Abstract
Loss of habitat area and structural heterogeneity through anthropogenic fragmentation poses a threat to the survival of wildlife, which may be exacerbated by urban pressures. Understanding the underlying ecological processes that influence species’ persistence in fragmented landscapes is vital for conservation. We described the effects of forest fragmentation via measures of patch size and isolation on the taxonomic richness and functional richness of forest bird communities in five Protected Areas within the urban-forest mosaic of the Durban Metropolitan Area, South Africa. We conducted 137 fixed-radius point-count surveys across 41 distinct forest patches during the austral breeding season. We quantified the avian taxonomic, guild and functional richness and measured at each survey patch. We measured the influence of patch size, Euclidean isolation distance, patch shape and habitat configuration (i.e. habitat amount) on each diversity measure. We then conducted a series of General Linear Models to determine how fragmentation and patch configuration influenced the diversity of forest birds. The explanatory variables in the top models had a significant effect on all avian diversity measures but habitat amount did not. The amount of habitat surrounding a patch was not significant for measures of bird diversity but based on the Akaike’s weight it was important for specialist species. Isolation distance did not have a significant effect on the measures of bird diversity. An increase in patch shape index increased species richness. These results show the importance of large forest fragments/patches for the conservation of forest birds and for maintaining ecosystem functioning and services of forests in increasingly urbanising landscapes, to the benefit of the environment and its human population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anthropogenic land-use change has detrimental effects on biodiversity worldwide (Gaston et al. 2003). Habitat loss as a result of conversions of natural environments for human activities is the main threat to the persistence and survival of several plants and animals (Andren 1994). The increase in the human population in urban areas has put direct pressure on natural environments as they are converted for human activities (Brown et al. 2014). Development (e.g. building of roads, houses) or land-use change within a natural habitat often pose threats to the survival of species and communities (Zurita et al. 2006; Maseko et al. 2017, 2019). Kale et al. (2012) found that urbanisation favours resource-generalist species, usually, exotics while negatively affecting overall richness in particular, native species. Understanding the effects that human-induced modifications pose on global biodiversity has become a key aspect in landscape ecology and conservation biology (Fischer and Lindenmayer 2007; Ehlers Smith et al. 2015). Despite the ongoing awareness and research on anthropogenic land-use change and fragmentation effects (e.g. Laurance 2008; Fahrig 2013; Haddad et al. 2015; Böhnert et al. 2016; Collins et al. 2017), the conversion of natural environments for human-induced modifications is continuing at an unprecedented rate. Studies predict that by 2100, at least half of the present biodiversity will be lost because of anthropogenic activities (Alvey 2006; Cushman 2006). Studies document the vital role that biodiversity plays in ecosystem functioning, and suggest that its loss may have significant impacts on the long-term functioning of ecosystems (Alvey 2006; Boyer and Jetz 2014; Brose and Hillebrand 2016).
Landscape modifications may cause a dramatic decline in species richness, which subsequently affects ecosystem functioning. Functional traits are individual species’ attribute features and requirements which are essential for survival and thriving in a given environment (Ricklefs 1991). Ecosystem functioning is not solely dependent on taxonomic richness, but instead is mainly driven by a wide diversity of functional traits contributed by all species within an ecosystem (Flynn et al. 2009). A diversity of traits indicates the availability of diverse resources and a variety of environmental characteristics such as diverse landscape matrices, land-cover types and thus, changes to traits may indicate changing environmental conditions (Ehlers Smith et al. 2017a, 2018a, b). Essentially, diverse traits are key indicators of a well-functioning ecosystem (Flynn et al. 2009; Mayfield et al. 2010); where there are changes to environmental conditions or landscape configuration, specialised niches and their occupying species are at risk of extinction (causing a decrease in functional diversity), consequently affecting ecosystem functioning (Tscharntke et al. 2005; Strohbach et al. 2013; Ehlers Smith et al. 2017a).
Many studies have documented the impacts of habitat fragmentation and its consequences on birds, detailing how bird communities are changed or affected (Andren 1994; McIntyre 1995). Fragmentation is a process of partitioning any contiguous habitat type into smaller and more isolated segments, often because of its conversion for human activities (Villard et al. 1999; Fahrig 2003). Most case-studies find that factors such as escalating human population growth and intensification of anthropogenic land-use are the major drivers of fragmentation (e.g. Andren 1994; McKinney 2002; Scharlemann et al. 2004). Fragmentation effects include: habitat loss; structural complexity, resource and niche diversity loss; increasingly smaller habitat patches; an increase in isolation distance between fragmented patches to the original/source habitat, and an increase in “edge” habitat at the expense of internal habitat structures (Andren 1994; Murcia 1995; Fahrig 2003). Subsequently, this results in increased dispersal and colonisation challenges because of habitat-patch isolation. It also results in a homogenisation of resources and niches in smaller patches through disturbance and “edge effects”; i.e. a decrease in the diverse structures and resources for nesting and foraging. This further leads to species in small patches becoming more susceptible to invasion of alien flora and fauna, and an increase in predation and competition (McIntyre 1995; Murcia 1995; Broadbent et al. 2008; Gaublomme et al. 2008; Husté and Boulinier 2011; Amaya-Espinel et al. 2019). These consequences often drive dramatic declines in species and functional diversity, and their abundance, within fragmented habitats.
