Abstract
Urban environments have become an unexpected and promising avenue for pollinator conservation. One group of pollinators, bumble bees, might be especially well suited to utilize the heterogeneous landscape of urban ecosystems. Weedy margins (pervious land adjacent to impervious surfaces such as roads and paved lots) offer plentiful flowers for bees, but are often overlooked in urban pollinator studies. If weedy margins buffer urban bees from loss and fragmentation of foraging habitat in cities, then urban development may not have as strong of a negative effect on bumble bees as previously thought. In this study we test the hypothesis that bumble bee abundance and species richness in these weedy margins will not be affected by the degree of urbanization. We surveyed bees and flowers within weedy plots of land in six industrial cities of the Midwestern USA. Bumble bee abundance and richness were not influenced by the proportion of intensely developed land in the surrounding landscape, but abundance increased with local floral abundance. Bumble bees were also significantly more abundant than other bee groups (honeybees and ‘other’ wild bees). We found that weedy margins and weedy plant species provide important resources to urban pollinators, and to bumble bees in particular. If small patches of habitat can sustain bees in the city, future efforts of urban pollinator conservation could focus on the installation of high-quality micro-meadows, increasing the availability of quality habitat for pollinators.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pollinators provide the essential ecosystem service of animal-facilitated pollination, which is required for reproduction by more than 85% of global flowering plants and roughly 35% of crop species (Ollerton et al. 2011). This ecosystem service has come under threat in recent years as pollinators have experienced global population declines (Kluser and Peduzzi 2007; Potts et al. 2010). In 2017 the United States placed the first bumble bee, Bombus affinis, on the federally endangered species list after its population declined by an estimated 87% in the past 20 years (Cameron et al. 2011; Szymanski et al. 2016). Though the exact cause of pollinator declines is unknown, it is likely a synergistic result of habitat loss (habitat removal, reduction, and fragmentation), increased use of pesticides, and introduced diseases, pests, and parasites (Colla and Packer 2008; Goulson et al. 2008; Grixti et al. 2009; Cameron et al. 2011; Goulson et al. 2015).
The importance of pollinators in combination with the recent publicity of their global population declines has sparked research around the world investigating pollinator habitat requirements, distribution, response to contaminants, and resource preferences. Recent studies have presented a novel and unexpected avenue for future pollinator conservation, urban pollinator habitat (Fortel et al. 2014; Glaum et al. 2017; Baldock et al. 2019). Urban greenspaces, such as city parks and community gardens, have recently been shown to support abundant and diverse pollinator communities (Fortel et al. 2014; McFrederick and LeBuhn 2006; Hall et al. 2017; Banaszak-Cibicka et al. 2018).
A defining feature of urban environments is their increased abundance of impervious surface (pavement, factories, buildings, and roads; Liu et al. 2014). This inhospitable habitat is typically most abundant near city centers and industrial complexes. However, urban environments also provide a variety of potentially rewarding and generally unrecognized bee habitats in the form of neglected pieces of pervious land (hereafter referred to as weedy margins), including roadsides, overgrown lots, and parking lot edges. Weedy margins are not considered urban greenspaces; they are pervious surfaces nestled alongside impervious surfaces and are not managed for ecological objectives. Weedy margins are generally too small to be differentiated from the surrounding impervious surfaces via land cover databases, which classify 30m2 pixels of land into various land cover types, this results in weedy margins often being overlooked in urban pollinator studies. However, weedy margins contribute to the heterogeneity of urban environments and likely provide important resources like foraging and nesting habitat for pollinators, beyond that provided by greenspaces and forest remnants (Lowenstein et al. 2019). Though many researchers have investigated the influence of urbanization on bee pollinators, few have investigated urban habitats outside of managed urban greenspaces (botanical gardens, urban farms, and community parks). Weedy margins may provide important, overlooked resources for urban pollinators, especially bumble bees.
