Abstract
Purpose
To identify mixed acid–base disorders, clinicians must estimate the value of partial pressure of carbonic dioxide (pCO2), complying with the reduced plasma bicarbonate concentration (HCO3). What is the most appropriate equation relating the two quantities in chronic hemodialysis patients remains unknown. Chronic hemodialysis patients remains unknown, which motivates our study.
Methods
Among a large database of blood gas analysis from chronic hemodialysis patients, we selected 291 blood samples showing HCO3 < 24 mmol/L and, among these, we further selected a subset of samples claimed for pure metabolic acidosis. A linear approximation based upon the least-square criterion was adopted to derive the best-fit equation. The differences between this and other commonly used formulas were computed in terms of root mean square (RMS) errors.
Results
In chronic hemodialysis patients, the reduction in pCO2 due to metabolic acidosis is better predicted multiplying by 1.2 the reduction in HCO3, or by using the expression pCO2 = HCO3 + 15; the two approaches lead to almost the same results. In contrast, the equation pCO2 = 1.5 × HCO3 + 8, known as Winters’ formula, exhibits larger errors.
Conclusions
The easy-to-use expression pCO2 = HCO3 + 15 seems suitable for the daily clinical practice in hemodialysis patients. However, if HCO3 value is lower than 12 mmol/L, a threshold at which different formulas return almost the same value, also Winters’ formula, derived in the 60’s from patients with low values of HCO3, could be used.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic acidosis is the more frequent acid–base derangement in hemodialysis patients as a consequence of kidney failure [1, 2]. However, mixed acid–base disorders are not a rare occurrence because respiratory diseases can complicate the clinical picture of this frail population. To identify the presence of additional acid–base disorder, clinicians must evaluate the physiologic respiratory response to metabolic acidosis or, in other words, they must estimate the value of partial pressure of carbonic dioxide (pCO2) complying with the reduced plasma bicarbonate concentration (HCO3). If the measured pCO2 value is larger or smaller than the “expected” one, then mixed disorder occurs. Incorrect calculations could lead to wrong diagnosis and inappropriate treatment, but a single and widely accepted formula to compute the pCO2 in the presence of metabolic acidosis seems not available in the literature. The most frequently used are: pCO2 = 1.5 × HCO3 + 8—known as Winters’ formula [3–5]—and the common practical rule that reads “the reduction in pCO2 with respect to its normal value equals 1.2 multiplied by the reduction in HCO3 with respect to its normal value” [6–9]. A rarely used alternative expression is pCO2 = HCO3 + 15 [4, 5, 9]. These formulas have been validated in simple acid–base disorders, but not in double and triple disorders [8] that often occur in hemodialysis patients featured by various comorbidities. Moreover, in this scenario, a specific HCO3 value may be due to more than one simple acid–base disorder, i.e., metabolic acidosis or respiratory alkalosis, as well as by several disorders simultaneously occurring. This notwithstanding, in daily clinical practice, clinicians have to predict the expected value of CO2 in order to identify acute illness overlapping chronic kidney disease. What is the most appropriate equation to be applied to chronic hemodialysis patients is unknown, and this motivates our analysis.
Materials and methods
Patients and data collection
The present study is based on the database of arterial blood gas analysis samples (henceforth, simply “samples”) collected at the hemodialysis unit of “Maria Rosaria” Clinic, Pompeii, Italy, over the period from January 2008 to December 2012. In this database, samples were labeled as “arterial” if the oxyhemoglobin saturation was equal to that displayed by a digital pulse oximeter placed on the hand without vascular access, or if the oxyhemoglobin saturation was larger than or equal to 97 %.
We have selected the samples drawn by the arteriovenous fistula needle, just before the treatment of patients on bicarbonate hemodialysis scheduled at rate of three sessions per week, and only patients with at least one available sample per year have been included in the analysis. To avoid the inclusion of patients with acute illness, samples withdrawn during the first six months of dialysis vintage, and those ones obtained during hospital admission, have been discarded, thus yielding a dataset of 339 samples taken from 30 patients, see Table 1. From each sample the values of pH, HCO3 and pCO2, were obtained. Note that the gas analyzer computes the value of the HCO3 by the measured values of pCO2 and pH. Among this dataset, we selected 291 blood samples showing HCO3 < 24 mmol/L. This cutoff value was chosen to compare our results with those reported in the literature [6, 7]. A further analysis was carried out in the subgroup of blood samples featuring pH < 7.38, HCO3 < 22 mmol/L and pCO2 < 38 mmHg, claimed for pure metabolic acidosis according to widely accepted criteria [10]. It is worth mentioning that the availability of a gas analyzer (OMNI S-4, Roche Diagnostic) at the hemodialysis unit made it possible to process the collected samples within few minutes from the withdrawn, thus ensuring the reliability of the measured values.
