Abstract
Niche partitioning reduces interspecific competition, facilitating coexistence. In urban ecosystems, however, habitat loss reduces species’ ability to spatially partition activity. Temporal partitioning may thus increase in urban areas as species, unable to avoid each other spatially, partition time to avoid competition. In Midwestern US cities, eastern gray squirrels (Sciurus carolinensis) and fox squirrels (S. niger) co-occur and compete for resources. We identified urban gray and fox squirrel activity patterns and how they vary with season, land cover, and among sites where they do and do not co-occur using camera-trap data. Both species’ activity patterns varied with season and canopy and impervious surface cover. Gray squirrel activity patterns varied in the presence of fox squirrels only in the fall, providing limited support for our temporal partitioning hypothesis. Temporal niche partitioning may thus play a role in supporting these species co-existence when competition is seasonally-elevated (e.g., fall hording), but appears less important in other seasons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Competitive interactions between species are important drivers of community composition (Goldberg and Barton 1992), which can affect other ecological processes (e.g., species distributions; Ritchie et al. 2009). The competitive exclusion principle states that if two species were to have identical niches and at least one limiting resource, the species with superior competitive ability would inevitably drive the other to extinction (Hardin 1960; Chesson 2000). Therefore, species have evolved niche partitioning mechanisms to avoid or reduce competition from other species and reach stable coexistence (Vance 1985), for example, via spatial (Shigesada et al. 1979), or temporal (by altering peak activity times) partitioning (Carothers and Jaksić 1984). Temporal partitioning may be particularly important in coexistence of ecologically-similar species (e.g., Di Bitetti et al. 2010).
Temporal niche partitioning may be particularly salient in urban environments, where habitat is lost or degraded due to human development and the ability of species to spatially segregate is eroded (Gallo et al. 2019). In addition to habitat loss, urban areas are key areas for the introduction of non-native species, including species not native to the continent and native species outside of their geographic range on a given continent. Fox squirrels (Sciurus niger) exemplify the latter situation. Fox squirrels are native to the open woodlands of the eastern United States but have been intentionally introduced to urban and suburban areas across the country, often to locations with other native squirrel species. In many areas east of the Mississippi River, these introductions bring fox squirrels into contact with native eastern gray squirrels (Sciurus carolinensis). Both squirrel species readily adapt to living in urban areas, are ecologically similar (Koprowski 1994a, b), and can co-occur at the same sites (van der Merwe et al. 2005); therefore there must exist some mechanism that enables their stable coexistence. However, few studies have examined this mechanism, leaving a gap in our understanding of not only the means whereby these two species coexist, but also in our broader understanding of factors that facilitate the co-existence of similar species more generally. Understanding the coexistence of close competitors remains a motivation for testing theories of diversity maintenance.
Coexistence of gray and fox squirrels in non-urban systems has been extensively studied. In the eastern US, forest patches occupied by fox squirrels are different structurally from patches also used by gray squirrels (Dueser et al. 1988), indicating that habitat partitioning is present between these species. In the Midwestern US, fox and gray squirrels were more likely to have conspecifics as neighbors (Armitage and Harris 1982), indicating possible microhabitat preferences or intolerance of heterospecifics. Other research has found both species have similar food preferences, therefore niche differences are likely related to differential habitat use and predator escape behavior (Smith and Follmer 1972). In urban environments, however, habitat patches are significantly smaller and thus contain fewer habitat types; native plant diversity is known to be lower in urban compared to rural habitat patches (Aronson et al. 2014). As such, our current understanding of the mechanisms that facilitate gray and fox squirrel coexistence in non-urban environments may not translate to urban habitats.
In this study, we sought to identify whether urban gray and fox squirrels exhibit temporal niche partitioning in locations where they co-occur in urban environments, focusing on the Iowa City metropolitan area of Iowa, USA. We hypothesized that gray squirrels, given their subordinance to fox squirrels in encounters over food (Brown and Batzli 1985), would shift their activity times to avoid peak fox squirrel activity, enabling both species to occur on the same sites. Gray squirrels on sites with fox squirrels would, therefore, have different activity patterns than gray squirrels on sites without fox squirrels. We also expected peak activity timing to change seasonally, as both species are well-known to vary activity patterns with changes in day length and average daily temperatures, and in response to local habitat variables. Given the importance of canopy cover in reducing raptor hunting success (Bechard 1982), squirrels on sites with low canopy cover should have different activity patterns from squirrels on sites with higher canopy cover to avoid predation. Impervious surface cover, a proxy for urbanization (Sutton et al. 2009), should also alter squirrel activity patterns as squirrels balance risk with the need to forage. By identifying the mechanisms that support the co-existence of these two species in urban environments, this study will help us to ascertain biotic mechanisms that could influence each species’ occurrence in urban environments. It will also further our understanding of urban ecological theory by determining whether niche partitioning observed in rural populations also occurs in urban settings.
