Abstract
Objective
The presence of antibodies against the M-type phospholipase A2 receptor (PLA2RAb) is considered to be a promising serological diagnostic biomarker of idiopathic membranous nephropathy (IMN). We compare the change in serum Cystain C (CysC), urea, creatinine (CREA), uric acid (UA), total protein (TP), albumin (ALB), IgG4 and 24-h urinary protein (proteinuria, PRO) between PLA2RAb+ and PLA2RAb− of IMN patients.
Materials and methods
The serum and urine samples were collected from 120 patients with IMN. The presence of circulating PLA2RAb was determined by indirect immunofluorescence, and their titer was quantified by ELISA. CysC, urea, CREA, UA, TP, ALB and 24-h PRO were examined by automatic biochemical analyzer.
Results
Serum IgG4 level was determined by specific protein analyzer. PLA2RAb-positive percentage by ELISA was higher than that by IIF, but no significant difference was found by McNemar’s Chi-square test. Serum IgG4 level and 24-h PRO level were significantly higher in PLA2RAb+ than in PLA2RAb− (P < 0.05). In PLA2RAb+ group, PLA2RAb is positively related to serum IgG4 and 24-h PRO and negatively related to serum TP and ALB (P < 0.01).
Conclusion
This is the first study to show that combined detection of IgG4 concentration and PLA2RAb was usable for IMN patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic membranous nephropathy (IMN) is a common cause of nephrotic syndrome in adults and has been identified as an autoimmune-mediated disease [1]. A number of studies have shown that 52–82 % of IMN sera have autoantibodies directed toward the M-type phospholipase A2 receptor [2, 3], a 180-kDa protein that is expressed by alveolar epithelial cells and neutrophils but is mainly restricted to podocytes within the kidney. The presence of antibodies against the M-type phospholipase A2 receptor (PLA2RAb) is considered to be a promising serological diagnostic biomarker of idiopathic membranous nephropathy (IMN). Anti-PLA2R autoantibody is detected mainly by Western blot (WB), indirect immunofluorescence (IIF), enzyme-linked immunosorbent assay (ELISA) and so on. In the initial publication, Beck et al. [1] used a Western blot after non-reducing SDS–PAGE. Despite unconfirmed descriptions about an improved variant of the method with a sensitivity of >90 % [4], it is difficult to use WB outside of an expert laboratory, and the method is not suited for the evaluation of large sample numbers. These limitations led to the development of a recombinant cell-based indirect immunofluorescence assay (RC-IFA) that uses the human cell line HEK293 over-expressing the full-length human PLA2R1 as substrate [5]. The assay can be used both as a diagnostic tool and for the approximation of antibody concentrations during monitoring of patients. More recently, an ELISA protocol was published that uses a recombinantly produced extracellular domain of PLA2R1 to form the solid phase [6]. The anti-PLA2R in serum samples from IMN were primarily IgG4 subclass [7].
More and more studies showed that PLA2RAb could play an important role in IMN. However, controversy remains about the diagnostic accuracy of serum PLA2RAb testing. So we would test PLA2RA by IIF and ELISA and then analyze their sensitivity. Renal function-related index could be abnormal in IMN patients. However, there were no associated reports on whether renal function index was the difference between PLA2RAb+ and PLA2RAb− in patients with IMN. No study about the serum IgG4 concentration has been described between PLA2RAb+ and PLA2RAb− in patients with IMN. Therefore, we compare biomarkers, such as serum Cystain C (CysC), urea, creatinine (CREA), uric acid (UA), total protein (TP), albumin (ALB), IgG4 and 24-h urinary protein (proteinuria, PRO), between PLA2RAb+ and PLA2RAb− of IMN patients, in order to seek the association with PLA2RAb level and biomarkers, which would try to find a potential way to elucidate the functional mechanism of PLA2RAb.