Globally, forests play a key role in provisioning and protecting biodiversity. However, forest habitats are being increasingly transformed, mainly for agriculture and human settlements (Scharlemann et al. 2004; Ehlers Smith 2014). Severe loss and fragmentation of forests is occurring in areas of high biodiversity value, with a detrimental effect within forest fragments utilised by forest-dependent species (Scharlemann et al. 2004; Aratrakorn et al. 2006). Changes in forest environments by human-induced modifications results in reduction of specific habitat structures and niches, thus threatening the survival of forest-dependent and specialised species (Bennun et al. 1996; McAlpine et al. 2006; Maseko et al. 2017). The mobility and visibility of birds, their wide array of functional traits, and therefore their varied response to land-use change, is key in documenting the impacts of human-induced modifications (McIntyre 1995).
Persistence of suitable habitat patches usually ensures that species dependent on them survive; however, the main drivers for colonisation of a particular patch by avian species are the size of a patch and isolation distances between them (Walker et al. 2003; Martensen et al. 2008). For many decades, the effects of fragmentation have been documented in reference to the Island Biogeography Theory (IBT) (MacArthur and Wilson 1967; Andren 1994; Norton et al. 2000; Cook et al. 2002), which states that species richness is directly influenced by size and isolation distance between patches or from “mainland” habitat patches (MacArthur and Wilson 1967; Cook et al. 2002). However, research conducted in the past two decades shows that factors such as the configuration and amount of habitat surrounding a patch also influences species richness in fragments as it aids dispersal and colonisation between patches (Norton et al. 2000; Fahrig 2001; Donnelly and Marzluff 2004; Suarez-Rubio and Thomlinson 2009; Fahrig 2013). For example, if the surroundings of a patch are buildings and roads, there will be more dispersal resistance than through a matrix of natural habitat with a diversity of structures surrounding the patch (Ehlers Smith et al. 2018b; Amaya-Espinel et al. 2019). Thus, the hostile matrix brought about by urbanisation can serve as a barrier to the movement of fauna even if patches of native vegetation are located closer to each other (Amaya-Espinel et al. 2019).
In many cases, the primary predictor for species richness is patch size (Walker et al. 2003; Martensen et al. 2008). Generally, specialist species avoid small patches as there is a high chance of extinction because of the lack of available resources and diversity of niches (Walker et al. 2003). Therefore, it is expected that the larger a patch, the greater the diversity of species that will occupy it, in comparison to that found in a small patch. In this study, we documented how forest fragmentation and patch configuration impacted the diversity of forest birds within the eThekwini Municipality, South Africa. Our main objectives were to investigate the effects of (1) patch size, (2) isolation of patches, and (3) the influence of patch shape on the taxonomic richness and functional diversity of forest-bird species in five forested Protected Areas. We predicted that an increase in isolation distance would negatively affect bird species richness in isolated patches, because patch size and increasing isolation reduce the probability of colonisation (Bender et al. 2003). Assuming larger patches provision more diverse ecological niches and resources (Soga et al. 2013), we predicted that these patches would contain the highest avian species richness, functional diversity and the most specialist species. According to the habitat amount hypothesis, the availability of suitable habitat patches around a given/surveyed patch would positively influence species richness in that patch (Fahrig 2013). Therefore, we predicted that the amount of habitat surrounding a surveyed patch would positively influence avian species richness and the number of forest specialist species because of a) an increase in availability of niches and resources (i.e. suitable habitat) and b) increasing dispersal opportunities with decreased dispersal resistance. We predicted that patch-shape irregularity would negatively affect forest-specialist species and functional diversity because it represents an increase of homogenised “edge” habitat and structures, in relation to interior habitat. Conversely, we predicted that patch shape irregularity would increase species richness as there would be an increase in edge-tolerant and generalist species sharing similar functional traits. Isolation distance negatively affects sedentary species when they try to disperse (from natal range to find territory, mates, etc.) because their functional traits prevent dispersal across wide isolation distances (Harris and Reed 2002; Ehlers Smith et al. 2018b). Therefore, we predicted that non-migratory species and forest-specialists would be affected by isolation distance.