The life history of bumble bees (Bombus) suggests that they might be especially capable of accessing and utilizing the resources distributed throughout urban environments. Bumble bees are eusocial, diet-generalist, large-bodied bees that develop colonies of a few hundred individuals, often within abandoned rodent or bird nests or other small cavities (Michener 2007; Goulson 2012a, d). The eusociality of bumble bees allows them to forage far from the nest for longer periods of time without leaving their nests open to invasion and predation (Goulson 2012b). The generalist diet of the vast majority of bumble bee species grants them access to the array of native and non-native floral resources available within urban environments throughout the duration of the colony life cycle. Furthermore, their large body size increases their capability to fly long distances (greater than 1 km) between patches of flowers (Goulson 2012c). The relatively small annual colonies of bumble bees can utilize cavities that are too small and inconspicuous for honeybees (Michener 2007; Goulson 2012a), the only other large, eusocial bees in North America. This combination of life history traits makes them well-suited to city life and may give bumble bees an advantage over other pollinators in exploiting resources dispersed throughout urban ecosystems.
In this study we investigated the influence of urbanization on bumble bees by surveying bee and flower abundances and species richness within weedy margins in urban environments. Study sites exhibited varying levels of intensely developed land spanning a gradient of urbanization. We hypothesized that weedy margins provide suitable habitat to support urban bees, especially bumble bees, so that bumble bees would be insensitive to the intensity of urban development. We predicted that (1) bumble bee abundance and species richness would be unaffected by the degree of intensely developed land and (2) bumble bees would be more abundant than other bee groups in these habitats.
Methods
Study system
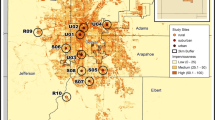
To investigate the influence of urbanization on bumble bees, we first quantified and defined an urban gradient based on abundance of impervious surface. We did this by analyzing the United States Geologic Survey (USGS) National Land Cover Database (NLCD) which categorizes 30m2 sections of the contiguous United States into 1 of 15 land cover categories (Homer et al. 2015). Four of these land cover categories are designated as various levels of development: open developed areas and low, medium, and high intensity developed areas. We created an “intensely developed” land cover category by combining two of their original developed categories (developed medium intensity: 50–79% impervious surface and developed high intensity: 80–100% impervious surface) allowing for us to define an urban gradient as the increase in proportion of land with greater than 50% impervious surface. Sites were distributed across the gradient of intensely developed urban land. To quantify the level of intensely developed land surrounding each site, we extracted land use within a 500 m radius buffer area from site centroid (Fortel et al. 2014; Geslin et al. 2016) from the 2016 USGS NLCD using ArcGIS (10.5.1) software (ESRI 2016). We surveyed weedy margins with >400m2 but <0.4 ha of flowering weedy vegetation (Fig. 1) so that we could accurately survey the site within the allotted time while reducing the chance of double counting individual bees. Additionally, we purposefully avoided locations that had been specifically planted with wildflowers in order to separate the effects of habitat restoration from land use intensity. We sampled in six cities located in the industrial Midwestern USA separated by 30–320 km (Akron - OH, Canton - OH, Cleveland - OH, Pittsburgh - PA, Toledo – OH, and Youngstown – OH; Fig. 2). Each site was separated by over 1.5 km, with roughly five sites per city.
Field surveys
We conducted bee surveys from mid – June to early – August 2018 on sunny, warm days (22.8–33.9 °C) between the hours of 10:00 and 17:00, when bees are most active (preliminary analyses showed no effect of time or temperature). Survey methods followed the States Fish and Wildlife Service, Rusty Patched Bumble Bee Survey Protocols of 2017. Each site was surveyed twice with a minimum of 13 days between each survey. During the second round of sampling nine locations did not have sufficient floral resources, so a nearby replacement was surveyed instead. In these cases, the distance between first and second sampling locations was on average 479 m ± 362 m with a maximum distance of 930 m.