Methods
Study area
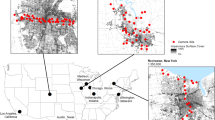
Our study area, the Iowa City metropolitan area of Iowa, USA, is a relatively small metropolitan area embedded in a predominantly agricultural landscape in the Midwestern United States. Iowa City is the fifth largest city in the state of Iowa, with an estimated population of 74,566 (U.S. Census Bureau 2018) in the city proper and, including surrounding smaller cities and unincorporated settlements, approximately 173,400 residents. The topography of the study area consists of gentle rolling hills with intermittent plains. The climate is categorized as humid temperate continental with average annual high and low temperatures of 16.6 °C and 4.7 °C, respectively. Average annual precipitation includes 956 mm of rainfall and 710 mm of snowfall. According to the 2011 National Land Cover Database (NLCD) Land Cover product (Homer et al. 2015), the predominant vegetation type in Johnson County (the county in which the Iowa City metropolitan area is located) is annual row crops, accounting for 51% of land cover. Impervious surfaces cover 12% of the county and eastern temperate forest, prairie, wetlands cover another 17.5%. Common tree species whose seeds serve as important food sources for squirrels include oaks (Quercus spp.), maples (Acer spp.), black walnuts (Juglans nigra), and hickories (Carya spp.).
Data collection
We used data gathered through a camera-trapping effort that is part of the Urban Wildlife Information Network (UWIN; Magle et al. 2019). A total of 39 camera sites were established across the urban-to-rural gradient in the Iowa City metropolitan area using UWIN protocols (Magle et al. 2019; MacDougall and Sander 2022). Sites were selected using a stratified random-sampling scheme whereby we first laid out transects across the urbanization gradient, then divided them into 10 equally sized 4 km by 5 km sections. We randomly generated four potential camera sites within each section. Sites included a wide range of habitat types, among them urban parks with few trees and lawn understory, suburban yards and open spaces with mature trees and shrubs, rural row crop landscapes, and protected areas with a closed canopy and dense understory. Randomly generated points that were not viable (e.g., in the middle of a house) were relocated to the nearest suitable site. To reduce the likelihood of capturing the same individual on multiple sites, all cameras were located at least 1 km away from each other.
One Bushnell Trophy Trail Camera (Bushnell Outdoor Products, Overland Parks, KS) was placed at each site for at least 30 days in four separate months (January, April, July, and October) beginning in 2017 and continuing to the present. Each camera was attached to a tree along a prominent game trail at a height of 1 m above the ground. Cameras were set to run continuously and take one, date- and timestamped photograph any time a warm object moved in the field of view with a 30 s quiet period between each photo if the motion sensor is continuously triggered. Species present in photographs were identified by trained graduate and undergraduate student researchers, with each photograph receiving at least two independent identifications. If two researchers did not agree on the identity of the species in a photograph, a third observer (another graduate student) was used as a “tie breaker”. Photographs of animals that could not be identified to species or that contained no animals were not included in this analysis (n = 22,986).
We used data collected over 10 sampling months (July 2017 – October 2019) to identify fox and gray squirrel “occupancy”. A site was considered “occupied” by a species for a sampling bout if it was detected during half or more of the weeks sampled. For example, if one photo of a fox squirrel was captured in both the first and second week of October, the site would be considered occupied by fox squirrels for the month of October. This method of determining occupancy was used to reduce the likelihood of classifying sites with transient squirrel individuals as truly occupied. Sites were categorized as having one of three occupancy states during each bout: “gray squirrel only” (only gray squirrels were detected), “fox and gray squirrel present” (both species were detected), and “fox squirrel only” (only fox squirrels were detected). Because occupancy status was determined independently for each bout, camera sites could change status between bouts. This definition is different from “occupancy” that uses complex statistical modeling (e.g., MacKenzie et al. 2002). Because our goal was not to identify what variables influence whether a squirrel species occupies a site, we utilize this coarse metric simply to characterize the site as containing one, both, or no squirrel species.
We used the date- and timestamp on each photograph to determine activity patterns for each species. Dates of photographs were used to determine the season in which an image was captured, such that photographs dated from January 1 to January 31 were categorized as “winter”, from April 1 to April 30 were categorized as “spring”, from July 1 to July 31 were categorized as “summer”, and from October 1 to October 31 were categorized as “fall”. We used the ‘fitact’ function in the R package ‘activity’ (Rowcliffe et al. 2014) to fit a kernel density estimate to the timestamps on the photos of each species. This function bootstrap samples (with replacement) the timestamp distribution then uses the calculated mean and standard error to fit a probability distribution function across a 24-h activity time axis. Peak activity times were estimated for each species for each season using 1,000 bootstrap repetitions. In addition, seasonal gray squirrel activity was estimated separately at sites where fox squirrels were present and at sites where fox squirrels were not present to identify whether the presence of fox squirrels was related to gray squirrel activity. We used the ‘compareAct’ command in the ‘activity’ package to run several pairwise Wald tests to assess differences in peak activity times between seasons for both gray and fox squirrels. We also used ‘compareAct’ to identify whether gray squirrel activity patterns differed on sites with and without fox squirrels for each season.