Methods
Study sites
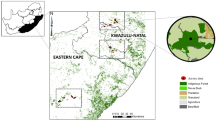
The study was conducted in five forested Protected Areas within eThekwini Municipality (Durban), KwaZulu-Natal (KZN), South Africa (Table 1, Zungu et al. 2019; Maseko et al. 2019). The city of Durban (29.8587° S, 31.0218° E) is on the eastern coast of KZN, within the Indian Ocean Coastal Belt (Fig. 1). Like many other Metropolitan areas in the world, the remaining natural environments in Durban are on the verge of being converted for agriculture, buildings, roads and human settlements. The area of eThekwini Municipality is 2297 km2, and most of the landscape is utilised for settlements and industries (eThekwini Municipality 2010). However, within the city there are open areas used for biodiversity conservation through the Durban Metropolitan Open Space System (D’MOSS) programme, which is concerned with either natural/Protected Areas or those restored back to natural environments with the main aim to protect fauna and flora (Boon et al. 2016; McPherson et al. 2016; Zungu et al. 2019; Maseko et al. 2019). Currently, ~ 4 million people (and rising) reside in Durban (eThekwini Municipality 2010; Boon et al. 2016), and with the current escalating human population growth, most of the remaining natural environments are likely to be affected because of urbanisation. D’MOSS areas mainly comprise of indigenous coastal scarp and coastal lowland forests, coastal thicket/dense bush (which may be considered secondary/regenerating indigenous forest; Ehlers Smith et al. 2017a, b), and grasslands (Zungu et al. 2019; Maseko et al. 2019). The region has a subtropical climate and receives an annual rainfall of ~1000 mm, the majority of which falls during summer (McPherson et al. 2016).
Data collection and initial analyses
We completed a total of 137 fixed-radius point-counts within 41 distinct forest patches in the five Protected Areas (Maseko et al. 2019). As per Ehlers Smith et al. (2017a, 2018a, b) we used the most recent land cover maps (GeoTerra Image 2014) to overlay a systematic point grid-system of 200 m × 200 m on the indigenous forest and coastal thicket/dense bush layers of our five Protected Area study sites (Table 1) using ArcGIS v10.2 (Environmental Systems Research Institute 2011). The cross-points of the grid lines were used as locations to conduct surveys (Fig. 1; Maseko et al. 2019) and were projected onto a global position system (Garmin GPSMAP 64 Mapping Handheld GPS) and located in the field. Some of the physical points were not geographically accessible, resulting in some identified points not being surveyed. However, we ensured that each sampled point was not 50 m further from the projected point and the 200 m inter-point distance was maintained. At each point, we conducted a fixed-radius point-count survey wherein we identified all bird species within a 100 m radius using visual and auditory cues. Surveys were done in the first 3 h after sunrise, and at each point we surveyed for 10 min. Each survey was consistently done by the same three people between October 2016 and March 2017, to cover the breeding season of southern Africa (Maseko et al. 2019). To ensure detectability of forest birds, we conducted the surveys on days when there was no rain and the wind was <4 km/h (Bibby et al. 2000). Any identification uncertainties were unrecorded.
We calculated the patch size of all the distinct patches surveyed within each of our Protected Area study sites (Table 1). We then selected the largest patch in our survey region as the regional “mainland”/core patch. We calculated the distance of all the other patches to the core patch as the isolation distance. To determine the species richness of each patch, we pooled the total number of species recorded in each survey point conducted within a particular patch. We then created a species presence/absence binary matrix for each distinct patch (Ehlers Smith et al. 2018a, b). We categorised birds according to their feeding guilds and determined the number of insectivores, carnivores, frugivores, nectarivores, omnivores and granivores within each patch (i.e. feeding guild diversity; c.f. Hockey et al. 2005). Additionally, we calculated the number of forest-dependent species in every patch (c.f. Oatley 1989; Hockey et al. 2005). Although Oatley’s (1989) list has been used in several studies (e.g. Ehlers Smith et al. 2017a, 2018b; Maseko et al. 2019) for defining forest dependent species, however there have been issues raised with some species being in above-mentioned list (see Brown 2006).
With reference to Hockey et al. (2005), we created a matrix of functional traits that included main diet (carnivory, frugivory, granivory, insectivory, nectarivory, omnivory), main foraging strategy (harvest [fruit/seed/nectar], terrestrial probe, arboreal probe, glean, hawk, perch and swoop, various [omnivory]), nesting substrate (cup/ball/bowl, cavity, ground, platform) and body mass in grams. We then calculated the functional diversity score of each surveyed patch in package FD using R v3.3.1 (R Core Team 2015). Functional diversity was calculated using the Functional Richness (FRic) calculation which describes functional richness as a convex hull of traits present in the community, determined using a Gower’s distance matrix (which accounts for a combination of categorical and continuous covariates; Gower 1966).
The average inter-patch distances for coastal thicket/dense bush, and indigenous forest patches is 0.66 km and 0.42 km, respectively (Olivier et al. 2013). Therefore, using ArcGIS v10.2 (Environmental Systems Research Institute 2011), we created a mean 0.54 km buffer around each survey patch to determine the number of available patches around our surveyed patches as a measure of patch-configuration connectivity and the total habitat amount (i.e. area of coastal thicket/dense bush and indigenous forests) which can be utilised by the birds within the 0.54 km buffer. Contrary to the IBT, landscape ecology highlights the importance of fragment shape (Laurance and Yensen 1991). Therefore, to determine fragment shape and its deviation from circularity we calculated the Shape Index (SI) using the model of Patton (1975). The ordinary perimeter to area/ratio only focuses on the size of the patch, whereas the SI examines both the patch size and irregularities of the patch perimeter (Hill and Curran 2003). The standard SI value of a circular patch is 1.0, and increases with irregularity /complexity of a patch (Laurance and Yensen 1991; Hill and Curran 2003; Ewers and Didham 2007). Perimeter and area of each fragment were calculated in ArcGIS v10.2 (Environmental Systems Research Institute 2011). The Shape Index (SI) (Patton 1975) used was SI = P / 200(π.TA)0.5 where P = Perimeter (m), and TA = Fragment area (ha).