To quantify bee abundance and diversity, on each site visit we conducted timed surveys in which a single observer (the same observer at all sites) walked methodically throughout the site for 15 min and recorded all bees visiting flowers. We identified all bumble bees and honeybees to species and grouped the remaining bees into an “other” category (Baldock et al. 2019). Individuals were either identified “on-the-wing”, or net-collected, placed in a vial for further inspection, and released on site upon identification. The time required to net and identify these individuals was not included in the 15-min of survey time. Two species of bumble bee were grouped together (Bombus auricomus and Bombus pensylvanicus) due to the difficulty of distinguishing between the two in the field (this grouping accounted for only 4 out of 1235 bumble bees). We also recorded the floral taxa on which each bee was observed foraging. Double counting of individuals was minimized by recording all bees at one flower patch and then moving to another; because our methods target larger bees, it is possible that we underestimated the abundance of smaller wild bees in the ‘other’ category.
During each site visit we positioned a 20 m × 1 m transect to represent the available floral resources. We identified all plants with insect-visited flowers to species (occasionally to genus) and counted the number of flowering units per species. We defined a flowering unit as a single flower or cluster of flowers that a bee could forage from without having to fly between separate units (Saville 1993); therefore a single plant stem may have more than one flowering unit.
Data analysis
To accurately represent the bee abundance at each site, the mean abundance for each bee group and species was calculated from both rounds of sampling. Bumble bee species richness was simply the total number of species observed during both rounds of sampling. To characterize the floral resources available at each site we calculated the mean floral units per m2 from both rounds of sampling and then extrapolated that representative density to the site boundaries (boundaries were determined either in the field or via satellite imagery). Floral abundance and species richness were evaluated for plant species on which we observed foraging bees in this study, or on which we observed foraging in a larger two-year bumble bee survey of approximately 25,000 bumble bee observations at over 300 sites (J Lanterman, K Goodell, P Reeher, R Mitchell unpublished data). Floral species richness was determined by simply tallying the total number of species observed during both rounds of sampling.
Statistical analyses were conducted using SAS 9.4 (SAS Institute Inc 2012). We used ANCOVA to test for differences in bumble bee abundance and species richness by percent intensely developed land, floral abundance, and floral species richness with site nested within city, and city incorporated as a random blocking factor. The assumptions of ANOVA and ANCOVA were tested using Levene’s test for homogeneity of variance and we investigated variance inflation factor values to ensure the absence of collinearity. Due to high collinearity with intensely developed land, additional land cover values like urban greenspace (Developed Open Space category in the USGS NLCD) and forest (combined value from the USGS NLCD categories of Deciduous Forest, Evergreen Forest, and Mixed Forest categories) could not be included in analyses (Pearson r = −0.7296, N = 33; r = −0.6464, N = 33 respectively) as each site buffer was composed of all land cover types within a 500 m radius of the site center. We analyzed the response of (a) all bees and (b) bumble bee abundance and (c) bumble bee species richness to land cover and floral resources using Generalized Linear Mixed Models (SAS PROC GLIMMIX) with a Gaussian distribution. We used ANOVA to test for differences in abundance for the three bee groups (honeybees, bumble bees, and ‘other’ wild bees) by comparing the mean abundance of each bee group for the 33 individual sites using the General Linear Model (SAS PROC GLM) with a Gaussian distribution and city incorporated as a random variable. Tukey’s post hoc test was used to determine which of the groups were significantly different in relative abundance.
Results
Across all 33 sites we observed 2776 total bees; these included 1235 (44.5%) bumble bees, 913 (32.9%) ‘other’ wild bees, and 628 (22.6%) honeybees. We identified 7 different bumble bee species within these urban environments (Table 1). The most common species of bumble bee, Bombus impatiens, accounted for 60% (740) of the observed bumble bees. Proportional abundance of bumble bee species and mean species richness was similar in these small urban sites (3.7 ± 1.3) as in a larger statewide survey (3.9 ± 1.2) encompassing a wide variety of habitats including larger wildflower meadows and other rural and urban habitats (J Lanterman, K Goodell, P Reeher, R Mitchell unpublished data, Table 1).
