Abstract
Urbanisation is causing rapid land-use change worldwide. Populations of freshwater turtles are vulnerable to impacts of urbanisation such as habitat loss, fragmentation and degradation, because many species require interconnected aquatic and terrestrial habitats. Understanding the processes that underpin survival in urban areas is critical in managing species that may vary in their responses to urbanisation. Here, we conducted a mark-recapture study of a common freshwater turtle (Chelodina longicollis) at 20 wetlands over five years across a broad geographical gradient in a large and expanding Australian city. Our aim was to examine relationships between survival and a broad suite of local and landscape environmental variables, and body condition. Using capture-recapture models, we found a positive relationship between the probability of survival of C. longicollis and the proportion of green open space in a 1-km radius around a wetland. There was a positive relationship between survival of female C. longicollis and body condition. Survival probabilities generally did not differ substantially among males, females or juveniles, or seasons. We found evidence of adult turtle mortality resulting from recreational fishing. Our results demonstrate the importance of terrestrial habitat surrounding wetlands for freshwater turtle survival in an urban environment. Our results suggest that management actions for C. longicollis in urban areas need to protect green spaces surrounding wetlands (e.g. parks and remnant vegetation) and discourage human actions that threaten turtle survival. Our study adds to mounting evidence that conserving freshwater turtle populations in urban areas requires managers to consider life cycle requirements over broad spatiotemporal scales.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urbanisation is rapidly transforming vast natural areas of the Earth’s surface with modified landscapes dominated by artificial (non-natural) surfaces, restructuring vegetation and endangering biodiversity (Czech et al. 2000; McKinney 2002; Shochat et al. 2006). By 2030 world urban populations are predicted to increase to 5 billion people, who will need an additional 5.87 million km2 of land to be converted to urban land use, and 20% will come from urban expansion (Seto et al. 2012). Freshwater wetlands are an ecosystem that is particularly vulnerable to land-use change and urbanisation at the land-water interface, with hydrology often dependent on surrounding catchments (Ehrenfeld 2000; Lee et al. 2006). Around half the global wetland area has been lost due to human activities, with around a third of the loss in developing countries attributed to urbanisation (Zedler and Kercher 2005). Urbanisation threatens wetland-dependent wildlife through habitat loss, habitat fragmentation and isolation, and habitat degradation (Hamer and McDonnell 2008).
Freshwater turtles are a relatively long-lived group of wetland-dependent animals that often require multiple habitats (aquatic and terrestrial) distributed over broad landscapes to fulfil life cycle requirements over many years (Burke et al. 2000; Joyal and McCollough 2001). Wetland loss and degradation due to urban development threatens many turtle species (Gibbons et al. 2000; Bodie 2001), in addition to the loss of terrestrial habitats that many species use for nesting, overwintering and movement (Mitchell and Klemens 2000; Steen et al. 2012). Moreover, many species are vulnerable to being killed on roads by vehicles while making overland movements (Aresco 2005; Crump et al. 2016), particularly species with high mobility and dependence on terrestrial habitats (Gibbs and Shriver 2002; Eskew et al. 2010). Female mortality incurred on land during nesting migrations may be the most significant threat to freshwater turtle population persistence (Gibbs and Shriver 2002; Steen et al. 2006, 2012). The life history characteristics of freshwater turtles, including high adult survival rates and longevity, delayed sexual maturity and high juvenile mortality (Brooks et al. 1991; Congdon et al. 1993, 1994), predisposes many populations to substantial declines if one or more life stages are negatively affected by urbanisation or roads (Burke et al. 2000; Beaudry et al. 2008).
Correlational studies incorporating land-use gradients to assess patterns of occupancy, abundance and diversity in freshwater turtle species with urbanisation showed mixed relationships, relating to different movement abilities and habitat requirements among species (Marchand and Litvaitis 2004; Rizkalla and Swihart 2006; DeCatanzaro and Chow-Fraser 2010). Moreover, some species are more abundant in degraded wetlands with poor water quality in human-altered landscapes due to the increased availability of food and nesting sites (Souza and Abe 2000; DeCatanzaro and Chow-Fraser 2010), although disease may affect individuals through contaminated water and sediments (Bishop et al. 2010). North American studies show that occupancy of wetland landscapes by freshwater turtle species increases with an increase in the amount of forest cover surrounding wetlands (Guzy et al. 2013; Quesnelle et al. 2013).
The eastern long-necked turtle (Chelodina longicollis) is a highly mobile freshwater turtle species that uses multiple wetlands throughout its life. In urban areas of south-eastern Australia, this common species is largely confined to lakes and reservoirs (Rees et al. 2009), whereas in relatively natural areas the species moves between permanent and ephemeral wetlands depending on water levels, and will aestivate on land for extended periods if nearby wetlands are dry (Roe and Georges 2007, 2008a, 2008b; Roe et al. 2009). The species is regarded as being relatively resilient to habitat degradation associated with urbanisation because it occupies a wide range of wetland types throughout its broad distribution, has a wide prey spectrum, and demonstrates behavioural plasticity in its response to changing environmental conditions (Kennett et al. 2009; Stokeld et al. 2014). Suburban waterbodies can be high quality habitat for C. longicollis during drought periods when other ponds are dry, as evidenced by higher growth rates and relative abundance (Roe et al. 2011). Owing to the frequency of movement and distances travelled in urban landscapes (Rees et al. 2009; Ferronato et al. 2017), C. longicollis is likely to be susceptible to road mortality while undertaking overland migrations, which can reduce survivorship (Ferronato et al. 2016). Population structures may be biased towards larger turtles at waterbodies surrounded by high road densities compared with lower densities, although differences may not be apparent in juvenile size classes (Hamer et al. 2016). These studies suggest that while inhabiting suburban waterbodies may be advantageous for C. longicollis, there are risks associated with terrestrial activities such as nesting and inter-wetland movements.