Data analyses
To avoid problems of multicollinearity we created a correlation matrix to test for significant correlations between explanatory covariates (i.e. patch size, isolation distance, habitat amount and shape index) with a correlation threshold of r ≥ 0.7 (Garden et al. 2010). No individual covariate exceeded this threshold; hence each explanatory covariate was retained. We created General Linear Models (GLMs) with an identity-link function to determine the importance of each explanatory variable both in isolation and as an interaction on our primary response variables (i.e. species richness, functional diversity, and forest specialist species). We used the Akaike’s Information Criterion values to rank and select the model of best fit (Burnham and Anderson 2002), with the highest model for each response variable being that containing the most significant explanatory variables. We calculated the Akaike Weight of each model to determine the strength of explanatory variables on response variables. To further elucidate factors pertaining to our hypotheses, we tested the significance of (i) patch size on individual feeding guilds (ii) isolation distance on sedentary species. All statistical analyses were conducted in IBM SPSS statistics version 20.0.
Results
The total avian species richness recorded across the five study sites was 75 (see Maseko et al. 2019 for bird species list). Based on model selection, GLMs showed that (i) patch size and shape index were the most significant covariates for species richness; (ii) patch size was the only significant covariate driving functional diversity, and (iii) shape index, habitat amount and patch size were the most significant covariates for forest specialists (Table 2). All explanatory covariates in the top models had a significant effect on all measures of bird diversity, except habitat amount on forest specialists (Table 3). Patch size had a significant effect on functional feeding guilds: insectivores (F = 82.140, P < 0.001, r2 = 0.678), carnivores (F = 16.657, P < 0.001, r2 = 0.30), frugivores (F = 28.571, P < 0.001, r2 = 0.42), nectarivores (F = 20.291, P < 0.001, r2 = 0.34), omnivores (F = 17.384, P < 0.001, r2 = 0.31) and granivores (F = 41.059, P < 0.001, r2 = 0.51). Patch size had a significant effect on (i) species richness (F = 95.305, P < 0.05, r2 = 0.71), (ii) functional diversity (F = 10.110, P < 0.05, r2 = 0.21) and (iii) forest specialists (F = 109.395, P < 0.05, r2 = 0.74). Isolation distance had no significant effect on (i) species richness (F = 0.03, P > 0.05, r2 = 0), (ii) functional diversity (F = 0.137, P > 0.05, r2 = 0), (iii) forest specialists (F = 0.671, P > 0.05, r2 = 0.02) or (iv) sedentary species (F = 0.071, P = 0.891, r2 = 0). Habitat amount had no significant effect on (i) species richness (F = 0.445, P > 0.05, r2 = 0), (ii) functional diversity (F = 0.260, P > 0.05, r2 = 0) or (iii) forest specialists (F = 1.710, P > 0.05, r2 = 0.04). However, habitat amount was important for explaining forest specialist species’ diversity, based on model selection (Table 3, Fig. 2). Shape index had a significant effect on species richness (F = 58.288, P < 0.05, r2 = 0.60) and forest specialists (F = 69.343, P < 0.05, r2 = 0.64) but not on functional diversity (F = 6.020, P > 0.05, r2 = 0.13).
Discussion
Our study region is highly threatened through fragmentation by anthropogenic activities including human settlement developments, industry and agriculture. Habitat patch-size is a key parameter for the persistence of species in fragmented habitats (Fahrig 2003; Uezu et al. 2005; Suarez-Rubio and Thomlinson 2009; Soga et al. 2013; Strohbach et al. 2013). Our results showed that avian species richness increased with increasing forest patch size (present study; Maseko et al. 2019), which supported our first prediction that larger forest patches would have more diverse ecological niches and resources, thus supporting a diverse avifaunal community (Maseko et al. 2019). Moreover, habitat-patch size was important for explaining the occurrence of forest-dependent species. Given our prediction that larger forest patches would have more diverse structures, resources and therefore ecological niches, we assumed that the strongly positive relationship between large forest patches and forest specialists was because of the availability of specific ecological niches (which are key for supporting a diverse avian community) within these patches (Maseko et al. 2019). This further supported our first prediction and highlights the importance of ensuring that these forest fragments are protected from any additional anthropogenic disturbances, ensuring its persistence and reduce the chances of losing forest-dependent species.