Bumble bee abundance was not significantly affected by the proportion of intensely developed land in the local landscape (p = 0.33, F 1,24 = 1.01, Fig. 3a), even though the degree of urbanization varied substantially between sites (percent cover of intensely developed land within a 500 m radius ranged from 6 to 94%). Floral abundance had a positive, significant influence on bumble bee abundance (p = 0.047, F 1,24 = 4.38, Fig. 3b), irrespective of land use. There was no correlation between floral abundance and percent intensely developed land (p = 0.86, F 1, 26 = 0.03; Fig. 3c). The influence of floral abundance on bumble bee abundance was further accentuated once the most common species, Bombus impatiens, was removed from the analysis (p < 0.01, F 1,24 = 9.43). Bumble bee abundance was not influenced by floral richness (p = 0.95, F 1,24 = 0.00).
Bumble bee species richness was not significantly influenced by percent intensely developed land (p = 0.38, F 1,24 = 0.81), floral abundance (p = 0.59, F 1,24 = 0.29), or floral richness (p = 0.13, F 1,24 = 2.45). However, mean bumble bee richness (3.7 species/site ±1.3 SD) for this urban study with necessarily small scale and short duration surveys (30-min total surveys per site, N = 33 sites) mirrored that of a larger, statewide (Ohio) study that surveyed a variety of habitat types with greater duration surveys (90-min total surveys per site, N = 318); (J Lanterman, K Goodell, P Reeher, R Mitchell unpublished data).
There were prominent temporal (e.g. phenological) and spatial patterns for some bumble bee species. Bombus bimaculatus individuals were exclusively observed during the first round of sampling (16 June – 17 July 2018), and never during the second round (25 July - 4 August). A similar pattern was found for individuals of B. perplexus; 18 out of 19 individuals were observed during the first round of sampling. Spatial variation was notable for two species. Nearly half of B. fervidus (27 out of 59 individuals) were observed in Pittsburgh, and over half of B. vagans (8 out of 15) occurred in Youngstown. Additionally, there was a notable difference in the mean overall bee and bumble bee abundances between two of the cities; Pittsburgh had an 83% greater mean abundance of bees than Toledo (325.5 and 135, respectively) and a 97% greater mean abundance of bumble bees (165 and 57, respectively).
The abundance of the three functional groups of bees (honeybees, bumble bees, and other bees) differed significantly (p < 0.01, F 2,91 = 9.97, Fig. 4), with bumble bees being significantly more abundant than either honeybees or ‘other’ wild bees (according to Tukey’s test).
We documented 66 different floral taxa across all 33 sites, with native plant species making up less than 10% of the floral abundance. We observed bees foraging on only 48 of the 66 different floral resources, and 93% of observed visits were on non-native, weedy, floral species. In fact, >50% of observations were on just 4 of these floral species (all non - native): Centaurea stoebe L. (Asteraceae), Cirsium arvense L. (Asteraceae), Dipsacus sp. L. (Dipsacaceae), and Securigera varia L. (Fabaceae).
Discussion
Our findings affirm both of our predictions: (1) bumble bees were unaffected by the degree of intensely developed land along an urban gradient and (2) bumble bees were more abundant than other bee groups in these urban environments. These results support the assertion that bumble bees have an advantage over other bee groups in utilizing the resources distributed throughout urban environments, presumably because of various life history traits like increased flight distance capabilities, generalist diet, sociality, and small, annual colony life cycles. This study also highlights weedy margins located outside of conventional urban greenspaces as important contributors to urban pollinator habitat.
