Abstract
Purpose
To clarify the association between clinically defined simple stress urinary incontinence (SUI) symptoms and urodynamic SUI, we examined the relationship between Valsalva leak point pressure (VLPP) as measured by the Q-tip test and Stamey grade in simple female SUI.
Methods
Two hundred grade I or II female SUI patients with SUI symptom were examined by reviewing medical history; physical examination; urethral mobility as assessed by Q-tip test; stress test; and cystometry, including VLPP measurement. On the basis of the VLPP, patients were classified into urethral hypermobility [UH, subdivided into anatomical incontinence (AI) and equivocal incontinence (EI)] or intrinsic sphincter deficiency groups for analysis of the relationship between VLPP and Stamey grade and Q-tip angle.
Results
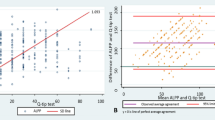
Seventy-eight patients were included, and the mean patient age was 54 ± 7.5 years, mean SUI symptom duration 2.8 years (range 0.5–6 years), mean VLPP 103.6 ± 18.4 cm H2O, and mean Q-tip angle 28.6° ± 7.2°. Fifty-three patients were categorized as Stamey grade I, 25 as Stamey grade II, 51 as AI, and 27 as EI. VLPP was found to be negatively correlated with Q-tip angle (Rs = −0.798, Y = −0.313X + 60.95, P < 0.001), and classifications of VLPP and Stamey grade have positive correlation (χ 2 = 4.9130, P = 0.0267).
Conclusions
In simple female SUI, VLPP is associated with the Q-tip angle and Stamey grade, which may help to reduce some of urodynamic items.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stress urinary incontinence (SUI) is defined by the International Continence Society (ICS) as involuntary leakage of urine with effort, exertion, sneezing, or coughing [1]. SUI is a common problem in women and has a profound negative effect on quality of life as well as a negative impact on society [2]. Female SUI has been attributed to 2 factors: urethral hypermobility (UH) and intrinsic sphincter deficiency (ISD) [3]. Although UH can typically be well treated by various surgical procedures that attempt to reposition and support the urethral sphincter [4], ISD generally cannot [5].
Urodynamics (UDS) is a popular means of evaluating SUI. Several studies have found that preoperative UDS improves objective and subjective surgical outcomes in SUI treatment focusing on performance of mid-urethral sling procedures [6, 7]. Preoperative UDS is believed to be particularly important and is thus recommended in women with SUI. However, other studies concluded that basic preoperative outpatient evaluation comprises a sufficient evaluation for women with SUI, having found that the incontinence surgery outcomes of women who had not undergone preoperative UDS were not inferior to those of women who had undergone preoperative urodynamic testing [8]. As the utility of UDS in SUI has continued to be debated over the years, urological researchers have attempted to identify associations between clinical factors and UDS parameters that may help clarify the clinical importance of UDS. Several studies that identified the clinical factors associated with ISD in women found that Stamey symptom grade was the only clinical factor predictive of ISD, with higher SUI grade found to be associated with higher risk of ISD [9, 10] and higher probability of low Valsalva leak point pressure (VLPP) [11].
However, to our knowledge, few studies have investigated the clinical factors associated with uncomplicated UH, a simple SUI defined as a VLPP of >60 cm H2O that can be well treated by mid-urethral sling surgery [4]. Such a research gap is troubling, as identification of the clinical factors could improve primary evaluation of simple SUI, and possibly outcome, as well as reduce the need to conduct several invasive tests. In addition, the reliability of basing prediction of urodynamic SUI on evaluation of clinically defined simple SUI symptoms and signs remains equivocal. To further check this troubling topic and improve prediction, we aimed to examine the association between clinically defined simple SUI symptoms and urodynamic SUI by evaluating the relationship between clinical factors, namely the Q-tip test angle, Stamey grade, and VLPP in female SUI patients.