We also examined the effects of tree canopy and impervious cover on squirrel behavior. We delimited 100 m radius buffers around each camera site to approximate the average home range of fox and gray squirrels (area = 3.14 ha, range = 2.39–7.56) in small woodlots and urban settings (Adams 1976; Tounzen et al. 2013). We calculated the mean percent impervious surface cover within each buffer using the 2009 High Resolution Land Cover for Johnson County, Iowa which we updated to 2015 conditions using tax assessor and planimetric data prior to calculations. We calculated average tree canopy cover using the 2016 NLCD Tree Canopy product (Coulston et al. 2012). We then categorized sites as having “high” or “low” cover in each of these categories by comparing a site’s cover with the mean canopy (6.90 ± 18.57%) or impervious (4.24 ± 14.26%) cover at the county level. We utilized county-level estimates because some of our sites extend beyond our urbanized areas and these values are more representative of land cover within our sampling area. Sites were categorized as “high” if their average tree canopy or impervious cover exceeded the average values reported for Johnson County and “low” if their average cover was below the average value of Johnson County. All spatial analysis was done in ArcMap 10.6.7 (ESRI, Redlands, CA). We used pairwise Wald tests to determine whether differences existed in peak activity times on sites with high and low canopy cover and high and low impervious surface cover separately. All analyses were performed, and Figs. 2–8 were created, in R version 3.6.1.
Results
Photos and occupancy
We used data from 39 camera sites that were surveyed 10 times each (2 winter, 2 spring, 3 summer, 3 fall), except when logistical constrains prevented camera placement for that month (n = 18 cameras not placed for one month across three years; 3 in April 2017, 1 each in April and July 2018, 3 in October 2018, 7 in January 2019, and 2 in April 2019), for a total of 372 sampling bouts from July 2017 to October 2019. The cameras were active for a total of 10,997 trap-days. A total of 14,075 photographs of squirrels were captured: 11,527 of gray squirrels and 2,548 of fox squirrel. Of these photographs, 15% (n = 2,164 photos) were captured in winter, 24% (n = 3,388) were captured in spring, 6% (n = 881) were captured in summer, and 54% (n = 7,642) were captured in fall. The average number of squirrel photos per site was 360.9 (SD = 280.2, range: 13–1,480). Gray squirrels were detected in 289 sampling bouts and fox squirrels were detected in 112 sampling bouts. There were 189 bouts classified as “only gray squirrel present”, 77 bouts as “both gray and fox squirrel present”, and 19 as “only fox squirrel present”, and 87 bouts where neither squirrel was detected. Gray squirrels were detected in at least one bout at all 39 camera sites while fox squirrels were detected at least once at 21 camera sites (Fig. 1).
We classified 33 sites as high canopy cover (cover > 6.90%) and 6 sites as low canopy cover. Fox squirrels occurred on 19 of the 33 high canopy-cover sites, but on only one of the 6 low-canopy cover sites. Fourteen sites were classified has having high impervious surface cover (cover > 4.24%) while 25 sites were classified as having low impervious cover. Fox squirrels were present on 3 of the 14 high impervious cover-sites and 17 of the 25 low impervious-cover sites. As stated above, gray squirrels were present at all sites. Total numbers of photographs captured of each species at each site type can be found in Online Resource 1.
Activity patterns
Fox squirrels exhibited a diurnal activity pattern, with peak activity in the late morning hours (approximately 09:00–10:00) that tapered off throughout the day until activity ceased at approximately 19:00–20:00 (Fig. 2). Fox squirrel activity patterns did not differ significantly between seasons (winter-spring W = 0.444, p = 0.50; winter-summer W = 0.389, p = 0.53; winter-fall W = 1.935, p = 0.16; spring–summer W = 0.921, p = 0.34; spring-fall W = 3.784, p = 0.058; summer-fall W = 0.002, p = 0.96). Because differences in activity patterns between seasons were not significant, fox squirrel activity data are presented as year-round activity in the remaining analyses.
Fox squirrel activity patterns differed significantly on sites with high impervious compared to low impervious surface cover (W = 10.812, p < 0.01; Fig. 3). Fox squirrel activity on sites with high impervious cover increased beginning at 06:00, with peak activity occurring near midday, before steadily decreasing throughout the evening. On sites with low impervious cover, fox squirrels exhibited steady activity during daylight hours. Fox squirrel activity patterns also differed significantly between sites with high and low tree canopy cover (W = 18.646, p < 0.01; Fig. 3) such that activity steadily increased during the morning, peaked at midday, then steadily decreased in the evening. On sites with high canopy cover, fox squirrels were more continuously active during daylight hours.
