/10.1007/s11255-015-1185-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-015-1185-8