Patients and methods
Subject selection
Two hundred female patients with proven SUI as assessed by clinical and urodynamic study in the outpatient clinics of our hospital from November 2012 to September 2013 served as the subjects of this study. The parameters of evaluation were identified or recorded by comprehensive retrospective review of medical history: patients complaints, physical and neurological examination, urine analysis, analysis of urethral mobility by Q-tip test [12], stress test, and cystometry, which included measurement of VLPP. Pelvic organ prolapse (POP) was assessed at rest in the dorsolithotomy position and during straining using the POP quantification system (POP-Q) [13]. The simple SUI was with (inclusion) (1) involuntary leakage of urine with effort, exertion, sneezing, or coughing, and (2) VLPP >60 cm H2O; and without (exclusion): (1) overactive bladder symptoms; (2) categorization as Stamey grade III, an indication of urinary incontinence resulting from very little or no intravesical pressure increase upon activities such as sitting, standing, slowly moving, and/or bending over; (3) previous urethral surgery, retropubic surgery, or anterior colporrhaphy; (4) history of urinary retention with a residual urine level over 200 mL/s; (5) active urinary tract infection or other urological disease; (6) use of drug treatment that could affect bladder function and urethral function; (7) use of alpha-adrenergic receptor agonists or antagonists; and/or (8) presence of any possible cause of neurogenic bladder; and (9) other lower urinary tract dysfunction.
Stamey grade assessment
The patients were classified according to urine leakage symptom resulting from different level of the increase in intravesical pressure by the Stamey grade. The Stamey (clinical symptom) grade was assessed subjectively according to patients’ descriptions of their experience and objectively by test findings, doing the movement to increase in intravesical pressure [14]. Grade I was defined as urinary incontinence resulting from an increase in intravesical pressure to a high level from everyday activities such as coughing, sneezing, and running; grade II as urinary incontinence resulting from an increase in intravesical pressure to a median level during daily activities such as fast walking and climbing stairs; and grade III, which was not examined in this study, as urinary incontinence resulting from a very slight increase in intravesical pressure from activities such as sitting, standing, slowly moving, and bending over.
Q-tip testing
The Q-tip test [12] was conducted by 2 highly experienced specialists in urology. Patients were examined with an empty bladder while in the dorsolithotomy position on a standard gynecological table. The Q-tip test was performed by placing a lubricated cotton swab transurethrally and then withdrawing it until resistance was experienced. A goniometer was used for measurement of the Q-tip angle along the horizontal plane.
Urodynamic testing
Multichannel UDS investigations were performed using the DUET Logic Urodynamic Instrument (Medtronic, Skovlunde, Denmark) in accordance with ICS protocol [15]. Symptoms and urodynamic parameters were recorded using standardized ICS terminology [3]. The urethral pressure profiles (UPPs) were performed after nonintubated uroflowmetry and prior to the filling cystometry in accordance with a standardized UPP protocol that conformed to the ICS definition of urethral pressure “as the fluid pressure needed to just open a closed (collapsed) urethra” as previous description [16]. Both the UPP and filling cystometry studies were performed with subjects in the supine position using a normal sterile saline solution (0.9 % NaCl, Industry Group, Sichuan Province, China). Filling cystometry was performed with an infusion rate of 50 mL/min with the subject using the same catheter and urodynamic system, but without continued perfused urethral pressure measuring system. VLPP was measured by increasing the total infused volume of saline to 200 mL while the patient was seated and then asking the patient to perform a Valsalva maneuver until urine loss could be directly observed. The initial VLPP measurement was recorded before the measurement was repeated to verify the initial finding. If urine leakage was not observed with the Valsalva maneuver, the patient was asked to cough. On the basis of the VLPP results, the patients were classified into 1 of 2 groups [17]: (1) UH group, comprised of patients with a VLPP >60 cm H2O and divided into 2 subgroups, the anatomical incontinence (AI) with a VLPP >90 cm H2O and the equivocal incontinence (EI) with a VLPP 60–90 cm H2O; and (2) the ISD group, comprised of patients with a VLPP ≤60 cm H2O, which will be excluded in this study.
Statistical analyses
SPSS version 17 for Windows (SPSS Inc., Chicago, IL, USA) was used to perform all statistical analyses. Data were analyzed by the t test and the Chi-square test, expressed as mean ± SD values. The bivariate analysis (linear regression analysis if possible) was performed to determine the correlations between VLPP and Q-tip test score. P values <0.05 were considered statistically significant.
Ethical considerations
The study protocol was approved by the institutional Medical Ethics Committee and accords with the 1983 Declaration of Helsinki. Informed consent was obtained from all patients.