Materials and methods
Patients and samples
Inclusion criteria for participating in this clinical study were histologic diagnosis of idiopathic MN and no immunosuppressive therapy prior to inclusion in the study. Secondary MN led to the exclusion of patients from the study. Patients were screened by serological tests for systemic lupus erythematosus, hepatitis, a detailed medical history and a screening for malignancies depending on the age of and risk factors for the patient. Patient serum samples were collected from the Affiliated Hospital of Jiujiang University. Following are clinical and socio-demographic characteristics of the study population: 135 cases approached, 8 cases refused participation and 7 cases were excluded because of immunosuppressive therapy. The study included sera from 120 IMN patients, and the majority of patients in our study cohort have nephrotic range proteinuria and are without immunosuppressive treatment, constituted the IMN study population (mean age 45 ± 11 years, 45 females and 75 males). Table 1 shows clinical and socio-demographic characteristics of the study for PLA2RAb. Blood from an antecubital vein was taken into a serum tube and serum was separated within 2 h for PLA2RAb, CysC, urea, CREA, UA, TP, ALB and IgG4 level test, and at the same time, 24-h urinary protein was collected for examination. The study was approved by our hospital ethics committee. Patients’ informed consent for the usage of sera was obtained prior to sample collection.
Detection of PLA2RAb by IFA Mosaic
The detection of circulating PLA2RAb was performed using an IFA Mosaic (Euroimmun, Germany). The presence of PLA2RAb was detected by conventional indirect IIF on 5-μm ‘biochip’ sections of a composite substrate containing human embryonic kidney (HEK)293 cells, which were transiently transfected with full-length complementary DNA encoding a PLA2R1, and non-transfected HEK293cells, using revealing reagent an anti-total human IgG–fluorescein isothiocyanate conjugate (Euroimmun, Germany), as previously described [5]. Briefly, serum samples were diluted from a dilution 1:10 in PBS–Tween. Thirty microliters of diluted serum was applied to each reaction field and incubated 30 min at room temperature (18–25 °C). The slides were rinsed with a flush of PBS–Tween using a beaker and immediately immersed in a cuvette containing PBS–Tween for at least 5 min. Then, 25 μL of fluorescein-labeled anti-human globulin was applied to each reaction field incubating for 30 min. After washing as above, they were placed onto a cover glass. Slides were observed using a fluorescence microscope (Olympus BX-51). The optimum cutoff titer was set at 1:10. The positivity should be judged by multiple researchers in blind manners, and the rate of reproducibility is above 95 %.
ELISA for PLA2RAb
ELISA (Euroimmun, Germany) for the detection of IgG-specific isotypes of PLA2RAb. The assay was performed according to the instruction. Calibrators (2, 20, 100, 500 and 1500 RU/mL), positive and negative controls, and diluted 1:100 patient sera were transferred into the individual microplate well, respectively; all samples were measured in duplicate, and then incubated for 30 min followed by washing three times with washing buffer. Bound antibodies were detected by incubating with anti-human-IgG–HRP conjugate (Euroimmun, Germany), diluting 1:1000 in sample buffer for 30 min, subsequent washing as described above and adding TMB substrate (Euroimmun) for 15 min. All incubation steps were carried out at room temperature. The optical density (OD) was read at 450 nm within 30 min after adding the stop solution using an automated spectrophotometer (Kehua, Shanghai, China). Optimum cutoff value for diagnostic use was set at 15 relative units (RU/mL).
Methods for biomarkers determination
The levels of serum CysC, urea, CREA, UA, TP, ALB, 24-h PRO were quantified using automatic biochemical analyzer (AU5800, Beckman). Serum IgG4 level was determined by specific protein analyzer (IMMGE800, Beckman).
Statistical analysis
Statistical analysis of the results was carried out by using McNemar’s Chi-square test, independent t test and Pearson’s correlation analysis with SPSS17.0 software. P values < 0.05 were considered significant.
Results
Examination of PLA2RAb
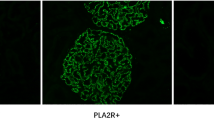
PLA2RAb pattern by immunofluorescence
Circulating PLA2R antibodies were detected by using an IFA Mosaic. The figure shows a biochip coated with HEK293 cells expressing the PLA2R protein on the surface. A specific cytoplasmic fluorescence of the transfected cells can be seen at a dilution of 1:10 (A). No cytoplasmic specific fluorescence can be detected at non-transfected HEK293 cells (B) (Fig. 1).