Here, we assessed a broad array of biotic and abiotic variables measured at multiple spatial scales to elucidate relationships with the survival of C. longicollis in a large Australian city. Our study is unique in that we sampled turtles across a relatively wide geographical gradient throughout the suburbs of Melbourne and over a relatively long time span. By implementing mark-recapture methods, we aimed to provide information on population demographics to better understand which local and landscape factors may be regulating the persistence of populations in urban areas. This study complements two previous studies on the occurrence/ abundance and population structure of C. longicollis in the study area (Stokeld et al. 2014; Hamer et al. 2016). Our general predictions were that survivorship of C. longicollis would decrease with increased landscape fragmentation and habitat degradation, reduced water levels and prey availability, greater conspecific competition, and with poorer body condition.
Methods
Study area
We conducted our study in the greater Melbourne region in Victoria, south-eastern Australia. The area was first settled by European people in 1835 and had a human population of 4.08 million in 2010, which is predicted to reach 6.5 million by 2051, with much of the growth concentrated in the outer suburbs (Ives et al. 2013). Melbourne has a temperate climate, with an annual mean temperature and precipitation of 15.5 °C and 639 mm, respectively, with rainfall distributed evenly throughout the year (Stern 2005). Stormwater retention wetlands have been constructed in new housing estates and adjacent to new motorways since the 1990’s. These wetlands are designed to collect and treat runoff from impervious surfaces such as roads (Victorian Stormwater Committee 1999), but also provide wildlife habitat (Hamer et al. 2012). Only 2% of the area of the inner city of Melbourne is still covered in remnant vegetation, whereas the outer suburbs retain nearly 16% remnant vegetation (McDonnell and Holland 2008).
Turtle surveys
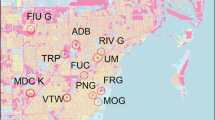
We conducted turtle surveys at 20 wetland sites distributed throughout the greater Melbourne region (Fig. 1). These sites were included in the previous study (Hamer et al. 2016), and were comprised largely of stormwater retention ponds and lakes, and some remnant wetlands. Sites were >2 km apart to ensure we were sampling demographically-independent populations. We conducted turtle surveys at the 20 sites over five seasons when C. longicollis was active, once a month from November to March from 2011/2012 to 2015/2016. No surveys were conducted in March 2016. We used three fyke traps and one cathedral trap at each wetland site (T and L Netmaking, Mooroolbark, Vic., Australia) baited with diced beef, to sample turtles at randomly-selected positions around the wetland perimeter (see Hamer et al. 2016 for details). In 2015/2016, a modified crab trap was used in place of a cathedral trap at some sites. Multiple trapping methods were used to increase capture probabilities of different turtle groups (males, females, juveniles; Tesche and Hodges 2015). Surveys were not conducted at a site if the water level was too shallow to deploy traps (e.g. if the site was being drained or due to summer draw-down), or if permission for access was denied. After accounting for these constraints, we conducted a total of 456 surveys at the 20 sites over the five seasons. Two different pairs of sites were sampled each day over one week of surveys each month. The weekday on which each pair of sites were sampled was randomised to account for differences in weather conditions. Traps were deployed in the morning (about 1000 h Australian Eastern Daylight Time) and removed after a minimum of 4 h (about 1400 h). Further details on site selection and sampling methods are in Hamer et al. (2016).
Location of the wetland sites sampled in the greater Melbourne region, Australia. The black square is the Melbourne central business district. Green open space represents remnant vegetation, parks, gardens and fields. Open space beyond the outer suburbs and study area was not mapped (see Leary and McDonnell 2001)
Turtles were removed from the traps and the straight-line carapace length (CL) of each individual was measured to the nearest 0.1 mm using 300 mm vernier callipers. Turtles were then weighed to the nearest 0.5 g using platform scales. The sex of each individual with CL ≥145 mm was determined using the curvature of the caudal area of the plastron (Kennett and Georges 1990). Individuals with CL <145 mm were classed as juveniles. Female C. longicollis do not become sexually mature until they are 165 mm (Kennett and Georges 1990), but can be differentiated from adult males between 145 and 165 mm as sub-adult females. Accordingly, we grouped all female turtles ≥145 mm as adult and sub-adult females. We uniquely marked each turtle by notching the marginal scutes of the carapace with a cordless rotary tool (DREMEL®). An alphabetic coding system was used to identify each notched individual (Cagle 1939). Turtles were released once notched or inspected for notches.
Environmental variables
A suite of environmental variables was measured to assess the influence of local and landscape covariates on turtle survivorship. Local habitat variables were recorded during each turtle survey at four sampling points corresponding to the cardinal points around each wetland. The percentage of the full water-holding capacity of each wetland site (WATER) was recorded during the turtle surveys. Chelodina longicollis often responds to fluctuating water levels in urban wetlands through movement (Rees et al. 2009), which may affect emigration patterns. Australian freshwater turtles can survive temporarily in saline waters, but increased salinity may impede longer-term survival (Bower et al. 2016). We therefore measured wetland salinity (as electrical conductivity, EC; μS/cm) at the water surface, 1 m from the shoreline using a handheld digital meter (Tracer Pocketester, LaMotte Company, Chestertown, Maryland, USA). Chelodina longicollis is an opportunistic carnivore with a wide prey spectrum (Georges et al. 1986). The relative abundance of potential prey items (PREY) was quantified at each sampling point using time-constrained (30 s) dip-netting with a triangular-framed dip-net (35 × 30 × 30 cm, mesh size 1.4 mm). Two people each vigorously swept a dip-net around submerged objects such as vegetation and rocks, through open water, and under the waterbody bank. Aquatic organisms (small fishes, tadpoles and macroinvertebrates) trapped in dip-nets were identified using field guides (Anstis 2002; Gooderham and Tsyrlin 2002; Allen et al. 2003) and counted in the field. Macroinvertebrates were identified to Family or Order.