Results
Seventy-eight female patients were proven simple SUI and met all the inclusion criteria and none of the exclusion criteria. The mean patient age was 54 ± 7.5 years (range 42–72 years), mean SUI symptom duration 2.8 years (range 0.5–6 years), and mean number of childbirth deliveries was 1.4. All patients were found to be urine-analysis negative and stress-test positive. Mild prolapse in POP-Q stage I was found in 33 patients (42.3 %) and bladder instability in 10 (12.8 %), although these latter 10 patients all had detrusor pressure (Pdet) <15 cm H2O and no complaint of urgency. No significant differences were found between the Stamey grade I and II groups regarding the urodynamic parameters of maximum flow rate (MFR), cystometry capacity of first desire to urinate (FD), maximum cystometry capacity (MCC), and detrusor pressure during maximum urine flow rate (PdetQmax; P > 0.05). However, a significantly lower maximum urethral closure pressure (MUCP) was found in the Stamey grade II group (P = 0.0267; Table 1). The mean VLPP was found to be 103.6 ± 18.4 cm H2O (range 76–132 cm H2O) and the mean Q-tip angle 28.6° ± 7.2° (range 14.2°–38.9°). Notably, the Q-tip angle found to be >30° in 40 patients. Statistical analysis revealed a negative linear relationship between VLPP and Q-tip angle (R =−0.798, Y = −0.313X + 60.95, P < 0.001; Fig. 1). On the basis of these results, 53 patients were classified as Stamey grade I and 25 as grade II. AI was identified in 51 patients, EI in 27, and ISD in 0 (Table 1; Fig. 2). The results of Chi-square testing revealed correlation between UH subgroups and Stamey grade groups (χ 2 = 4.9130, P = 0.0267, Fig. 2).
Discussion
Defined as the involuntary loss of urine during increased abdominal pressure in the absence of detrusor contraction [18], SUI occurs when the intravesical pressure exceeds the urethral pressure resulting from increased intra-abdominal pressure. Many factors have been correlated with the development of SUI, including aging, previous pelvic surgery, and prolonged menopausal status associated with estrogen withdrawal [19], all of which contributed to 2 pathogenesis factors, UH and ISD, being believed primarily responsible for its development [4]. UDS, a popular means of evaluating SUI for diagnosis of UH and ISD [6, 20], is particularly recommended before surgery in women with SUI [7, 21]. However, several studies concluded that a basic preoperative outpatient evaluation comprises a sufficient evaluation for women with SUI, having found their incontinence surgery outcomes not inferior to those of women who had undergone preoperative UDS [9]. As the clinical importance of UDS in SUI has continued to be debated over the years, researchers have attempted to identify the associations between clinical factors and UDS parameters.
Several studies have found that a positive relationship between Stamey grade and risk of ISD and cumulative association between symptom severity and previously low VLPP [9, 10]. Meanwhile, Nitti and Combs reported that high Stamey grade is highly predictive of low VLPP measurement (i.e., Stamey III within 63–83 %) [11]. However, few reports had examined the clinical factors associated with uncomplicated UH, a simple SUI, which has VLPP >60 cm H2O. When evaluating patients for the simple SUI, it is thus necessary to exclude patients with the risk factors for ISD: previous hysterectomy, prolapse surgery, and radical pelvic surgery may lead to severe defects in urethral function owing to denervation, devascularization, and extensive urethral fibrosis and scarring of the urethra [22], so we excluded patients who had undergone previous urethral surgery, retropubic surgery, or anterior colporrhaphy; complained of urinary urgency or frequency; or had been classified as Stamey grade III. Exclusion of these patients successfully avoided inclusion of any patients with ISD, VLPP <60 cm H2O.
UH, caused by a poorly supported proximal urethra, is the key factor in the development of simple SUI, and results in mild or moderate incontinence compared with ISD [23, 24]. To explain the mechanism of SUI, DeLancey proposed the “the hammock theory,” according to which fascial attachments connect the periurethral tissue and anterior vaginal wall to the arcus tendineus at the pelvic side wall, while muscle attachments connect the periurethral tissue to the medial border of the levator ani muscle. The resulting musculofascial support provides a hammock-like supporting layer onto which the urethra is compressed during increases in intra-abdominal pressure, and this compression induces an increase in urethral closure pressure during a stress maneuver or coughing [25]. On the basis of this theory, two minimally invasive mid-urethral sling procedures are commonly used to produce urethral suspension: the tension-free vaginal tape procedure [26] and the tension-free vaginal tape-obturator procedure (TVT-O) [27]. Many studies reported that mid-urethral sling placement outcome is determined by the extent of UI [28].