Fox squirrel activity patterns on sites with (a) high and low impervious surface cover and (b) high and low tree canopy cover. Activity patterns were significantly different between sites with high and low impervious surface cover (W = 10.812, p < 0.01) and high and low tree canopy cover (W = 18.646, p < 0.01)
Gray squirrels exhibited a crepuscular activity pattern, with peak activity at approximately 09:00 followed by a midday decrease in activity, then another activity peak around 17:30. Gray squirrel activity ceased at approximately 19:00–20:00 (Fig. 4). Gray squirrel activity patterns differed significantly between most seasons, (winter-spring W = 52.053, p < 0.01; winter-summer W = 65.305, p < 0.01; winter-fall W = 89.254, p < 0.01; spring–summer W = 5.797, p = 0.02; summer-fall W = 4.134, p = 0.04), but not between spring and fall (spring-fall W = 0.535, p = 0.46). Winter activity peaked at approximately 09:00, then declined through the day until just after sunset (approximately 17:30) when activity peaked again before declining for the night. Spring activity was similar to winter activity but with wider peak activity intervals, especially in the late afternoon just before sunset. Summer activity patterns were more continuously diurnal, with activity increasing until approximately 11:00, then slowly decreasing until around 18:00 when activity declined sharply. Fall activity was similar to summer activity, but with slight increases in morning and late afternoon activity. Although spring and fall activity patterns did not differ significantly, the data for these seasons were not combined and were treated separately in further analyses.
Gray squirrel activity was significantly different on sites with high canopy cover compared to low canopy cover (W = 12.134, p < 0.01; Fig. 5). Gray squirrels on sites with low canopy cover were more active in the morning, with peak activity occurring between 09:00 and 12:00, and less active in the evening compared to sites with high canopy cover. No interactive effects of season and canopy cover on squirrel activity times occurred (all comparisons p > 0.05). Gray squirrel activity was significantly different on sites with low compared to high impervious surface cover in the winter (W = 4.444, p = 0.04), spring (W = 9.896, p < 0.01), and fall (W = 25.301, p < 0.01), but not in the summer (W = 2.672, p = 0.10; Fig. 6). In winter, gray squirrels on high impervious cover sites are more active in mid-morning than gray squirrels on low impervious cover sites. In the spring and fall, however, gray squirrels at high impervious sites are less active in the mid-morning and more active at midday/late afternoon than gray squirrels at sites with low impervious cover.
Gray squirrel activity patterns on sites with high and low impervious surface cover in (a) winter, (b) spring, (c) summer, and (d) fall. Activity patterns were significantly different on sites with low compared to high impervious surface cover in all seasons except summer (winter: W = 4.444, p = 0.04; spring: W = 9.896, p < 0.01; summer: W = 2.672, p = 0.10; fall: W = 25.301, p < 0.01)
Activity overlap
Gray squirrel activity patterns on sites with fox squirrels did not differ significantly in winter and spring seasons, but did differ significantly in fall and were close to significantly different in summer (winter activity W = 1.264, p = 0.26, spring activity W = 2.44, p = 0.12; summer activity: W = 3.754, p = 0.055; fall activity W = 8.545, p < 0.01; Fig. 7). In winter, gray squirrels on sites with and without fox squirrels exhibited an activity peak in the morning near 09:00, followed by a decrease in activity until sunset (approximately 17:30) when activity peaked once again before ceasing for the day. Spring activity was similar to winter activity, with a wider peak of activity time in the morning. In summer, gray squirrels on sites with fox squirrels exhibited a morning activity peak followed by declining activity. On sites without fox squirrels, gray squirrel peak activity occurred at midday and lasted several hours. In fall, gray squirrels on sites with fox squirrels experienced an activity peak right after sunrise followed by declining activity until another activity peak occurred about an hour before sunset. On sites without fox squirrels, gray squirrels remained continuously active through the day.
Activity patterns of gray squirrels in the presence and absence of fox squirrels in (a) winter, (b) spring, (c) summer, and (d) fall based on kernel density estimates. Dotted lines indicate sites on which both gray and fox squirrels were present, dashed lines indicate sites where only gray squirrels were present. Activity patterns of fox squirrels (gray solid lines) are provided for reference. Gray squirrel activity was nearly significantly different in summer (p = 0.055) and significantly different in fall (p < 0.01), but not in winter (p = 0.26) and spring (p = 0.12)
On sites where the two squirrel species co-occurred, their seasonal activity patterns differed significantly in winter (W = 19.643, p < 0.01), spring (W = 4.300, p = 0.04), and fall (W = 5.275, p = 0.02), but not summer (W = 1.696, p = 0.193; Fig. 8). In winter, gray squirrel activity peaked in the morning, at noon, and again just before sunset. In spring, gray squirrels experienced peak activity around 09:00, then activity tapered through the day with a small activity peak around 18:00. Summer gray squirrel activity patterns resembled spring activity patterns; however, no peak activity spike occurred in the evening. Fall gray squirrel activity was characterized by relatively continuous all-day activity. Fox squirrels exhibited relatively constant activity levels from 09:00 to 18:00 year-round.