Our results showed that patch irregularity increased with forest patch size. This indicated that larger forest patches had an increase in habitat area within its increased “edges”. This was likely to support a range of generalist and edge-tolerant avian species, hence avian species richness increased (Germaine et al. 1997; Bender et al. 2003; Ewers and Didham 2007; Maseko et al. 2019), supporting our prediction of increasing avian species richness with patch shape irregularity. Conversely, smaller forest patches with an increase in ratio of edge to interior were more likely to have more generalists and fewer specialists, and low overall functional diversity (Germaine et al. 1997; Soga et al. 2013). Isolation distance had no significant effect on the overall diversity measure in our study. Given the timing of our surveys, i.e. during the breeding season, we assumed initially that the presence of Palearctic, intra-African and altitudinal avian migrants (Hockey et al. 2005) in our data set drove the resilience to the isolation effect. However, after testing for the significance of isolation distance on the avian community with Palearctic, intra-African and altitudinal avian migrants removed isolation distance was still insignificant. A possible explanation for this is functional resilience to isolation during the dispersal process given the biogeographic evolution of the community in a naturally fragmented forest-grassland mosaic; however, a previous study showed the strong negative effect of isolation on sedentary forest specialists in the same biome (Ehlers Smith et al. 2018b). Thus, surrounding land use is likely to play a significant role in dispersal ability. We highlight that although general trends are emerging in the effects of anthropogenic fragmentation on avian forest communities, certain complexities in the drivers of decline are yet to be elucidated. Thus, we recommend further research into the effects of anthropogenic fragmentation on the phylogenetic diversity of the IOCB avian community.
Our results showed that functional diversity increased with patch size, thus supporting our prediction that larger forest patches would have high functional diversity, presumably provisioned for by the diversity of structures, resources and niches available in less-disturbed, larger patches (Maseko et al. 2019). Critically, changes in taxonomic richness and functional diversity were correlated, indicating that as functional traits were pressured and ultimately lost by patch size, resource and niche reduction, the species that possessed vulnerable traits became locally extinct. This is problematic as it results in a homogenisation of avian communities (Morelli et al. 2016), with the success of generalist species with similar traits at the expense of a diverse community with many specialised traits. For conservation purposes, this result is key because it shows that larger patches are not only crucial for species richness, but also for the functional diversity essential for healthy ecosystem functioning. Its loss has significant and critical impacts on the long term-functioning of ecosystems (Alvey 2006; Boyer and Jetz 2014; Brose and Hillebrand 2016). For example, loss of seed-dispersing bird species can have detrimental effects on the long-term survival of plants and food availability for many species, thus affecting the functioning and persistence of an ecosystem (Cordeiro and Howe 2003). Via ecosystem services provided by birds, crucial survival and financial needs for the survival of humans are met (Alho 2012). For instance, through seed dispersal and pollination services, birds ensure that plant species are produced, which is crucial to humans for agricultural crops and medicine/drugs (Alho 2012). Natural spaces, including forests, are critical for the spiritual and mental well-being of humans, particularly in heavily-urbanised areas (Miura et al. 2015). Through recreational activities such as hiking and bird-watching humans benefit mentally and financially, as people gain employment for their skills in wildlife guiding (Greenwood 2007). Critically, the richness of all functional avian feeding guilds (insectivores, carnivores, frugivores, nectarivores, omnivores and granivores) increased with increasing patch size. Various food sources in larger forest patches provisioned for all avian feeding guilds to persist and thrive, hence we observed a positive relationship between larger forest patches and all avian feeding guilds (Uezu et al. 2005). Therefore, we concluded that larger forest patches played a significant role in ensuring the survival of diverse avian communities in an urbanised landscape.
Current studies show the importance of the habitat matrix (i.e. the amount of habitat and the configuration of natural habitat patches in the landscape surrounding fragmented forest landscapes) in facilitating immigration and colonisation, which allow species to disperse and persist (Laurance 2008; Fahrig 2013; Neuschulz et al. 2013). We predicted that an increasing amount of habitat available around a surveyed forest patch would have a positive effect on avian species richness and the diversity of forest specialists, but our results showed that there was no significant effect on either avian species richness nor forest specialist species. Fahrig (2013) discussed that a relationship between avian species richness and habitat amount is dependent on matrix quality surrounding the surveyed patch: high quality matrix influences the relationship between habitat amount and avian species richness whereas low quality matrix may result in the two variables having no relationship. Therefore, considering the study region (relatively high-density housing), we expected that the low quality of the matrix surrounding our forests drove the lack of relationship between habitat amount and overall avian species richness. However, the Akaike weight of habitat amount showed its importance for forest specialist species, suggesting that for this sedentary guild, habitat configuration in the matrix was key, supporting the results of Ehlers Smith et al. (2018b).
In conclusion, our study highlighted the crucial role that forests and forest fragments play in protecting and providing habitat for various bird communities. We show the importance of larger forest patches in providing and maintaining ecosystem functioning, and the likelihood of disappearance of forest specialist species in small patches. Therefore, because of the crucial role these forest fragments play in conservation of various bird communities, we urge South African policy makers and all stakeholders involved in decision-making to ensure that the protection of natural environments (particularly forests) against anthropogenic activities is prioritised. Our results have general relevance for ensuring that future generations in heavily urbanised environments benefit through ecosystem services provided by species occupying embedded natural habitats.