Statistical analysis
Preliminary, we check the Gaussianity of the datasets by resorting to a standard Chi-square goodness-of-fit test. Then, we focus on the following quantities: mean value, standard deviation (SD), median value, quartiles and percentiles. To obtain mathematical models relating sets of data, we adopt the linear approximation method based upon the least-square criterion. Accordingly, the differences between the prediction of the model and the data are computed in terms of root mean square (RMS) errors.
Results
As shown in Table 1, our dataset consists of aged patients. Thanks to the aforementioned criteria for selecting the samples, our study involves patients with less severe comorbidities and, as a consequence, with long staying in dialysis. Acid–base parameters (see Table 2) showed numbers consistent with compensated mild metabolic acidosis. HCO3 values are typical of modern dialysis population with mean value not so far from the normal value. Values of pCO2 are slightly reduced as a result of respiratory compensation. These values agree with compensated mild metabolic acidosis and, alone or superimposed, respiratory alkalosis. However, inspection of individual data suggests that all the four acid–base disorders could be present.
In this dataset, the relationship between pCO2 and HCO3 as linear best-fit equation was found in the form: pCO2 = 1.13 × HCO3 + 11.96, with RMS error amounting to 3.07 mmHg (see Table 3, left column). As shown in Table 4 (left column), Winters’ formula applied to these data yields an RMS error of 4.85 mmHg. With regard to the common practical rule, if 40 mmHg and 24 mmol/L are the normal values of pCO2 and of HCO3, respectively, then such practical rule reads: pCO2 = 40 − [24 − HCO3] × 1.2, which can be equivalently rewritten as pCO2 = 1.2 × HCO3 + 11.2. The RMS error pertaining to this expression is 3.14 mmHg. Finally, if we use the expression pCO2 = HCO3 + 15, then the RMS error amounts to 3.09 mmHg.
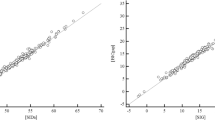
At this point, we focus our analysis to the narrowed dataset consisting of samples for which pH < 7.38, HCO3 < 22 mmol/L and pCO2 < 38 mmHg, which is the typical picture of metabolic acidosis. We found 65 samples from 21 patients matching these criteria. In this group of samples, the mean value (±SD) of pH is 7.353 (±0.02), that of HCO3 is 19.1 (±1.3) mmol/L, with values ranging from 15.1 to 21.2 mmol/L. Finally, the mean value of the pCO2 value is 35.0 ± 2.1 mmHg. The statistical correlation between HCO3 and pCO2 is relevant (Pearson’s r coefficient is 0.82), and the best-fit linear equation is found as: pCO2 = 1.28 HCO3 + 10.57 mmHg (see Table 3, right column, and Fig. 1). The RMS error pertaining to this formula is 1.20 mmHg. By applying Winters’ formula, the “common practical rule” and the “alternative formula” yield RMS errors amounting to 2.06, 1.50 and 1.56 mmHg, respectively (see Table 4, right column).
Discussion
Metabolic acidosis is the most common acid–base disorder in hemodialysis patients, due to kidney failure. Secondary ventilatory response occurs to compensate reduction in HCO3 yielding a reduction in pCO2 [8]. However, in this population, different acid–base disorders can arise as isolated disturbance or as mixed disorders, thus affecting the pCO2 value. This notwithstanding, in the complex and heterogeneous scenario of daily clinical practice, clinicians, facing with low HCO3 value, try to predict the expected value of CO2 in order to identify acute illness overlapping chronic kidney disease. Acute respiratory disorders can be life threatening and could require rapid actions, often bedside. Therefore, the availability of reliable and easy-to-use equations is essential. However, a single and widely accepted formula seems not available in the literature. Despite its popularity, Winters’ formula [3–5] seems not suitable to infer mixed acid–base disorders in the clinical context of hemodialysis units because of large RMS error (see Table 4, left column) even in the case of pure metabolic acidosis (see Table 4, right column). This is not an unexpected finding since Winters’ formula was derived by a patient population of the 60’s, with a mean value of HCO3 of 9.9 mmol/L, while modern hemodialysis population [2], and also the patients under investigation (see Table 2), show a mean value of HCO3 which is approximately the double of that.