Gray squirrel and fox squirrel activity patterns in (a) winter, (b) spring, (c) summer, and (d) fall based on kernel density estimation. Fox squirrel activity patterns did not differ significantly in different seasons. Gray and fox squirrel activity differed significantly in winter (p < 0.01), spring (p = 0.04), and fall (p = 0.02)
Discussion
We sought to identify urban gray and fox squirrel activity patterns and whether they vary with season, land cover, and among sites where these species occur alone and together, thereby assessing spatiotemporal variation in squirrel activity across urban environments and the potential for temporal niche partitioning to facilitate co-occurrence of these species. Both species’ activity patterns differed among sites with high and low levels of tree canopy and impervious surface cover, with important seasonal variation and interactions between season and imperious cover for gray squirrels. We found only limited support for our hypothesis regarding temporal niche partitioning such that gray squirrel activity patterns varied in the presence of fox squirrels only in the fall. Fall was the only season in which gray squirrels at sites with and without fox squirrels had significatly different activity patterns, and gray and fox squirrels had significantly different activity patterns. In the other seasons, there was either not a significant difference in the activity of gray squirrels at sites with and without fox squirrels (winter, spring), or gray and fox squirrel activity patterns were not significantly different (summer). Thus, while temporal niche partioning may allow these species to co-occur in fall, it is unlikely to influence co-occurrence patterns overall.
Our results suggest that, in this system, tree squirrel activity patterns differ among sites with above versus below average tree and impervious surface cover more than they do among sites where the species we examined do and do not co-occur. Fox squirrels were more active at similar times on sites with above-average impervious surface cover (late morning) and with low canopy cover (midday; Fig. 3). Sites with above-average impervious cover are indicative of higher development intensity than average in the study area while low canopy sites include recent development and grassy areas as well as highly impervious sites. We observed few fox squirrels on these sites (n = 28 photos on low canopy sites, n = 289 photos on high impervious sites); thus, such environments may represent poor habitat for this species. Although some high impervious sites did have high canopy cover (e.g., older residential neighborhoods with mature trees), fox squirrels were also rare on these sites (n = 261 photos). High impervious and low canopy sites may offer reduced amounts of food or protection from predators, and higher activity may occur in late morning/midday to minimize predation risk (e.g., from crepuscular predators). In contrast, gray squirrels were photographed more often on high impervious (n = 6,674 photos) and low canopy (n = 2,301 photos) sites. This finding agrees with studies in Chicago that found gray squirrels are more likely to occur in areas with high human densities compared to fox squirrels (van der Merwe et al. 2005) and in Missouri where gray squirrels replaced fox squirrels in a new suburban housing development as food resources increased (Sexton 1990); therefore, gray squirrels may have a competitive advantage on these sites which have fewer trees given more efficient use of limited food resources. However, it is also possible that gray squirrels are competitively excluded from sites with high canopy and low impervious surfaces. Future studies that explore mechanisms such as resource use and interactions among gray and fox squirrels may be better able to identify such relationships.
Gray squirrels exhibited significant differences in activity patterns with respect to tree canopy cover. No significant interaction existed between canopy cover and season such that gray squirrels were more active in the morning and less active in the evening on sites with low canopy cover across all seasons (Fig. 5). Sites that have low canopy cover include large grassy areas such as meadows, agricultural fields, and lawns, and areas with high impervious surface cover. Squirrels perceive areas with low tree canopy or shrub cover as areas of increased predation risk (Thorson et al. 1998), thus gray squirrel activity may shift to avoid peak predator activity times in these areas as predation risk is one of the largest foraging costs experienced by small mammals (Preisser et al. 2005). Predation risk may also explain seasonal variation in gray squirrel response to impervious surface cover. Gray squirrels were more active in the morning and less active midday or in the evening on low impervious cover sites in the spring and fall, but less active in the mornings and more active in the evenings on low impervious cover sites in the winter (Fig. 6). In spring and fall when, given the deciduous nature of most trees in the study area (Zhao and Sander 2018; Sander and McCurdy 2021), all sites have yet to grow or have started to lose canopy cover, gray squirrels might be active early in the morning to avoid aerial predators (Bildstein 1978). However, predation risk may decline with increasing impervious cover as predator abundance is often lower in more intense urban environments (Dénes et al. 2017), allowing gray squirrel activity to be more evenly distributed. For example, Bowers and Breland (1996) show that gray squirrels spend more time foraging in more urban compared to rural sites. When trees leaf out in summer, aerial predation risk decreases at any site with high canopy cover, potentially causing lack of significant activity pattern changes in this season.
It is possible that the observed difference in winter activity patterns on high and low impervious surface cover sites may be related to the urban heat island effect (Imhoff et al. 2010). Given that impervious surfaces retain and re-radiate heat, gray squirrels in areas with high impervious surface cover may be able to start foraging earlier in the day as ambient tempetaures would be warmer than in low impervious surface environments. Gray squirrels are relatively small mammals (Koprowski 1994a) and warmer ambient temperatures caused by impervious surfaces would allow gray squirrels to reduce some of the metabolic costs associated with winter activity. This possibility warrants exploration in future studies.