Each wetland site was digitised as a polygon using a Geographical Information System (ArcGIS 10.2.2, Environmental Systems Research Institute Inc., Redlands, California, USA), and landscape-scale variables measured. Wetland surface area (AREA) was calculated. Large wetlands may have greater aquatic habitat resources available for freshwater turtles than smaller waterbodies, but capture probabilities might be lower. We recorded the proportion cover of land mapped as green open space (GREEN) in a 1-km radius around the site using a spatial layer composed of parks and gardens, remnant patches of native vegetation, and recreational fields (‘Open Space 2002’, Australian Research Centre for Urban Ecology 2003). Green open space represents terrestrial habitats that are important for nesting, aestivation and movement of C. longicollis (Roe and Georges 2007), which may affect both survival and emigration. Road density (ROADS; km/km2) was calculated for each site by summing the total length of roads in a 1-km radius around the wetland (Data Source: ‘Road Network 1:25,000 (version 1 December 2011)’, Department of Sustainability and Environment, Victoria). Freshwater turtles are vulnerable to road mortality when undertaking overland movements (Steen et al. 2006; Langen et al. 2012). The straight-line distance from a site to the nearest three wetlands (edge-to-edge of each respective wetland; WETDIST) was measured. Inter-wetland distances in urban areas affect movement rates of C. longicollis among multiple wetlands (Rees et al. 2009), which may influence emigration from wetlands. The age of each wetland (WETAGE) was determined from GIS layers (e.g. ‘Melbourne Water wetlands, June 2009’) or from personal communications with land managers. The year of wetland construction was subtracted from the mid-year of the study (2014). Older wetlands in the study area were found to have larger and hence older turtles (Hamer et al. 2016), which may reflect enhanced adult survival at these sites.
Statistical analyses
Catch-per-unit effort (CPUE) of C. longicollis each season was calculated by dividing the number of unique turtles captured at a site by the total number of traps deployed at the site each season. Turtle density (DENSITY; individuals trap−1 ha−1) for each season was then calculated by dividing CPUE by wetland area. Population density in waterbodies may affect turtle survival through increased intra-specific competition for food resources. The CPUE of prey organisms for each survey at a site was calculated by dividing the total number of prey organisms captured by the total number of minutes spent dip-netting during that survey (no. prey min−1). Only those organisms recorded in the diet of C. longicollis were included in the variable PREY (Chessman 1984; Georges et al. 1986). A body condition index (BCI) was calculated following the methods of Jakob et al. (1996) and Lecq et al. (2014), using the residuals taken from a linear regression between the logarithms of body mass and carapace length. Body condition can be regarded as an indicator of the energetic state of an individual, and hence its prospects of survival (Schulte-Hostedde et al. 2005). Body measurements used in calculating BCI were those recorded upon initial capture. Capture histories of all individual turtles were compiled over the 24 surveys. Individuals were assigned to one of three groups (males, adult and sub-adult females, juveniles), according to carapace length at initial capture.
Relationships between the probability of survival (φ) and the covariates were examined using a Cormack-Jolly-Seber (CJS) model (Lebreton et al. 1992) fitted to the recapture histories of turtles (males, females and juveniles together), and females separately. Female C. longicollis were examined separately because of their direct influence on population regulation through reproduction (Ferronato et al. 2016). The probability of survival is ‘apparent’ because the CJS model does not distinguish mortality from permanent emigration (Pollock and Alpizar-Jara 2005), and so φ is contingent upon an individual surviving and remaining in the study area. The CJS model was fitted to the data for the two groups using Bayesian methods described by McCarthy (2007). This approach allows for variation in the time interval between surveys and between seasons by rescaling the probability of survival between occasions (φ) to a daily probability of survival (φdaily) by adjusting for the number of days between each season (see Heard et al. 2012). The capture history data was analysed using two models: (1) to examine relationships between the daily probability of survival and the environmental covariates and BCI (model 1); and (2) to investigate differences in the probability of survival among groups and seasons (model 2). The CJS model enables estimation of the probability of recapture (p) which was also derived in each model.
In model 1, a linear equation and logistic link function was used for estimating the probability of individual i surviving between surveys j – 1 and j (φ i,j ), and the probability of recapture for individual i on survey j (p i,j ):
where: α1 and α2 are intercept terms, and β1 and β2 are regression coefficients for covariate x recorded at the site of capture for individual i. Time-varying covariates (x i,j ) were included in models where variables were recorded during each survey. One covariate was assessed per model to avoid potential overestimation of beta coefficients due to correlations among the variables (Online Resource 1), and because of relatively low recapture rates. The following covariates were log10(x)-transformed: PREY, EC, AREA, ROADS, WETDIST, WETAGE, while DENSITY was log10(x + 1)-transformed. Continuous covariates were centred by subtracting the mean and dividing by 2 standard deviations. Missing values of time-varying covariates were substituted by the mean value for that site, or in the case of BCI, with the mean value from that group. The survival status was modelled at each time interval using a Bernoulli random variable where φ i,j is raised to the power of the number of days in the interval j – 1 to j; recapture status was modelled similarly with probability p i,j being contingent on individual i remaining alive at survey j (see Heard et al. 2014). Uninformative normal priors (Normal[0, 0.01]) were used for α1, α2, β1 and β2.