References
Alho CJ (2012) The importance of biodiversity to human health: an ecological perspective. Estud Av 26:151–166
Alvey AA (2006) Promoting and preserving biodiversity in the urban forest. Urban For Urban Green 5:195–201
Amaya-Espinel JD, Hostetler M, Henríquez C, Bonacic C (2019) The influence of building density on neotropical bird communities found in small urban parks. Landsc Urban Plan. https://doi.org/10.1016/j.landurbplan.2019.05.009
Andren H (1994) Effects of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: a review. Oikos 71:355–366
Aratrakorn S, Thunhikorn S, Donald PF (2006) Changes in bird communities following conversion of lowland forest to oil palm and rubber plantations in southern Thailand. Bird Conserv Int 16:71–82
Bender DJ, Tischendorf L, Fahrig L (2003) Using patch isolation metrics to predict animal movement in binary landscapes. Landsc Ecol 18:17–39
Bennun L, Dranzoa C, Pomeroy D (1996) The forest birds of Kenya and Uganda. J East Afr Nat Hist 85:23–48
Bibby C, Burgess N, Hill D (2000) Bird census techniques. Academic Press, London
Böhnert T, Wenzel A, Altenhöve C, Beeretz L, Tjitrosoedirdjo SS, Meijide A, Kreft H (2016) Effects of land-use change on vascular epiphyte diversity in Sumatra Indonesia. Biol Conserv 202:20–29
Boon R, Cockburn J, Douwes E, Govender N, Ground L, Mclean C, Slotow R (2016) Managing a threatened savanna ecosystem KwaZulu-Natal sandstone Sourveld in an urban biodiversity hotspot: Durban, South Africa. Afr Biodivers Conserv 46:1–12
Boyer AG, Jetz W (2014) Extinctions and the loss of ecological function in island bird communities. Glob Ecol Biogeogr 23:679–688
Broadbent EN, Asner GP, Keller M, Knapp DE, Oliveira PJ, Silva JN (2008) Forest fragmentation and edge effects from deforestation and selective logging in the Brazilian Amazon. Biol Conserv 141:1745–1757
Brose U, Hillebrand H (2016) Biodiversity and ecosystem functioning in dynamic landscapes. Philos Trans R Soc B 371:1–9
Brown M (2006) Annual and seasonal trends in avifaunal species richness in a coastal lowlands forest reserve in South Africa. Ostrich 77:58–66
Brown ML, Donovan TM, Schwenk WS, Theobald DM (2014) Predicting impacts of future human population growth and development on occupancy rates of forest-dependent birds. Biol Conserv 170:311–320
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Collins CD, Banks-Leite C, Brudvig A, Foster BL, Cook WM, Damschen EI, Holt RD (2017) Fragmentation affects plant community composition over time. Ecography 40:119–130
Cook WM, Lane KT, Foster BL, Holt RD (2002) Island theory, matrix effects and species richness patterns in habitat fragments. Ecol Lett 5:619–623
Cordeiro NJ, Howe HF (2003) Forest fragmentation severs mutualism between seed dispersers and an endemic African tree. Proc Natl Acad Sci USA 100:14052–14056
R Core Team (2015) R: a language and environment for statistical computing R Foundation for statistical computing. Austria, Vienna
Cushman SA (2006) Effects of habitat loss and fragmentation on amphibians: a review and prospectus. Biol Conserv 128:231–240
Donnelly R, Marzluff JM (2004) Importance of reserve size and landscape context to urban bird conservation. Conserv Biol 18:733–745
Ehlers Smith DA (2014) The effects of land-use policies on the conservation of Borneo’s endemic Presbytis monkeys. Biodivers Conserv 23:891–908
Ehlers Smith YC, Ehlers Smith DA, Seymour CL, Thébault E, van Veen FJF (2015) Response of avian diversity to habitat modification can be predicted from life-history traits and ecological attributes. Landsc Ecol 30:1225–1239
Ehlers Smith DA, Ehlers Smith YC, Downs CT (2017a) Indian Ocean coastal thicket is of high conservation value for taxonomic and functional diversity of forest-dependent bird communities in a landscape of restricted forest availability. For Ecol Manag 390:157–165
Ehlers Smith YC, Ehlers Smith DA, Ramesh T, Downs CT (2017b) The importance of microhabitat structure in maintaining forest mammal diversity in a mixed land-use mosaic. Biodivers Conserv 26:2361–2382
Ehlers Smith DA, Si X, Ehlers Smith YC, Kalle R, Ramesh T, Downs CT (2018a) Patterns of avian diversity across a decreasing patch-size gradient in a critically endangered subtropical forest system. J Biogeogr 45:2118–2132
Ehlers Smith DA, Si X, Ehlers Smith YC, Downs CT (2018b) Seasonal variation in avian diversity and tolerance by migratory forest specialists of the patch-isolation gradient across a fragmented forest system. Biodivers Conserv 27:3707–3727. https://doi.org/10.1007/s10531-018-1622-y
ESRI Environmental Systems Research Institute (2011) ArcGIS Desktop:10