As we predicted, gray squirrel activity patterns differed among seasons (Fig. 4); however, fox squirrel activity patterns did not (Fig. 2). It is not surprising that we observed seasonal changes in gray squirrel activity patterns, given that gray squirrels are known to exhibit different activity levels in different seasons (Thompson 1977). What is surprising is that our data did not identify significant changes in fox squirrel activity patterns among seasons. Rather than being a true biological phenomenon of Iowa City fox squirrels, we suspect our inability to detect statistically significant differences among seasons was related to sampling design and sample size. Our cameras were deployed outside of important breeding windows for fox squirrels (peak breeding occurs in December and June; Koprowski 1994b), which prevented us from capturing reproduction-related changes in activity. If the cameras collected data for longer time intervals each season, we might capture short-term seasonal changes in fox squirrel behavior. The sample size of fox squirrel photos was also smaller than gray squirrels, which may limit our statistical power and ability to identify seasonal behavior patterns and differences among them.
Gray and fox squirrel activity patterns differed significantly in winter, spring, and fall (Fig. 8). However, gray squirrels on sites with fox squirrels had significantly different activity patterns from gray squirrels on sites without fox squirrels only in fall (Fig. 7). Thus, contrary to our hypothesis, gray squirrels did not appear to shift their daily activity patterns specifically to avoid fox squirrrels during most seasons. Fox and gray squirrels may thus only vary their activity patterns to avoid interspecific competition in the fall when there is intense inter- and intraspecific competition to collect food to cache through the winter. It as been demonstrated that, at least for females, fox squirrels are capable of displacing gray squirrels from food sources (Brown and Batzli 1985), and these aggressive encounters may increase during the fall hording season. Thus increased gray squirrel activity in the early morning and evening and reduced activity in the afternoon in the fall could minimize direct encounters with fox squirrels during peak fox squirrel afternoon activity times.
The results of this study should be considered with respect to certain limitations. Firstly, camera trapping poses limitations that could affect our findings. Trail cameras are limited in their ability to capture animal behavior, in the sense that cameras do not perfectly detect all animals (Burton et al. 2015). Cameras cannot detect animals that occur on a site but do not pass in front of the camera, thus a small possibility exists that squirrel species were present on sites but not observed and their activity time not recorded. Even when animals are detected by a camera, if the animal is out of focus or obscured by vegetation, identification to species is challenging. As such, it is possible that fox and gray squirrels co-occur on some sites where only one species was observed or that photos of gray and fox squirrels were captured but discarded because the human observers could not confidently identify the animal. Additionally, it is possible gray squirrels avoid fox squirrels for a few minutes at a time during direct confrontations, but this difference would not be captured by current methods of activity pattern modeling because activity patterns are smoothed over a 24-h curve. Setting trail cameras to record video or to take several photos in quick succession could improve detections and identifications of animals, as well as record behavioral changes that occur on the order of minutes. The use of GPS collars that record highly-detailed location data at short time intervals could also futher elucidate squirrel activity patterns and their relationships with land use and intra- and interspecific competition. Additionally, we did not examine relationships between gray or fox squirrel activity patterns and land-use and land-cover composition or species preferences for particular environments, nor did we assess the effects of tree and impervious surface cover measured at multiple spatial scales. Examining these relationships in future studies would help to further identify the degree to which the conditions represented by different land use and cover and urban environments affect the activity patterns of these species and at what scale these conditions are most relevant. Given that the presence of predators could affect squirrel activity patterns, future studies should also explore these relationships in order to identify the role of predators in shaping such patterns.
Conclusions
Urban environments pose challenges that impact species interactions and activity patterns. Temporal niche partitioning in urban environments may allow species to co-occur in ways that are similar to non-urban settings. While we found that temporal niche partitioning may contribute to the coexistance of gray and fox squirrels in our study area, the strength of these interspecific interactions appears to be modulated by seasonal environmental changes and urban development. It appears both species react more strongly to differences in tree canopy and impervious surface cover than to competition with each other. Even when gray and fox squirrels coexist on the same site, there is no evidence that gray squirrels alter their activity patterns, except when competition is seasonally elevated (fall). Although fox squirrels may dominate gray squirrels in direct confrontations over resources, gray squirrels are more efficient foragers (Brown and Batzli 1985) and more tolerant of human development (Parsons et al. 2018), which has likely promoted the stable coexistence of these two species in our study system. This increased tolerance of human development could lead to local replacement of fox squirrels by gray squirrels as urbanization increases across their range (Sexton 1990). Replacement of fox squirrels by gray squirrels may lead to a reduction in tree seedling recruitment given that gray squirrels are better able to locate buried seeds than fox squirrels (Brown and Batzli 1985). Gray squirrels also occur in higher densities in urban areas (Parker and Nilon 2008; Hansen et al. 2020), therefore, given their high rate of cache retrieval, fewer cached seeds may germinate in urban areas. As scatterhoarding by rodents is an important dispersal mechanism for some tree species (Theimer 2009), this could have imortant implications for urban forest dynamics. Understanding mechanisms of niche partitioning between these two species will therefore help us to understand the mechanisms that can preserve coexistance at sites where both species still occur.