The common practical rule “the reduction in pCO2 equals 1.2 multiplied by the reduction in HCO3” and its equivalent form pCO2 = 40 − [24 − HCO3] × 1.2 are widely used [6–9]. However, we would like to stress that, despite labeled as practical, it requires some calculation and, above all, while there is general agreement on the normal value of pCO2, the same is not true for that of HCO3. At any rate, the common practical rule always exhibits a smaller RMS error than that obtained with Winters’ formula (see Table 4) and therefore should be preferred in this population. The aforementioned formulas might seem conflicting with each other, but it is not necessarily so. In fact, as pointed out by Bushinsky et al. [6], the relationship between pCO2 and HCO3 is curvilinear with a slope of 1.5 (the same of Winters’ formula) up to a value of HCO3 of 10 mmol/L, and with a slope close to 1.2 (which resembles both the practical rule and our best-fit equations) for larger values of HCO3. Accordingly, the appropriateness of the formulas depends upon the range which the value of HCO3 belongs to, or in other words, no linear approximation may be appropriate for all possible values of HCO3.
Surprisingly, the little-known and rarely used equation pCO2 = HCO3 + 15, also found in some textbook, applied to our data showed almost the same (low) error of the common practical rule (see Table 4). It also has the evident advantage of simplicity, and hence has to be preferred in modern chronic hemodialysis patients.
However, also in this population a quarter of samples exhibits HCO3 values <20 mmol/L (see Table 2), and in daily clinical practice more severe metabolic acidosis can also occur. By setting HCO3 = 12 mmol/L, all formulas yield almost the same value of pCO2. Thus, a possible recommendation for bedside acid–base interpretation could be to use the simpler expression pCO2 = HCO3 + 15 when the value of HCO3 is above 12 mmol/L, and to resort to Winters’ formula for lower values of HCO3, as already suggested elsewhere [11]. The results of the present study are certainly biased by the retrospective and single-center nature of the investigation, implying that a validation in terms of a larger, prospective, and multi-center data collection would be certainly desirable. This represents part of our ongoing research.
References
Oh MS, Uribarri J, Weinstein J et al (2004) What unique acid-base considerations exist in dialysis patients? Semin Dial 17(5):351–364
Vashistha T, Kalantar-Zadeh K, Molnar MZ, Torlén K, Mehrotra R (2013) Dialysis modality and correction of uremic metabolic acidosis: relationship with all-cause and cause-specific mortality. Clin J Am Soc Nephrol 8(2):254–264
Albert MS, Dell RB, Winters RW (1967) Quantitative displacement of acid-base equilibrium in metabolic acidosis. Ann Intern Med 66(2):312–322
Du Bose TD Jr (2008) Acid-base disorders. In: Brenner BM (ed) Brenner and Rector’s the Kidney, 8th edn. Saunders, Philadelphia, pp 505–546
Du Bose TD Jr (2011) Acidosis and alcalosis. In: Longo DL, Fauci A, Kasper D, Mauser S, Jameson JL, Loscalzo J (eds) Harrison’s principles of internal medicine, 18th edn. McGraw Hill, New York, pp 363–373
Bushinsky DA, Coe FL, Katzenberg C, Szidon JP, Parks JH (1982) Arterial pCO2 in chronic metabolic acidosis. Kidney Int 22(3):311–314
Gennari FJ (2008) Acid-base homeostasis in dialysis. In: Nissenson AR, Fine RN (eds) Handbook of dialysis therapy. Saunders, Philadelphia, pp 673–684
Adrogué HJ, Madias NE (2010) Secondary responses to altered acid-base status: the rules of engagement. J Am Soc Nephrol 21(6):920–923
Palmer BF, Alpern RJ (2010) Metabolic acidosis. In: Floege J, Johnson RJ, Feehally J (eds) Comprehensive clinical nephrology, 4th edn. Saunders, St.Louis Missouri, pp 155–166
Berend K, de Vries AP, Gans RO (2014) Physiological approach to assessment of acid-base disturbances. N Engl J Med 371(15):1434–1445
Marano M (2014) On the use of Winters’ formula in chronic metabolic acidosis. Rev Psiquiatr Salud Ment. doi:10.1016/j.rpsm.2014.07.006
Acknowledgments
The authors declare no grant or other funding.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marano, M., D’Amato, A. & Marano, S. A very simple formula to compute pCO2 in hemodialysis patients. Int Urol Nephrol 47, 691–694 (2015). https://doi.org/10.1007/s11255-015-0913-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-015-0913-4