Exclusively investigating weedy margins gave us a unique view on the influence of urbanization on bee communities. Though other researchers have studied the relationship between urbanization and pollinators, they have done so by primarily surveying within city parks, community and botanical gardens, and urban farms (McFrederick and LeBuhn 2006; Ahrné et al. 2009; Glaum et al. 2017; Banaszak-Cibicka et al. 2018, Bennett and Lovell 2019). Our study indicates that weedy margins, often excluded from the urban greenspace category, provide resources to urban pollinators and bumble bee species. However, weedy margins may represent unique challenges to urban bees. It might be valuable for future studies to investigate whether urban pollinators foraging in weedy margins have greater exposure to vehicle collisions and contaminants compared to those foraging in other urban greenspaces. Although we restricted interest to weedy margins, bumble bee species richness in our study was similar to that of a larger regional survey (J Lanterman, K Goodell, P Reeher, R Mitchell unpublished data), despite differences in sampling effort, suggesting that bumble bee species richness may be fairly homogenous in any habitat with adequate flowers. This also supports the adequacy of our sampling approach (short duration small-scale surveys) for evaluating bumble bee communities in small patches of urban habitat like weedy margins.
Within our study, ‘weedy’, non-native floral species accounted for greater than 90% of floral abundance and observed bee visits. These findings add to the growing body of literature indicating that ‘weedy’, non-native floral species are important foraging resources for pollinators within urban ecosystems (MacIvor et al. 2014, Larson et al. 2014, Lowenstein et al. 2019). In places where plant diversity is low and native species are scarce, non-native floral species extend the foraging season for pollinators by increasing the duration of resource availability by often being the first and last species to bloom (Lowenstein and Minor 2016). Further research investigating the nutritional value provided by non-native, ‘weedy’ and native plant species grown along an urban gradient could guide urban pollinator conservation efforts.
The results of our study indicate that bumble bee abundance and species richness are not influenced by the percent of surrounding intensely developed land, however, this could reflect the fact that all of the cities within this study are considered a “shrinking city” (Shetty 2009). Shrinking cities are defined as those that have experienced mass human emigration or the mass movement of city residents to permanently establish residence elsewhere, outside of the city (Shetty 2009; Pallagst et al. 2011). In comparison to stable and growing cities, shrinking cities often have increased levels of vacancy and reduced maintenance regimes, resulting in an increase of vegetation in weedy margins. Other studies have found similar results of shrinking cities supporting abundant and species rich pollinator and bumble bee assemblages suggesting the need for comparative studies between pollinator communities within shrinking and non-shrinking cities (Glaum et al. 2017; Sivakoff et al. 2018). Understanding how shrinking cities can support abundant and diverse pollinators could potentially provide insight on how to create urban environments that are more pollinator friendly through simple adjustments to maintenance regimes or landscape planting schemes. Additionally, many cities, including a few within this study, have implemented pollinator habitat programs/initiatives where flowering prairie plants are purposefully planted and managed. Comparing the abundance and species richness of urban wildflower plantings to urban weedy margins with comparable levels of surrounding intensely developed land could provide biologically important information. This information could help pollinator conservation organizations and city planners determine where to focus their efforts.
To minimize the variation in quantity and quality of floral resources between sites we selected sites that had greater than 400 m2 but less than 0.4 ha of exclusively weedy flowering vegetation allowing us to concentrate primarily on the influence of intensely developed land on bumble bee abundance and richness. Despite our efforts to control for variation in quantity of floral resources, there was a significant increase in bumble bee abundance in sites with greater flower abundance. In contrast, landscape-scale features like percent of surrounding intensely developed land had no effect despite substantial variation among sites (6–97%). These results suggest that the local-scale features of urban environments, like floral abundance, have a greater influence on bee abundance than landscape-scale features, like percent intensely developed land. This implies that weedy margins, with reduced maintenance, can provide nesting and foraging habitat capable of supporting bumble bee populations.