The Q-tip test, the result of which is expressed as the Q-tip angle, is a valuable tool for specialized urological physical examination, and commonly used to determine urethral mobility in SUI patients. Karram and Bhatia, who standardized the technique used to perform the Q-tip test, emphasize the importance of proper placement of the Q-tip at the urethrovesical junction and of proper examination [29]. The Q-tip test is regarded the simplest and most reliable clinical tool for quantification of loss of support of the urethrovesical junction in clinical practice, and the Q-tip angle as an important clinical factor in determining UH in women with stress incontinence and in predicting surgical outcome by professional urologists [30].
To help resolve the equivocal situation regarding the need for invasive UDS in simple SUI and the benefit of clinical primary evaluation in SUI outcome, we examined whether several clinical factors are associated with UDS parameters in this study. We found the clinical factor of Q-tip angle, which we assessed to quantify the loss of support of the urethrovesical junction and indicate the extent of urethral mobility, negatively correlated with VLPP, important urodynamic parameter in SUI (Fig. 1). Moreover, 2 UH subgroups also correlated with Stamey grade groups (Fig. 2).
In accordance with previous studies, we found maximum urethral closure pressure to be lower in grade II compared to grade I patients [20, 21]. We identified mild prolapse in POP-Q stage I, which was verified by Cogan to be highly associated with the Q-tip straining angle of urethral mobility in 33 patients [31]. Some urologist recommended to use POP-Q instead of Q-tip, but the POP-Q is more complicated and hard to handle. Q-tip test is much more convenient for fast and firstly evaluation in simple SUI. Although we identified mild bladder instability in 10 included patients, and none complained of urgency. Follow-up of these 10 patients over 6 months after incontinence treatment by TVT-O revealed that 7 no longer experienced BI while 2 continued to have Pdet <15 cm H2O, with 1 patient lost to follow-up.
Assessment of SUI should firstly begin with the taking and review of patient history and performance of physical examination. Collection and review of data regarding clinical factors benefits primary evaluation of SUI and possibly outcome, and may reduce the need to perform several invasive tests. As basing prediction of pure urodynamic SUI on evaluation of clinically defined simple SUI symptoms and signs remains equivocal, we examined the association between clinically defined simple SUI factors and urodynamic SUI. Our findings indicate the existence of a negative relationship between VLPP-associated Q-tip angle (i.e., that higher VLPP is associated with lower Q-tip angle) in simple female SUI. However, as the number of patients in our study was relatively small, further research should be conducted by urologists to confirm our findings, which might help to reduce the need for urodynamic testing in some simple SUI patients.
In conclusion, the major finding of our study, the existence of a negative relationship between VLPP-associated Q-tip angle and correlation between classifications of VLPP UI groups-symptom grade groups in simple female SUI, suggests that firstly performance of Q-tip angle measurement in Stamey I and II SUI patients might help to reduce the need for urodynamic testing or some of its items.
References
Haylen BT, de Ridder D, Freeman RM et al (2010) An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J 21:5–26
Montesino-Semper MF, Jimenez-Calvo JM, Cabases JM et al (2013) Cost-effectiveness analysis of the surgical treatment of female urinary incontinence using slings and meshes. Eur J Obstet Gynecol Reprod Biol 171(1):180–186
Abrams P, Cardozo L, Fall M et al (2002) The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 21:167–178
Tantanasis T, Daniilidis A, Pantelis A et al (2013) Minimally invasive techniques for female stress urinary incontinence, how, why, when. Arch Gynecol Obstet 288:995–1001
Shah SM, Gaunay GS (2012) Treatment options for intrinsic sphincter deficiency. Nat Rev Urol 9:638–651
Serati M, Cattoni E, Siesto G et al (2013) Urodynamic evaluation: can it prevent the need for surgical intervention in women with apparent pure stress urinary incontinence? BJU Int 112:E344–E350
Serati M, Ghezzi F, Cattoni E et al (2012) Tension-free vaginal tape for the treatment of urodynamic stress incontinence: efficacy and adverse effects at 10-year follow-up. Eur Urol 61:939–946