Model 2 was similar to model 1, except there were no linear equations, and the daily probability of survival was allowed to vary between the three groups (males, females, juveniles), and either remain constant or vary from season 1 to season 5. The daily probability of survival was also allowed to vary over the winter interval when C. longicollis is relatively dormant. Estimates of the probability of surviving each season (φseason1, φseason2,… φseason5) were calculated by raising φdaily to the power of the number of days in each sampling period (November to March):
where \( {\varphi}_{daily_i} \) is the daily probability of survival during season i (see Heard et al. 2012). The annual probability of survival for each season i was calculated as:
where the probability of surviving the winter intervals (φwinter1, φwinter2,… φwinter4) was calculated by raising φdaily to the power of the number of days in the winter interval (April to October, 235 days). Uniform priors (U[0, 1]) were used for estimation.
All models were fitted using JAGS v4.2.0 (Plummer 2016) through program R (R2jags; Su and Yajima 2015) to estimate posterior distributions of parameters via Markov chain Monte Carlo (MCMC) simulation. Models were run using two parallel chains of 100,000 iterations after an initial burn-in of 50,000 samples, thinned by 10. Model convergence was assessed using the Gelman-Rubin statistic where \( \widehat{R} \) <1.1 was an acceptable threshold of convergence (Gelman et al. 2004).
In model 1, 20 linear models were used to assess relationships between φdaily and ten covariates for turtles and females separately, where each covariate assessed a different hypothesis about turtle survival. Seven models included a covariate (x) for both φ and p (φ[x] p[x]); ten models included a covariate for φ only (φ[x] p[.]); one model was a ‘null’ model (φ[.] p[.]); one model included the number of days since the start of each season to assess potential seasonal effects on p only (φ[.] p[DAYS]), while another model included season number as a covariate of φ and days for p (φ[SEASON] p[DAYS]) to assess potential yearly and seasonal differences in φ. In model 2, two models were derived using either constant or seasonal variation in φdaily. The Deviance Information Criterion (DIC; Spiegelhalter et al. 2002) was used to compare models. We considered the best-supported models to be those with a ΔDIC <2 (ΔDIC = DIC – minimum [DIC]), models with a ΔDIC of 2–7 were considered to have a moderate level of support, although any model with a ΔDIC <10 was regarded as being potentially supported (McCarthy 2007). In model 1, the posterior distributions of estimated parameters for models with a ΔDIC <10 were assessed based on the magnitude of the regression coefficients and the precision of the estimates. Bayesian 95% credible intervals (CIs) were obtained from the 2.5th and 97.5th percentiles of the posterior distribution; parameter estimates with 95% CIs that did not overlap zero were considered to be more certain than estimates with overlapping CIs.
Results
Turtle captures
We made 735 captures of 578 unique individuals comprised of 190 males, 250 adult and sub-adult females and 138 juveniles (see Online Resource 2 for numbers captured per wetland and per season). Of the 578 individuals marked, 124 were recaptured on one or more occasions (naïve recapture rate = 0.21). Only seven individuals (1% of the marked population) were recaptured more than twice, 98 individuals were recaptured only once (17%) and 454 individuals (79%) were never recaptured. Of the 250 sub-adult and adult females marked, 59 were recaptured at least once (naïve recapture rate = 0.24). No marked turtles were detected moving between wetlands.
Water levels, turtle density and relative prey abundance
Mean water levels over five seasons at the 20 wetlands ranged 55–97% of the maximum full capacity, with a grand mean of 87% (95% CI: 83–92%). Mean water levels in 2012/2013 (79%) were substantially lower than in 2011/2012 (90%), based on non-overlapping 95% CIs (Fig. 2). Water levels at one stormwater retention wetland dropped to 3% of full capacity during one survey, but no wetlands dried out over the five seasons. Mean rainfall recorded over the five seasons was 34–69 mm (mean = 44 mm); mean precipitation in season 1 was 33% above the long-term average for November to March, while seasons 2 to 5 were 15–34% below the long-term average (Online Resource 3). The mean density of C. longicollis at the 20 sites over five seasons was 0.39 individuals trap−1 ha−1 (95% CI: 0.19–0.59). Mean turtle density did not differ substantially between seasons (Fig. 2). The mean relative abundance of prey (CPUE) over five seasons was 71 prey captured min−1 of dip-netting (range: 18–154; 95% CI: 56–86). Of the total number of prey organisms captured, 83.8% were aquatic invertebrates, 15.9% were small fish and 0.2% were tadpoles.
Daily probability of survival
The best-ranked model of the daily probability of survival (φdaily) and probability of recapture (p) for C. longicollis included the proportion of green open space for both regression parameters (model 1; Table 1). There was considerably less support for a model that included electrical conductivity at the wetland of capture as a covariate (model 2; ΔDIC = 8.8). The best-ranked model for adult and sub-adult female C. longicollis included BCI as a covariate for φdaily while holding p constant (model 1; Table 1). A model including BCI as a covariate for both φdaily and p had considerably less support (model 2; ΔDIC = 8.9). There was no support for models containing water levels, turtle density or the relative abundance of prey.