The temporal and spatial variation in bumble bee abundance and species composition observed in this study provides valuable biologic information about the various species’ current phenology and regional distributions. The observation of B. bimaculatus and B. perplexus almost exclusively within June and early July reinforces their accepted phenologies. Both species are recognized as being among the earliest to begin and end colony growth (Williams et al. 2014; Lanterman et al. 2019). The spatial variation observed for B. fervidus and B. vagans might provide potential “hotspot” locations of more robust populations or indicate environmental “sweet spots” that might host a greater abundance of their preferred nesting habitat for these less common species. Descriptive information for these less common bee species is vital for accurate documentation of abundance, potentially indicating changes in population size, and providing information on the response of these organisms to environmental changes like urbanization, agricultural expansion (Wood et al. 2019), and climate change (Kerr et al. 2015; Wood et al. 2019).
We found that bumble bees were the most abundant group of bees in these weedy urban patches, with honeybees only 51% as abundant, and ‘other’ wild bees approximately 74% as abundant. It is clear that bumble bees thrive in these weedy urbanenvironments and are a major portion of the pollinator fauna there. These findings are further supported when compared to the relative abundances of these same bee groups within the larger statewide, Ohio survey mentioned earlier (J Lanterman, K Goodell, P Reeher, R Mitchell unpublished data) where honeybees are 99% as abundant, and ‘other’ wild bees approximately 46% as abundant as bumble bees. These findings support the suggestion that Bombus may be especially suited to urban environments. However, the high abundance of ‘other’ wild bees in combination with their increased relative abundance to bumble bees in urban environments indicates that Bombus might not be the only genus able to thrive within developed ecosystems. Future research targeting other genera could provide a more comprehensive understanding of urban bee communities.
The two most distant and topographically divergent cities, Pittsburgh and Toledo, differed dramatically in abundance of bumble bees. This may reflect the topographic differences between the two cities. Pittsburgh and the surrounding areas are nestled on the outskirts of the Appalachian Mountains within the Appalachian Plateau, with elevations ranging from roughly 213 – 421 m above sea level. Toledo, on the other hand, is on an ancient glacial lakeshore with a roughly consistent elevation of 180 m. Pittsburgh’s steep slopes provide an abundance of undisturbed and undeveloped land where bumble bees and other pollinators might be able to establish nests and forage. The low abundance of bees in weedy margins of Toledo may also relate to efforts to provide more bee habitat in that city. The city of Toledo and the Toledo Zoo participate in the Wild Toledo project, in which vacant lots and/or managed lawns in urban environments are converted into prairie habitat (“pocket prairies”). These restoration habitats aim to attract bees, and therefore may have redirected bees that would have otherwise been foraging in weedy margins.
Conclusion
Our study confirms that bumble bee abundance is not influenced by the level of surrounding intensely developed land in cities, and instead responds more strongly to the abundance of local floral resources. This supports pollinator conservation efforts promoting both habitat restoration and reduced maintenance regimes resulting in weedy margins. Our research also indicates that bumble bees are more abundant than other bee groups in urban environments and that the relative abundances of urban bumble bee species reflects those found within the greater region. These results add to the growing body of literature indicating that urban environments are capable of supporting abundant and diverse pollinators.