van Leijsen SA, Kluivers KB, Mol BW et al (2012) Can preoperative urodynamic investigation be omitted in women with stress urinary incontinence? A non-inferiority randomized controlled trial. Neurourol Urodyn 31:1118–1123
Kim SO, Kim YJ, Yoo DH et al (2011) Clinical factors associated with low valsalva leak point pressure among women with stress urinary incontinence. Int Neurourol J 15:211–215
Cummings JM, Boullier JA, Parra RO et al (1997) Leak point pressures in women with urinary stress incontinence: correlation with patient history. J Urol 157:818–820
Nitti VW, Combs AJ (1996) Correlation of Valsalva leak point pressure with subjective degree of stress urinary incontinence in women. J Urol 155(1):281–285
Kim SO, Jung HS, Jang WS et al (2013) Measurement of the Q-tip angle before and after tension-free vaginal tape-obturator (TVT-O): preoperative urethral mobility may predict surgical outcome. Int Urogynecol J 24:1005–1009
Visco AG, Wei JT, McClure LA et al (2003) Pelvic Floor Disorders Network. Effects of examination technique modifications on pelvic organ prolapse quantification (POP-Q) results. Int Urogynecol J Pelvic Floor Dysfunct 14:136–140
Stamey TA (1980) Endoscopic suspension of the vesical neck for urinary incontinence in females. Report on 203 consecutive patients. Ann Surg 192:465–471
Schäfer W, Abrams P, Liao L et al (2002) Good urodynamic practices: uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn 21:261–274
Nager CW, Kraus SR, Kenton K et al (2010) Urodynamics, the supine empty bladder stress test, and incontinence severity. Neurourol Urodyn 29:1306–1311
Türker P, Kilic G, Tarcan T (2010) The presence of transurethral cystometry catheter and type of stress test affect the measurement of abdominal leak point pressure (ALPP) in women with stress urinary incontinence (SUI). Neurourol Urodyn 29:536–539
Osborn DJ, Strain M, Gomelsky A et al (2013) Obesity and female stress urinary incontinence. Urology 82:759–763
Wehrberger C, Madersbacher S, Jungwirth S et al (2012) Lower urinary tract symptoms and urinary incontinence in a geriatric cohort—a population-based analysis. BJU Int 110:1516–1521
Kawasaki A, Wu JM, Amundsen CL et al (2012) Do urodynamic parameters predict persistent postoperative stress incontinence after midurethral sling? A systematic review. Int Urogynecol J 23:813–822
McGuire EJ, Lytton B, Kohorn EI et al (1980) The value of urodynamic testing in stress urinary incontinence. J Urol 124:256–258
Mashidori T, Yamanishi T, Yoshida K et al (2007) Continuous urinary incontinence presenting as the initial symptoms demonstrating a contractile detrusor and intrinsic sphincter deficiency in multiple system atrophy. Int J Urol 14:972–974
Hosker G (2009) Is it possible to diagnose intrinsic sphincter deficiency in women? Curr Opin Urol 19(4):342–346
Anger JT, Scott VC, Kiyosaki K et al (2013) Development of quality indicators for women with urinary incontinence. Neurourol Urodyn 32:1058–1063
DeLancey JO (1994) Structural support of the urethra as it relates to stress urinary incontinence: the hammock hypothesis. Am J Obstet Gynecol 170(6):1713–1723
Ulmsten U, Henriksson L, Johnson P et al (1996) An ambulatory surgical procedure under local anesthesia for treatment of female urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 7:81–85
Delorme E (2001) Transobturator urethral suspension: mini-invasive procedure in the treatment of stress urinary incontinence in women. Prog Urol 11:1306–1313
Fritel X, Zabak K, Pigne A et al (2002) Predictive value of urethral mobility before suburethral tape procedure for urinary stress incontinence in women. J Urol 168:2472–2475
Karram MM, Bhatia NN (1988) The Q-tip test: standardization of the technique and its interpretation in women with urinary incontinence. Obstet Gynecol 71:807–811
Bergman A, Koonings PP, Ballard CA (1989) Negative Q-tip test as a risk factor for failed incontinence surgery in women. J Reprod Med 34:193–197
Cogan SL, Weber AM, Hammel JP (2002) Is urethral mobility really being assessed by the pelvic organ prolapse quantification (POP-Q) system? Obstet Gynecol 99:473–476
Acknowledgments
We thank Dr. Tian Fang Li at Rush University Medical Center (Chicago, IL, USA) for editing the manuscript.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Y., Wen, J.G., Shen, H. et al. Valsalva leak point pressure-associated Q-tip angle and simple female stress urinary incontinence symptoms. Int Urol Nephrol 46, 2103–2108 (2014). https://doi.org/10.1007/s11255-014-0772-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-014-0772-4