There was a positive relationship between φdaily of C. longicollis and the proportion of green open space within 1 km of a wetland site (Table 2). Conversely, there was a negative relationship between p and the proportion of green open space (Table 2). Mean estimates of φdaily over the range of green open spaces measured around the 20 wetland sites were predicted to increase from 0.998 at the site with the lowest proportion of green open space (0.026) to 1.000 at the site with the highest proportion (0.641; Fig. 3a). Mean estimates of p were predicted to decrease from 0.086 at the site with the lowest proportion of green open space, to 0.011 at the site with the highest proportion (Fig. 3b). There was a positive relationship between φdaily and electrical conductivity (Table 2).
Relationships between green open space and a daily probability of survival (φ), and b probability of recapture (p) of Chelodina longicollis (males, females, juveniles). Predicted mean estimates (solid lines) were derived from model 1 (see Table 1, turtles). Dashed lines are 95% credible intervals
There was a positive relationship between φdaily of adult and sub-adult female C. longicollis and BCI, although the 95% CI overlapped zero slightly (model 1; Table 2). The mean estimate for BCI in model 2 was higher and the 95% CI did not overlap zero. There was a negative relationship between p and BCI, although the 95% CI overlapped zero (model 2; Table 2). Mean estimates of φdaily over the range of BCI recorded for 250 adult and sub-adult females were predicted to increase from 0.997 for the individual with the lowest BCI (−0.218, poor body condition) to 0.999 for the individual with the highest BCI (0.234, good body condition; Fig. 4).
Relationship between body condition index and the daily probability of survival (φ) of adult and sub-adult female Chelodina longicollis. The predicted mean estimate (solid line) was derived from model 1 (see Table 1, females). Dashed lines are 95% credible intervals. Positive BCI values indicate good body condition; negative values indicate poor condition
Seasonal probability of survival
The best-ranked model of the seasonal probability of survival (φseason) and p for C. longicollis included time-varying daily probability of survival and recapture (DIC = 1392.6, ΔDIC = 0.0) compared to a model with constant φdaily and p (DIC = 1485.6, ΔDIC = 93.0). There was no difference in the mean estimates of φseason between male, female and juvenile C. longicollis each season, with broadly overlapping 95% CIs (Fig. 5). However, there was a difference between the mean estimate of φseason for juveniles in 2011/2012 and the estimate in 2013/2014, with no overlapping 95% CIs (Fig. 5). There was no difference in φseason between seasons for males and females. There were no differences in p among the three groups due to broadly overlapping 95% CIs (Online Resource 4).
Probability of Chelodina longicollis surviving each season (φseason) from 2011/2012 to 2015/2016. Point estimates and 95% credible intervals are presented for males (open circles), females (closed circles) and juveniles (grey circles). Estimates were obtained from the time-varying model of the probability of survival
Annual probability of survival
There was no difference in the mean estimates of φannual between the four yearly periods for males, females and juveniles, and there were no differences between the three groups each year, with broadly overlapping 95% CIs (Table 3). Mean estimates of φannual (range of 95% CI) for males, females and juveniles were 0.244 (0.000–0.801), 0.506 (0.079–0.913) and 0.314 (0.000–0.930), respectively.
Discussion
This is the first study to examine relationships between a suite of environmental variables and the survival of a freshwater turtle species in an Australian city over a broad spatial and temporal scale. Our results demonstrate a clear positive relationship between the proportion of green open space (i.e. terrestrial habitat) surrounding wetlands for up to 1 km and the probability of survival for a freshwater turtle in an urban landscape. Guzy et al. (2013) highlighted the importance of greenspace (aquatic and terrestrial areas) in urban landscapes for maintaining populations of semi-aquatic turtles, specifically that increasing connectance of greenspace was positively related to turtle occupancy at suburban ponds. Populations of other wetland-dependent taxa (e.g. frogs) show a strong positive association with green open space in the Melbourne region (Hamer and Parris 2011).
Chelodina longicollis occupies a broad range of wetland types in the study area, and a previous study found no single habitat variable could explain wetland occupancy (Stokeld et al. 2014). Here, we found that the survival of C. longicollis at wetlands was clearly related to the availability of terrestrial habitat, which is used for movement and nesting, and aestivation if wetland conditions deteriorate (Stott 1987; Roe and Georges 2008a, 2008b). However, all the wetlands included in this study generally retained high water levels each season and the study was not conducted during a drought, and so there was no incentive for turtles to aestivate (Roe et al. 2008). Rees et al. (2009) did not detect C. longicollis aestivating in suburban locations where water levels in urban wetlands remained high. We did not find any aestivating turtles, but we detected gravid females searching for nesting sites or digging nests in open grassy areas that were within 50 m of a wetland. Therefore, we are certain that terrestrial habitat surrounding some wetlands in the study area is used for movement and nesting. Further, there may be a more normal size-class distribution of C. longicollis at sites surrounded by a high proportion cover of green open space if nesting is more successful than at wetlands with less green space (Online Resource 6).