Availability of data and material
All data are presented in the manuscript. Copies of the dataset are available upon request from lead author.
Code availability
Not applicable.
References
Adams CE (1976) Measurement and characteristics of fox squirrel, Sciurus niger rufiventer, home ranges. Am Midl Nat 95:211–215. https://doi.org/10.2307/2424250
Armitage KB, Harris KS (1982) Spatial patterning in sympatric populations of fox and gray squirrels. Am Midl Nat 108:389–397. https://doi.org/10.2307/2425501
Aronson MFJ, La Sorte FA, Nilon CH et al (2014) A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc R Soc B Biol Sci 281:20133330. https://doi.org/10.1098/rspb.2013.3330
Bechard MJ (1982) Effect of vegetative cover on foraging site selection by Swainson’s Hawk. Condor 84:153. https://doi.org/10.2307/1367658
Bildstein KL (1978) Behavioral ecology of red-tailed hawks (Buteo jamaicensis), rough-legged hawks (B. lagopus), northern harriers (Circus cyaneus), American kestrels (Falco sparverius) and other raptorial birds wintering in south central Ohio. Dissertation, The Ohio State University
Bowers MA, Breland B (1996) Foraging of gray squirrels on an urban-rural gradient: use of the GUD to assess anthropogenic impact. Ecol Appl 6:1135-1142. https://doi.org/10.2307/2269597
Brown BW, Batzli GO (1985) Foraging ability, dominance relations and competition for food by fox and gray squirrels. Trans Illinois Acad Sci 78:61–66
Burton AC, Neilson E, Moreira D et al (2015) Wildlife camera trapping: a review and recommendations for linking surveys to ecological processes. J Appl Ecol 52:675–685. https://doi.org/10.1111/1365-2664.12432
Carothers JH, Jaksić FM (1984) Time as a niche difference: the role of interference competition. Oikos 42:406. https://doi.org/10.2307/3544413
Chesson P (2000) Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst 31:343–366. https://doi.org/10.1146/annurev.ecolsys.31.1.343
Coulston JW, Moisen GG, Wilson BT et al (2012) Modeling percent tree canopy cover: a pilot study. Photogram Eng Remote Sens 78:715–727
Dénes FV, Sólymos P, Lele S et al (2017) Biome-scale signatures of land-use change on raptor abundance: Insights from single-visit detection-based models. J Appl Ecol 54:1268–1278. https://doi.org/10.1111/1365-2664.12818
Di Bitetti MS, De Angelo CD, Di Blanco YE, Paviolo A (2010) Niche partitioning and species coexistence in a Neotropical felid assemblage. Acta Oecologica 36:403–412. https://doi.org/10.1016/j.actao.2010.04.001
Dueser R, Dooley J, Taylor G (1988) Habitat structure, forest composition and landscape dimensions as components of habitat suitability for the Delmarva fox squirrel. In: Szaro RC, Severson KE, Patton DR (eds) Management of amphibians, reptiles, and small mammals in North America. U.S. Fish and Wildlife Service Technical Report RM-166, Fort Collins, CO, pp 414–421
Gallo T, Fidino M, Lehrer EW, Magle SB (2019) Urbanization alters predator-avoidance behaviours. J Anim Ecol 88:793–803. https://doi.org/10.1111/1365-2656.12967
Goldberg DE, Barton AM (1992) Patterns and consequences of interspecific competition in natural communities: a review of field experiments with plants. Am Nat 139:771–801. https://doi.org/10.1086/285357
Hansen CP, Parsons AW, Kays R, Millspaugh JJ (2020) Does use of backyard resources explain the abundance of urban wildlife? Front Ecol Evol 8:570771. https://doi.org/10.3389/fevo.2020.570771
Hardin G (1960) The competitive exclusion principle. Science 131:1292–1297. https://doi.org/10.1126/science.131.3409.1292
Homer CG, Dewitz J, Yang L et al (2015) Completion of the 2011 National Land Cover Database for the conterminous United States – Representing a decade of land cover change information. Photogram Eng Remote Sens 81:345–354
Imhoff ML, Zhang P, Wolfe RE, Bounoua L (2010) Remote sensing of the urban heat island effect across biomes in the continental USA. Remote Sens Environ 114:504–513. https://doi.org/10.1016/j.rse.2009.10.008
Koprowski JL (1994a) Sciurus carolinensis. Mamm Species 480:1–9. https://doi.org/10.2307/3504224
Koprowski JL (1994b) Sciurus niger. Mamm Species 479:1–9. https://doi.org/10.2307/3504263
MacDougall B, Sander H (2022) Mesopredator occupancy patterns in a small city in an intensively agricultural region. Urban Ecosys. https://doi.org/10.1007/s11252-022-01214-x
MacKenzie DI, Nichols JD, Lachman GB et al (2002) Estimating site occupancy rates when detection probabilities are less than one. Ecology 83:2248–2255. https://doi.org/10.1890/0012-9658(2002)083[2248:ESORWD]2.0.CO;2