EThekwini Municipality (2010) EThekwini Municipality Annual Report 2009−2010. eThekwini Municipality. http://www.durban.gov.za/City_Services/electricity/About%20Us/Pages/Annual-Reports.aspx 2009 10. Accessed 20 Sep 2017
Ewers RM, Didham RK (2007) The effect of fragment shape and species’ sensitivity to habitat edges on animal population size. Conserv Biol 21:926–936
Fahrig L (2001) How much habitat is enough? Biol Conserv 100:65–74
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Fahrig L (2013) Rethinking patch size and isolation effects: the habitat amount hypothesis. J Biogeogr 40:1649–1663
Fischer J, Lindenmayer DB (2007) Landscape modification and habitat fragmentation: a synthesis. Glob Ecol Biogeogr 16:265–280
Flynn DF, Gogol-Prokurat M, Nogeire T, Molinari N, Richers BT, Lin BB, DeClerck F (2009) Loss of functional diversity under land use intensification across multiple taxa. Ecol Lett 12:22–33
Garden JG, McAlpine CA, Possingham HP (2010) Multi-scaled habitat considerations for conserving urban biodiversity: native reptiles and small mammals in Brisbane, Australia. Landsc Ecol 25:1013–1028
Gaston KJ, Blackburn TM, Goldewijk KK (2003) Habitat conversion and global avian biodiversity loss. Proc R Soc Lond B Biol Sci 270:1293–1300
Gaublomme E, Hendrickx F, Dhuyvetter H, Desender K (2008) The effects of forest patch size and matrix type on changes in carabid beetle assemblages in an urbanized landscape. Biol Conserv 141:2585–2596
GeoTerra Image (2014) KZN Province land-cover mapping (from SPOT5 satellite imagery circa 2013) prepared for Ezemvelo KZN wildlife (biodiversity research), South Africa
Germaine SS, Vessey SH, Capen DE (1997) Effects of small forest openings on the breeding bird community in a Vermont hardwood forest. Condor 99:708–718
Gower, J. C. (1966). Some distance properties of latent root and vector methods used in multivariate analysis. Biometrika, 53, 325–338. https://doi.org/10.1093/biomet/53.3-4.325
Greenwood JJ (2007) Citizens, science and bird conservation. J Ornithol 148:77–124
Haddad NM, Brudvig LA, Clobert J, Davies KF, Gonzalez A, Holt RD, Cook WM (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv 1:1–9
Harris RJ, Reed JM (2002) Behavioral barriers to non-migratory movements of birds. Ann Zool Fenn 39:275–290
Hill JL, Curran PJ (2003) Area, shape and isolation of tropical forest fragments: effects on tree species diversity and implications for conservation. J Biogeogr 30:1391–1403
Hockey PAR, Dean WRJ, Ryan PG (2005) Roberts birds of southern Africa, 7th edn. John Voelcker Bird Book Fund, Cape Town
Husté A, Boulinier T (2011) Determinants of bird community composition on patches in the suburbs of Paris, France. Biol Conserv 144:243–252
Kale M, Dudhe N, Kasambe R, Chakane S, Bhattacharya P (2012) Impact of urbanization on avian population and its status in Maharashtra state, India. IJAES 7:59–76
Laurance WF (2008) Theory meets reality: how habitat fragmentation research has transcended island biogeographic theory. Biol Conserv 141:1731–1744
Laurance WF, Yensen E (1991) Predicting the impacts of edge effects in fragmented habitats. Biol Conserv 55:77–92
MacArthur RH, Wilson EO (1967) The theory of island biogeography. Princeton, New Jersey
Martensen AC, Pimentel RG, Metzger JP (2008) Relative effects of fragment size and connectivity on bird community in the Atlantic rain forest: implications for conservation. Biol Conserv 141:2184–2192
Maseko MST, Ramesh T, Kalle R, Downs CT (2017) Response of crested Guinea-fowl (Guttera edouardi), a forest specialist, to spatial variation in land use in iSimangaliso Wetland Park, South Africa. J Ornithol 158:469–477
Maseko MST, Zungu MM, Ehlers Smith D, Ehlers Smith Y, Downs CT (2019) High microhabitat heterogeneity drives high functional diversity of forest birds in five protected areas of Durban, South Africa. Glob Ecol Conserv. https://doi.org/10.1016/j.gecco.2019.e00645
Mayfield MM, Bonser SP, Morgan JW, Aubin I, McNamara S, Vesk PA (2010) What does species richness tell us about functional trait diversity? Predictions and evidence for responses of species and functional trait diversity to land-use change. Glob Ecol Biogeogr 19:423–431
McAlpine CA, Rhodes JR, Callaghan JG, Bowen ME, Lunney D, Mitchell DL, Possingham HP (2006) The importance of forest area and configuration relative to local habitat factors for conserving forest mammals: a case study of koalas in Queensland, Australia. Biol Conserv 132:153–165
McIntyre NE (1995) Effects of forest patch size on avian diversity. Landsc Ecol 10:85–99
McKinney ML (2002) Urbanization, biodiversity, and conservation: the impacts of urbanization on native species are poorly studied, but educating a highly urbanized human population about these impacts can greatly improve species conservation in all ecosystems. Bioscience 52:883–890