We found that wetland sites with a high proportion cover of green open space also had a lower probability of recapture, which may indicate that there is temporary emigration of individuals occurring at these sites. For instance, individuals may be either moving to nearby wetlands temporarily, or residing on land for brief periods, before returning to the wetland of capture. Moreover, wetlands with a high proportion cover of green open space were situated relatively close to surrounding wetlands (r = −0.49, Online Resource 1), and shorter inter-wetland distances facilitate greater movements by C. longicollis in urban landscapes (Rees et al. 2009; Ferronato et al. 2016, 2017). For example, marked individuals were detected moving to and from two relatively small and closely-spaced wetlands (~30 m apart) in the study area (A. Hamer, unpublished data). Movement among multiple wetlands may allow C. longicollis to exploit habitat resources that are variable in both space and time (e.g. prey availability; Roe and Georges 2008a, 2008b), thereby leading to higher survival rates of individuals within patchy populations (Roe et al. 2009). Wetland connectivity that facilitates inter-wetland movement is essential for the persistence of freshwater turtles in fragmented landscapes generally (Bowne et al. 2006).
Female C. longicollis with a higher body condition index had a higher probability of survival. Trends in body condition indices have been shown to correspond with survival patterns within populations of other freshwater turtle species (Canessa et al. 2016), and well fed (satiated) individuals are expected to have a higher BCI (Jakob et al. 1996). Here, we found no strong correlation between the relative abundance of prey and BCI (r = 0.12, Online Resource 1), indicating there may be other site or fitness-related variables we did not measure that are affecting the health of female turtles (e.g. heavy metals; Bishop et al. 2010). Alternatively, individuals may be immigrating from nearby wetlands where food availability may be higher. A higher BCI may also indicate reproductive output among female C. longicollis, as a positive relationship between body condition and clutch mass and egg size has been demonstrated in another freshwater turtle species (Litzgus et al. 2008). The negative relationship between the probability of recapture and BCI could reflect the increased vigour of turtles with a high BCI, and satiated individuals may be less likely to be attracted to trap bait.
There was a positive relationship between the daily probability of survival of C. longicollis and wetland conductivity (salinity). The closely-related C. expansa can tolerate short-term exposure to brackish water (15 ppt) with no obvious adverse effects, however, individuals reduce feeding which may affect body condition and longer-term survival (Bower et al. 2016). In our study, the highest mean conductivity recorded was close to the upper value examined by Bower et al. (2016), at a site that received saline groundwater. Stokeld (2012) found that body condition of C. longicollis increased with increasing water conductivity at sites in the study area, but the relationship was concave-down and body condition was predicted to decrease above around 6000 μS/cm, well above the maximum reading recorded in this study. It is unclear how increasing salinity at sites increased the probability of survival of C. longicollis, although salinity may be correlated with some other unmeasured habitat variable that covaries positively with turtle survival.
Our estimates of the annual probability of adult survival in C. longicollis over five seasons ranged between 0.064 and 0.681 (Table 3), compared to estimates between 0.408 and 0.470 derived by Ferronato et al. (2017). Similar survivorship estimates were obtained in a heavily urbanised region in North America for the eastern mud turtle (Kinosternon subrubrum), a semi-aquatic species that relies on terrestrial habitats (Eskew et al. 2010). Our estimates of juvenile survivorship were generally lower than for adult turtles, however, this trend is characteristic of many populations of freshwater turtles (Congdon et al. 1993, 1994). Rees et al. (2009) and Ferronato et al. (2016, 2017) surmised that road densities and traffic volumes were contributing to C. longicollis mortality. However, contrary to expectation, we found no support for a model of survivorship assessing road density. Moreover, we did not find any road-killed C. longicollis on roads surrounding our study sites. It would thus appear that landscape variables relating to the availability and connectivity of remnant terrestrial habitat are of greater importance than road effects for the persistence of C. longicollis in the study area.
Although our study included a wide array of environmental variables, there may be other variables affecting turtle survival that we did not measure. For instance, fishing and illegal fish nets were observed at several wetlands, and the shell of a dead female C. longicollis was found next to discarded fishing line. Steen and Robinson (in press) suggested that ingestion of fish hooks and recreational fishing are potential threats to freshwater turtles (Steen et al. 2014), and fishing and human recreation can impair the long-term survival of turtle populations (Garber and Burger 1995). Urban populations of C. longicollis may therefore be experiencing mortality from recreational fishing. Predation by the red fox (Vulpes vulpes) on eggs and turtles may also contribute to population declines (Kennett et al. 2009). Relatively small increases (e.g. 2–3%) in mortality rates of adult freshwater turtles can have dramatic effects on population growth and persistence (Congdon et al. 1993, 1994; Spencer et al. in press), particularly if immigration rates are low (Brooks et al. 1991). Moreover, fish hooks can inflict sub-lethal effects on turtle health (Steen and Robinson in press), and reduce body condition. Because body condition of female C. longicollis is strongly related to survival, recreational fishing could increase female mortality, which may reduce population viability given the more direct influence of female fecundity on the stability of freshwater turtle populations (Congdon et al. 1994).
Management implications
A recent review of urban biodiversity studies highlighted the importance of preserving green spaces within cities and towns for maintaining species populations (Norton et al. 2016). Here, we found that green open space was correlated with the survival of C. longicollis in an urban landscape. The role of terrestrial habitat has important implications for conserving freshwater turtle habitat (Buhlmann and Gibbons 2001; Ficetola et al. 2004). Because any loss of green open space will be correlated with terrestrial habitat loss and fragmentation, constructing new wetlands will not be sufficient to sustain turtle populations if the surrounding terrestrial habitats are destroyed or heavily fragmented. Moreover, protecting existing aquatic habitats has been shown to have negligible effects on preventing declines in some freshwater turtle species if the catchment around wetlands becomes increasingly urbanised (Browne and Hecnar 2007). In this instance, restoration and reconnection of the matrix surrounding protected core wetlands would be more effective (Quesnelle et al. 2013). Restricting urban development within wide buffer zones around wetlands may protect the terrestrial habitats used by C. longicollis; Roe and Georges (2007) recommended a 425-m wide vegetated buffer.