Magle SB, Fidino M, Lehrer EW et al (2019) Advancing urban wildlife research through a multi-city collaboration. Front Ecol Environ 17:232–239. https://doi.org/10.1002/fee.2030
Parker TS, Nilon CH (2008) Gray squirrel density, habitat suitability, and behavior in urban parks. Urban Ecosyst 11:243–255. https://doi.org/10.1007/s11252-008-0060-0
Parsons AW, Forrester TD, Baker-Whatton MC et al (2018) Mammal communities are larger and more diverse in moderately developed areas. eLife 7:e38012. https://doi.org/10.7554/eLife.38012
Preisser EL, Bolnick DI, Benard MF (2005) Scared to death? The effects of intimidation and consumption in predator-prey interactions. Ecology 86:501–509. https://doi.org/10.1890/04-0719
Ritchie EG, Martin JK, Johnson CN, Fox BJ (2009) Separating the influences of environment and species interactions on patterns of distribution and abundance: Competition between large herbivores. J Anim Ecol 78:724–731. https://doi.org/10.1111/j.1365-2656.2008.01520.x
Rowcliffe JM, Kays R, Kranstauber B et al (2014) Quantifying levels of animal activity using camera trap data. Methods Ecol Evol 5:1170–1179. https://doi.org/10.1111/2041-210x.12278
Sander HA, McCurdy JD (2021) Urban vegetation and songbird nesting guilds: Relationships and implications for conservation and management. Urban For Urban Green 67:127308. https://doi.org/10.1016/j.ufug.2021.127308
Sexton OJ (1990) Replacement of fox squirrels by gray squirrels in a suburban habitat. Am Midl Nat 124:198–205. https://doi.org/10.2307/2426096
Shigesada N, Kawasaki K, Teramoto E (1979) Spatial segregation of interacting species. J Theor Biol 79:83–99. https://doi.org/10.1016/0022-5193(79)90258-3
Smith CC, Follmer D (1972) Food preferences of squirrels. Ecology 53:82–91. https://doi.org/10.2307/1935712
Sutton PC, Anderson SJ, Elvidge CD et al (2009) Paving the planet: Impervious surface as proxy measure of the human ecological footprint. Prog Phys Geogr Earth Environ 33:510–527. https://doi.org/10.1177/0309133309346649
Theimer TC (2009) Rodent scatterhoarders as conditional mutualists. In: Seed fate: predation, dispersal and seedling establishment. CABI, pp 283–295
Thompson DC (1977) Diurnal and seasonal activity of the grey squirrel (Sciurus carolinensis). Can J Zool 55:1185–1189. https://doi.org/10.1139/z77-153
Thorson JM, Morgan RA, Brown JS, Norman JE (1998) Direct and indirect cues of predatory risk and patch use by fox squirrels and thirteen-lined ground squirrels. Behav Ecol 9:151–157. https://doi.org/10.1093/beheco/9.2.151
Tounzen MR, Epperson D, Taulman JF (2013) Home range and habitat selection of Eastern gray squirrels (Sciurus carolinensis) in a small urban hardwood forest. Trans Kansas Acad Sci 115:89–101. https://doi.org/10.1660/062.115.0301
U.S. Census Bureau (2018) American Community Survey. data.census.gov. Accessed 26 Jul 2020
van der Merwe M, Brown JS, Jackson WM (2005) The coexistence of fox (Sciurus niger) and gray (S. carolinensis) squirrels in the Chicago metropolitan area. Urban Ecosyst 8:335–347. https://doi.org/10.1007/s11252-005-4865-9
Vance RR (1985) The stable coexistence of two competitors for one resource. Am Nat 126:72–86. https://doi.org/10.1086/284397
Zhao C, Sander HA (2018) Assessing the sensitivity of urban ecosystem service maps to input spatial data resolution and method choice. Landsc Urban Plan 175:11–22. https://doi.org/10.1016/j.landurbplan.2018.03.007
Acknowledgements
We would like to thank Brandon MacDougall for assistance collecting data and tagging photographs. We would also like to thank numerous undergraduates at the University of Iowa for tagging photographs.
Funding
This research was funded in part by IGERT grant number 0966130 from the US National Science Foundation.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by Rachel Larson. The first draft was written by Rachel Larson and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This research has been approved by the Institutional Animal Care and Use Committee of the University of Iowa (protocol #7052015).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest
The authors declare no conflicts of interest that are relevant to the content of this article.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Larson, R.N., Sander, H.A. Seasonal activity patterns of sympatric eastern gray squirrels (Sciurus carolinensis) and fox squirrels (Sciurus niger) in a Midwestern metropolitan region. Urban Ecosyst 25, 1527–1539 (2022). https://doi.org/10.1007/s11252-022-01245-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-022-01245-4