References
Ahrné K, Bengtsson J, Elmqvist T (2009) Bumble bees (Bombus spp) along a gradient of increasing urbanization. PLoS One 4(5):e5574
Baldock KCR, Goddard MA, Hicks DM, Kunin WE, Mitschunas N, Morse H, Osgathorpe LM, Potts SG, Robertson KM, Scott AV, Staniczenko PPA, Stone GN, Vaughan IP, Memmott J (2019) A systems approach reveals urban pollinator hotspots and conservation opportunities. Nat Ecol Evol 3(3):363–373
Banaszak-Cibicka W, Twerd L, Fliszkiewicz M, Guejdasz K, Langowska A (2018) City parks vs. natural areas – is it possible to preserve a natural level of bee richness and abundance in a city park? Urban Ecosyst 21:599
Bennett AB, Lovell S (2019) Landscape and local site variables differentially influence pollinators and pollination services in urban agricultural sites. PLoS One 14(2):e0212034
Cameron SA, Lozier JD, Strange JP, Koch JB, Cordes N, Solter LF, Griswold TL (2011) Patterns of widespread decline in north American bumble bees. PNAS 108:662–667
Colla SR, Packer L (2008) Evidence for decline in eastern North American bumblebees (Hymenoptera: Apidae), with special focus on Bombus affinis Cresson. Biodivers Conserv 17(6):1379–1391
ESRI (2016) ArcGIS desktop: release 10.3.0.4322 Environmental Systems Research Institute, Redlands, California
Fortel L, Henry M, Guilbauld L, Guirai AL, Kuhlmann M et al (2014) Decreasing abundance, increasing diversity, and changing structure of the wild bee community (Hymenoptera: Anthophila) along an urbanization gradient. PLoS One 9(8):104679
Geslin B, Le Féon V, Folschweiller M, Flacher F, Carmignac D, Motard E, Perret S, Dajoz I (2016) The proportion of impervious surfaces at the landscape scale structure wild bee assemblages in a densely populated region. Ecol Evol 6(18):6599–6615
Glaum P, Simao M-C, Vaidya C, Fitch G, Iulinao B (2017) Big city Bombus: using natural history and land-use history to find significant environmental drivers in bumble-bee declines in urban development. R Soc Open Sci 4:170156
Goulson D (2012a) Chapter 1: introduction. In: Bumblebees behavior, ecology, and conservation, 2nd edn. Oxford University Press, Oxford, pp 1–12
Goulson D (2012b) Chapter 3: social organization and conflict. In: Bumblebees behavior, ecology, and conservation, 2nd edn. Oxford University Press, Oxford, pp 21–43
Goulson D (2012c) Chapter 6: foraging economics. In: Bumblebees behavior, ecology, and conservation, 2nd edn. Oxford University Press, Oxford, pp 81–99
Goulson D (2012d) Chapter 8: choice of flower species. In: Bumblebees behavior, ecology, and conservation, 2nd edn. Oxford University Press, Oxford, pp 113–129
Goulson D, Lye GC, Darvill B (2008) Decline and conservation of bumble bees. Annu Rev Entomol 53:191–208
Goulson D, Nicholls E, Botias C, Rotheray EL (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347(6229):1255957–1255957
Grixti JC, Wong LT, Cameron SA, Favret C (2009) Decline of bumble bees (Bombus) in the north American Midwest. Biol Conserv 142(1):75–84
Hall DM, Camilo GR, Tonietto RK, Ollerton J, Ahrné K, Arduser M, Ascher JS, Baldock KCR, Fowler R, Frankie G, Goulson D, Gunnarsson B, Hanley ME, Jackson JI, Langellotto G, Lowenstein D, Minor ES, Philpott SM, Potts SG, Sirohi MH, Spevak EM, Stone GN, Threlfall CG (2017) The city as a refuge for insect pollinators. Conserv Biol 31(1):24–29
Homer CG, Dewitz JA, Yang L, Jin S, Danielson P, Xian G, Coulston J, Herold ND, Wickham JD, Megown K (2015) Completion of the 2011 National Land Cover Database for the conterminous United States-representing a decade of land cover change information. Photogrammetric Engineering and Remote Sens 81:345–354
Kerr JT, Pindar A, Galpern P, Packer L, Potts SG, Roberts SM, Rasmont P, Schweiger O, Colla SR, Richardson LL, Wagner DL, Gall LF, Sikes DS, Pantoja (2015) Climate change impacts on bumblebees converge across continents. Science 349(6244):177–180