To maintain freshwater turtle populations in urban landscapes, we need to prevent negative impacts on the health and survival of individuals at the local scale, in addition to protecting fine-scale habitat elements (e.g. nesting sites), wetland complexes and landscape connectivity (Klemens 2000; Bodie 2001). Long-term actions need to target all life stages (Congdon et al. 1993, 1994; Burke et al. 2000). Populations of freshwater turtles in some human-dominated landscapes may be increasing due to the availability of artificial waterbodies (Rees et al. 2009; Roe et al. 2011; Stokeld et al. 2014). However, under increasing urban development and human population growth, these seemingly stable populations may experience declines that could take decades to become noticeable due to the relative longevity of turtles (Browne and Hecnar 2007). A challenging goal in conserving urban populations of freshwater turtle species that require large areas of terrestrial habitat will be to ensure the ongoing protection of existing parks and to protect unreserved lands from future urban development. Achieving the latter goal would require planners and managers to consider options such as land sparing and compact urban development (Sushinsky et al. 2013; Soga et al. 2014), although protected areas are also impacted by urbanisation (McDonald et al. 2008). This approach would help minimise the extinction debt (Tilman et al. 1994) of populations of long-lived species in rapidly urbanising regions.
References
Allen GR, Midgley SH, Allen M (2003) Field guide to the freshwater fishes of Australia. Western Australian Museum, Perth, Australia
Anstis M (2002) Tadpoles of south-eastern Australia: a guide with keys. Reed New Holland, Sydney
Aresco MJ (2005) Mitigation measures to reduce highway mortality of turtles and other herpetofauna at a North Florida lake. J Wildl Manag 69:549–560
Australian Research Centre for Urban Ecology (2003) Open space 2002 (ArcView Shapefile). Australian Research Centre for Urban Ecology, Melbourne
Beaudry F, deMaynadier PG, Hunter ML Jr (2008) Identifying road mortality threat at multiple spatial scales for semi-aquatic turtles. Biol Conserv 141:2550–2563
Bishop BE, Savitzky BA, Abdel-Fattah T (2010) Lead bioaccumulation in emydid turtles of an urban lake and its relationship to shell disease. Ecotoxicol Environ Saf 73:565–571
Bodie JR (2001) Stream and riparian management for freshwater turtles. J Environ Manag 62:443–455
Bower DS, Scheltinga DM, Clulow S, Clulow J, Franklin CE, Georges A (2016) Salinity tolerances of two Australian freshwater turtles, Chelodina expansa and Emydura macquarii (Testudinata: Chelidae). Conserv Physiol 4:cow042. https://doi.org/10.1093/conphys/cow042
Bowne DR, Bowers MA, Hines JE (2006) Connectivity in an agricultural landscape as reflected by interpond movements of a freshwater turtle. Conserv Biol 20:780–791
Brooks RJ, Brown GP, Galbraith DA (1991) Effects of a sudden increase in natural mortality of adults on a population of the common snapping turtle (Chelydra serpentina). Can J Zool 69:1314–1320
Browne CL, Hecnar SJ (2007) Species loss and shifting population structure of freshwater turtles despite habitat protection. Biol Conserv 138:421–429
Buhlmann KA, Gibbons JW (2001) Terrestrial habitat use by aquatic turtles from a seasonally fluctuating wetland: implications for wetland conservation boundaries. Chelonian Conserv Biol 4:115–127
Burke VJ, Lovich JE, Gibbons JW (2000) Conservation of freshwater turtles. In: Klemens MW (ed) Turtle conservation. Smithsonian Institution Press, Washington, DC, pp 156–179
Cagle FR (1939) A system of marking turtles for future identification. Copeia 1939:170–173
Canessa S, Genta P, Jesu R, Lamagni L, Oneto F, Salvidio S, Ottonello D (2016) Challenges of monitoring reintroduction outcomes: insights from the conservation breeding program of an endangered turtle in Italy. Biol Conserv 204:128–133
Chessman BC (1984) Food of the snake-necked turtle, Chelodina longicollis (Shaw) (Testudines, Chelidae) in the Murray Valley, Victoria and New South Wales. Aust Wildl Res 11:573–578
Congdon JD, Dunham AE, van Loben Sels RC (1993) Delayed sexual maturity and demographics of Blanding’s turtles (Emydoidea blandingii): implications for conservation and management of long-lived organisms. Conserv Biol 7:826–833
Congdon JD, Dunham AE, van Loben Sels RC (1994) Demographics of common snapping turtles (Chelydra serpentina): implications for conservation and management of long-lived organisms. Am Zool 34:397–408
Crump PS, Robertson SJ, Rommel-Crump RE (2016) High incidence of road-killed freshwater turtles at a lake in east Texas, USA. Herpetol Conserv Biol 11:181–187
Czech B, Krausman PR, Devers PK (2000) Economic associations among causes of species endangerment in the United States. Bioscience 50:593–601
DeCatanzaro R, Chow-Fraser P (2010) Relationship of road density and marsh condition to turtle assemblage characteristics in the Laurentian Great Lakes. J Gt Lakes Res 36:357–365