Determination of PLA2RAb
Among 120 IMN patients’ sera, 89 PLA2RAb+ and 34 PLA2RAb− were tested by ELISA and 87 PLA2RAb+ and 36 PLA2RAb− were tested by IIF. The diagnosis sensitivity of PLA2RAb was 71.67 % (86/120) by ELISA and 70.0 % (84/120) by IIF, respectively. PLA2RAb-positive percentage by ELISA was higher than that by IIF, but no significant difference was found by McNemar’s Chi-square test (Table 2).
Comparison of biomarkers between two groups
No significant difference was found in the concentrations of CysC, urea, CREA, UA, TP, ALB (P > 0.05) between PLA2RAb+ and PLA2RAb−. But serum IgG4 level and 24-h PRO level were significantly higher in PLA2RAb+ than in PLA2RAb− (P < 0.05) (Table 3).
Correlation analysis
In PLA2RAb+ group, there were no significant correlation between PLA2RAb level and the concentrations of CysC, urea, CREA, UA, TP, ALB (P > 0.05); PLA2RAb is positively related to serum IgG4 and 24-h PRO and negatively related to TP and ALB (P < 0.01) (Table 4).
Discussion
Most cases of primary membranous nephropathy (MN), a relatively common cause of nephrotic syndrome in adults, were considered idiopathic. We now recognize that MN is an organ-specific autoimmune disease, in which circulating autoantibodies bind to an intrinsic antigen on glomerular podocytes and form deposits of immune complexes in situ in the glomerular capillary walls [8]. Recent studies showed that membranous nephropathy (MN) is the leading cause of nephrotic syndrome in adults. PLA2RAb is found in most patients with IMN worldwide, which facilitate a better understanding of IMN pathogenesis and may provide a new tool to its diagnosis, differential diagnosis, risk evaluation, response monitoring and patient-specific treatment [9]. Positive rate of anti-phospholipase A2 receptor antibodies varies according to various geographic and ethnic groups [10, 11]. Detection of PLA2RAb can be used in serological diagnosis of idiopathic membranous nephropathy (IMN), but there are limited data about the sensitivity and specificity of its diagnostic values [12]. PLA2RAb has been identified as one of the target antigens of the autoimmune response in IMN. The prevalence of anti-PLA2R antibodies in patients with IMN is around 70 %, but this varies in accordance with geographic region, and until present, anti-PLA2R has not been shown to be associated with any particular clinical profile of the disease. PLA2RAb were found in Japanese patients with IMN. The recent identification of circulating autoantibodies directed toward the PLA2R has been a major advancement in the serological diagnosis of idiopathic membranous nephropathy (IMN) [13]. Patients with high PLA2RAb levels achieved remission of proteinuria significantly later than patients with low PLA2R antibody levels. PLA2R antibody levels fell over time in patients with spontaneous remission but remained elevated in patients who did not show a reduction in proteinuria [14, 15]. Our data confirmed that a decrease in PLA2R antibody level is associated with a decrease in proteinuria in patients with primary MN.
In the present study, firstly, we investigated the correlation and agreement between two different immunoassays for the detection of anti-PLA2R antibodies in IMN patients. This is the first report that it had good sensitivity between ELISA and IFA for the detection of anti-PLA2R antibodies. The diagnosis sensitivity of PLA2RAb was 71.67 % (86/120) by ELISA and 70.0 % (84/120) by IIF, respectively. PLA2RAb-positive percentage by ELISA was higher than that by IIF, but no significant difference was found by McNemar’s Chi-square test. Additionally, IIF is semiquantitative, and quantitative ELISA would have an advantage because they provide a more accurate reflection of changes in the antibody titers. Secondly, we found no significant difference in the concentrations of CysC, urea, CREA, UA, TP, ALB (P > 0.05) between PLA2RAb+ and PLA2RAb−. But serum IgG4 level and 24-h PRO level were significantly higher in PLA2RAb+ than in PLA2RAb− (P < 0.05). Thirdly, in PLA2RAb+ group, there was no significant correlation between PLA2RAb level and the concentrations of CysC, urea, CREA, UA, TP, ALB (P > 0.05), and PLA2RAb is positively related to serum IgG4 and 24-h PRO and negatively related to TP and ALB, which suggested that PLA2RAb is strongly associated with serum IgG4 and 24-h PRO.