Kluser S, Peduzzi P (2007) Global pollinator decline: a literature review. UNEP/GRID Europe https://www.researchgate.net/publication/239903454_Global_Pollinator_Decline_A_Literature_Review. Accessed 27 May 2017
Lanterman J, Reeher P, Mitchell RJ, Goodell K (2019) Habitat preference and phenology of nest seeking and foraging spring bumble bee queens in northeastern North America (Hymenoptera: Apidae: Bombus). Am Midl Nat 182(2):131–159
Larson JL, Kesheimer AK, Potter DA (2014) Pollinator assemblages on dandelions and white clover in urban and suburban lawns. J Insect Conserv 18(5):863–873
Liu Z, He C, Zhou Y, Wu J (2014) How much of the world’s land has been urbanized, really? A hierarchical framework for avoiding confusion. Landsc Ecol 29(5):763–771
Lowenstein DM, Matteson KC, Minor ES (2019) Evaluating the dependence of urban pollinators on ornamental, non-native, and ‘weedy’ floral resources. Urban Ecosyst 22:293–302
MacIvor JS, Cabral JM, Packer L (2014) Pollen specialization by solitary bees in an urban landscape. Urban Ecosyst 17:139–147
McFrederick QS, LeBuhn G (2006) Are urban parks refuges for bumble bees Bombus spp. (Hymenoptera: Apidae)? Biol Conserv 129(3):372–382
Michener CD (2007) Chapter 119: tribe Bombini. In: The bees of the world, 2nd edn. The John Hopkins Press, Baltimore, pp 785–802
Ollerton J., Winfree R., Tarrant S (2011) How many flowering plants are pollinated by animals? Oikos 120(3):321 – 326
Pallagst KM, Wiechmann T, Martinez-Fernandez C (2011) Shrinking cities; international perspectives and policy implications. Routledge, New York
Potts SC, Beismeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE (2010) Global pollinator declines: trends, impacts and drivers. Trends in Ecol Evol 25(6):345–353
SAS Institute Inc (2012) SAS/ACCESS® 9.4 Interface to ADABAS: Reference. SAS Institute Inc, Cary
Saville NM (1993) Bumblebee ecology in woodlands and arable farmland. University of Cambridge, Dissertation
Shetty S (2009) Shrinking cities in the Industrial Belt: a focus on small and mid-size cities in northwestern Ohio. University of Toledo Urban Affairs Center. https://www.utoledo.edu/centers/urban-affairs/publications/WORC-UAC-Shrinking-Cities.pdf. Accessed 11 May 2017
Sivakoff FS, Prajzner SP, Gardiner MM (2018) Unique bee communities within vacant lots and urban farms results from variation in surrounding urban intensity. Sustainability 10(6):1926
Szymanski, J, Smith, T, Horton, A, Parking, M, Raga, L, Masson, G, Olson, E, Gifford, K, Hill, L (2016) Rusty patched bumble bee (Bombus affinis) species status assessment. Final report version 1. USFWS. https://www.fws.gov/midwest/Endangered/insects/rpbb/pdf/SSAReportRPBBwAdd.pdf
Williams P, Thorp R, Richardson L, Colla S (2014) An identification guide to bumble bees of North America. Princeton University Press, Princeton
Wood TJ, Gibbs J, Graham KK, Isaacs R (2019) Narrow pollen diets are associated with declining Midwestern bumble bee species. Ecology 100(6):e02697
Acknowledgements
We are grateful to Elizabeth DiCesare and Sarah Schmucker for their help with data collection. We thank Karen Goodell for her assistance in obtaining funding and guidance. We also thank the Ohio Department of Transportation and the University of Akron for funding this research.
Funding
This study was partially funded by the Ohio Department of Transportation, grant number 30571, and by the University of Akron Biology Department.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Reeher, P., Lanterman Novotny, J. & Mitchell, R.J. Urban bumble bees are unaffected by the proportion of intensely developed land within urban environments of the industrial Midwestern USA. Urban Ecosyst 23, 703–711 (2020). https://doi.org/10.1007/s11252-020-00965-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-020-00965-9