McPherson SC, Brown M, Downs CT (2016) Crowned eagle nest sites in an urban landscape: requirements of a large eagle in the Durban metropolitan open space system. Landsc Urban Plan 146:43–50
Miura S, Amacher M, Hofer T, San-Miguel-Ayanz J, Ernawati, Thackway R (2015) Protective functions and ecosystem services of global forests in the past quarter-century. For Ecol Manag 352:35–46
Morelli F, Benedetti Y, Ibáñez-Álamo JD, Jokimäki J, Mänd R, Tryjanowski P, Møller AP (2016) Evidence of evolutionary homogenization of bird communities in urban environments across Europe. Glob Ecol Biogeogr 25:1284–1293
Murcia C (1995) Edge effects in fragmented forests: implications for conservation. Trends Ecol Evol 10:58–62
Neuschulz EL, Brown M, Farwig N (2013) Frequent bird movements across a highly fragmented landscape: the role of species traits and forest matrix. Anim Conserv 16:170–179
Norton MR, Hannon SJ, Schmiegelow FK (2000) Fragments are not islands: patch vs landscape perspectives on songbird presence and abundance in a harvested boreal forest. Ecography 23:209–223
Oatley TB (1989) Biogeography of the forest avifauna in South Africa. In: Geldenhuys CJ (ed) Biogeography of the mixed evergreen forests of southern Africa occasional report no 45. CSIR, Pretoria, pp 48–59
Olivier PI, van Aarde RJ, Lombard AT (2013) The use of habitat suitability models and species-area relationships to predict extinction debts in coastal forests, South Africa. Divers Distrib 19:1353–1365
Patton DR (1975) A diversity index for quantifying habitat “edge”. Wildl Soc Bull 3:171–173
Ricklefs RE (1991) Structures and transformations of life histories. Funct Ecol 5:174–183
Scharlemann JP, Green RE, Balmford A (2004) Land-use trends in endemic bird areas: global expansion of agriculture in areas of high conservation value. Glob Chang Biol 10:2046–2051
Soga M, Kanno N, Yamaura Y, Koike S (2013) Patch size determines the strength of edge effects on carabid beetle assemblages in urban remnant forests. J Insect Conserv 17:421–428
Strohbach MW, Lerman SB, Warren PS (2013) Are small greening areas enhancing bird diversity? Insights from community-driven greening projects in Boston. Landsc Urban Plan 114:69–79
Suarez-Rubio M, Thomlinson JR (2009) Landscape and patch-level factors influence bird communities in an urbanized tropical island. Biol Conserv 142:1311–1321
Tscharntke T, Klein AM, Kruess A, Steffan-Dewenter I, Thies C (2005) Landscape perspectives on agricultural intensification and biodiversity and ecosystem service management. Ecol Lett 8:857–874
Uezu A, Metzger JP, Vielliard JM (2005) Effects of structural and functional connectivity and patch size on the abundance of seven Atlantic Forest bird species. Biol Conserv 123:507–519
Villard MA, Trzcinski MK, Merriam G (1999) Fragmentation effects on forest birds: relative influence of woodland cover and configuration on landscape occupancy. Conserv Biol 13:774–783
Walker RS, Novaro AJ, Branch LC (2003) Effects of patch attributes, barriers, and distance between patches on the distribution of a rock-dwelling rodent Lagidium viscacia. Landsc Ecol 18:185–192
Zungu MM, Maseko MST, Kalle R, Ramesh T, Downs CT (2019) Fragment and life-history correlates of extinction vulnerability of forest mammals in an urban-forest mosaic in EThekwini municipality, Durban, South Africa. Anim Conserv. https://doi.org/10.1111/acv.12470
Zurita GA, Rey N, Varela DM, Villagra M, Bellocq MI (2006) Conversion of the Atlantic Forest into native and exotic tree plantations: effects on bird communities from the local and regional perspectives. For Ecol Manag 235:164–173
Acknowledgements
We would like to thank University of KwaZulu-Natal (ZA), National Research Foundation (ZA), Oppenheimer Memorial Trust (ZA) and D’RAP eThekwini Municipality (ZA) for funding the project. We thank the Ford Wildlife Foundation (ZA) for vehicle support. We are grateful to eThekwini Municipality and Ezemvelo KZN Wildlife for allowing us to work in their Protected Areas. We thank Moses Chibesa, David Phiri, Sambulo Cele and Pumla Dlamini for assisting with fieldwork. We are most grateful for the constructive comments of the reviewers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The Authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Maseko, M.S.T., Zungu, M.M., Ehlers Smith, D.A. et al. Effects of habitat-patch size and patch isolation on the diversity of forest birds in the urban-forest mosaic of Durban, South Africa. Urban Ecosyst 23, 533–542 (2020). https://doi.org/10.1007/s11252-020-00945-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-020-00945-z