To sum up, we confirmed that PLA2RAb was significantly associated with 24-h PRO and indicated that serum PLA2RAb concentration was related to serum IgG4 level. Combined detection of PLA2RAb, serum IgG4 and 24-h PRO would improve diagnosis accuracy of IMN.
References
Beck LH Jr, Bonegio RG, Lambeau G et al (2009) M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361(1):11–21
Hofstra JM, Wetzels JF (2012) Anti-PLA2R antibodies in membranous nephropathy: ready for routine clinical practice? Neth J Med 70(3):109–113
Svobodova B, Honsova E, Ronco P et al (2013) Kidney biopsy is a sensitive tool for retrospective diagnosis of PLA2R-related membranous nephropathy. Nephrol Dial Transplant 28(7):1839–1844
Qin W, Beck LH Jr, Zeng C et al (2011) Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol 22:1137–1143
Hoxha E, Harendza S, Zahner G et al (2011) An immunofluorescence test for phospholipase-A2-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant 26(8):2526–2532
Hofstra JM, Debiec H, Short CD et al (2012) Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol 23(10):1735–1743
Wang C, Lu H, Yang C et al (2014) Progress on the M-type phospholipase A2 receptor in idiopathic membranous nephropathy. Chin Med J 127(10):1960–1963 (Engl)
Beck LH Jr, Salant DJ (2014) Membranous nephropathy: from models to man. J Clin Investig 124(6):2307–2314
Wang C, Lu H, Yang C et al (2014) Progress on the M-type phospholipase A2 receptor in idiopathic membranous nephropathy. Chin Med J 127(10):1960–1963 (Engl)
Akiyama S, Akiyama M, Imai E et al. (2014). Prevalence of anti-phospholipase A2 receptor antibodies in Japanese patients with membranous nephropathy. Clin Exp Nephrol. [Epub ahead of print]
Segarra-Medrano A, Jatem-Escalante E, Quiles-Pérez MT et al (2014) Prevalence, diagnostic value and clinical characteristics associated with the presence of circulating levels and renal deposits of antibodies against the M-type phospholipase A2 receptor in idiopathic membranous nephropathy. Nefrologia 34(3):353–359
Hu SL, Wang D, Gou WJ et al (2014) Diagnostic value of phospholipase A2 receptor in idiopathic membranous nephropathy: a systematic review and meta-analysis. J Nephrol 27(2):111–116
Behnert A, Schiffer M, Müller-Deile J et al (2014) Antiphospholipase A2 receptor autoantibodies: a comparison of three different immunoassays for the diagnosis of idiopathic membranous nephropathy. J Immunol Res 2014:143274
Hladunewich MA, Cattran D, Beck LH et al (2014) A pilot study to determine the dose and effectiveness of adrenocorticotrophic hormone (H.P. Acthar® Gel) in nephrotic syndrome due to idiopathic membranous nephropathy. Nephrol Dial Transplant 29(8):1570–1577
Hoxha E, Thiele I, Zahner G et al (2014) Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrol 25(6):1357–1366
Acknowledgments
The project received the financial support from the Bureau of Public Health of Jiangxi Province (20143246) and The Education Department Youth Fund of Jiangxi Province IGJJ12625).
Conflict of interests
There is no conflict of interest in this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xun, C., shuai, L., Wang, W. et al. Comparison of biomarkers between PLA2RAb+ and PLA2RAb− in patients with idiopathic membranous nephropathy. Int Urol Nephrol 47, 831–835 (2015). https://doi.org/10.1007/s11255-015-0956-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-015-0956-6