Ehrenfeld JG (2000) Evaluating wetlands within an urban context. Ecol Eng 15:253–265
Eskew EA, Price SJ, Dorcas ME (2010) Survival and recruitment of semi-aquatic turtles in an urbanized region. Urban Ecosyst 13:365–374
Ferronato BO, Roe JH, Georges A (2016) Urban hazards: spatial ecology and survivorship of a turtle in an expanding suburban environment. Urban Ecosyst 19:415–428
Ferronato BO, Roe JH, Georges A (2017) Responses of an Australian freshwater turtle to drought-flood cycles along a natural to urban gradient. Austral Ecol 42:442–455
Ficetola GF, Padoa-Schioppa E, Monti A, Massa R, De Bernardi F, Bottoni L (2004) The importance of aquatic and terrestrial habitat for the European pond turtle (Emys orbicularis): implications for conservation planning and management. Can J Zool 82:1704–1712
Garber SD, Burger J (1995) A 20-yr study documenting the relationship between turtle decline and human recreation. Ecol Appl 5:1151–1162
Gelman A, Carlin JB, Stern HS, Rubin DB (2004) Bayesian data analysis, 2nd edn. Chapman and Hall/CRC, Boca Raton
Georges A, Norris RH, Wensing L (1986) Diet of the freshwater turtle Chelodina longicollis (Testudines, Chelidae) from the coastal dune lakes of the Jervis Bay territory. Aust Wildl Res 13:301–308
Gibbons JW, Scott DE, Ryan TJ, Buhlmann KA, Tuberville TD, Metts BS, Greene JL, Mills T, Leiden Y, Poppy S, Winne CT (2000) The global decline of reptiles, déjà vu amphibians. Bioscience 50:653–666
Gibbs JP, Shriver WG (2002) Estimating the effects of road mortality on turtle populations. Conserv Biol 16:1647–1652
Gooderham J, Tsyrlin E (2002) The waterbug book. A guide to the freshwater macroinvertebrates of temperate Australia. CSIRO Publishing, Collingwood
Guzy JC, Price SJ, Dorcas ME (2013) The spatial configuration of greenspace affects semi-aquatic turtle occupancy and species richness in a suburban landscape. Landsc Urban Plan 117:46–56
Hamer AJ, McDonnell MJ (2008) Amphibian ecology and conservation in the urbanising world: a review. Biol Conserv 141:2432–2449
Hamer AJ, Parris KM (2011) Local and landscape determinants of amphibian communities in urban ponds. Ecol Appl 21:378–390
Hamer AJ, Smith PJ, McDonnell MJ (2012) The importance of habitat design and aquatic connectivity in amphibian use of urban stormwater retention ponds. Urban Ecosyst 15:451–471
Hamer AJ, Harrison LJ, Stokeld D (2016) Road density and wetland context alter population structure of a freshwater turtle. Austral Ecol 41:53–64
Heard GW, Scroggie MP, Malone BS (2012) The life history and decline of the threatened Australian frog, Litoria raniformis. Austral Ecol 37:276–284
Heard GW, Scroggie MP, Clemann N, Ramsey DSL (2014) Wetland characteristics influence disease risk for a threatened amphibian. Ecol Appl 24:650–662
Ives CD, Beilin R, Gordon A, Kendal D, Hahs AK, McDonnell MJ (2013) Local assessment of Melbourne: the biodiversity and social-ecological dynamics of Melbourne, Australia. In: Elmqvist T, Fragkias M, Goodness J, Güneralp B, Marcotullio PJ, McDonald RI, Parnell S, Schewenius M, Sendstad M, Seto KC, Wilkinson C (eds) Urbanization, biodiversity and ecosystem services: challenges and opportunities: a global assessment. Springer, Dordrecht, pp 385–407
Jakob EM, Marshall SD, Uetz GW (1996) Estimating fitness: a comparison of body condition indices. Oikos 77:61–67
Joyal LA, McCollough M, Hunter ML Jr (2001) Landscape ecology approaches to wetland species conservation: a case study of two turtle species in southern Maine. Conserv Biol 15:1755–1762
Kennett RM, Georges A (1990) Habitat utilization and its relationship to growth and reproduction of the eastern long-necked turtle, Chelodina longicollis (Testudinata: Chelidae), from Australia. Herpetologica 46:22–33
Kennett R, Roe J, Hodges K, Georges A (2009) Chelodina longicollis (Shaw 1784) – eastern long-necked turtle, common long-necked turtle, common snake-necked turtle. In: Rhodin AGJ, Pritchard PCH, van Dijk PP, Saumure RA, Buhlmann KA, Iverson JB, Mittermeier RA (eds) Conservation biology of freshwater turtles and tortoises: a compilation project of the IUCN/SSC tortoise and freshwater turtle specialist group. Chelonian res Monogr no. 5, pp 031.1–031, p 8. https://doi.org/10.3854/crm.5.031.longicollis.v1.2009 http://www.iucn-tftsg.org/cbftt/
Klemens MW (2000) From information to action: developing more effective strategies to conserve turtles. In: Klemens MW (ed) Turtle conservation. Smithsonian Institution Press, Washington, DC, pp 239–258
Langen TA, Gunson KE, Scheiner CA, Boulerice JT (2012) Road mortality in freshwater turtles: identifying causes of spatial patterns to optimize road planning and mitigation. Biodivers Conserv 21:3017–3034
Leary E, McDonnell M (2001) Quantifying public open space in metropolitan Melbourne. Parks Leisure Aust 4:34–36
Lebreton J-D, Burnham KP, Clobert J, Anderson DR (1992) Modeling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecol Monogr 62:67–118
Lecq S, Ballouard J-M, Caron S, Livoreil B, Seynaeve V, Matthieu L-A, Bonnet X (2014) Body condition and habitat use by Hermann's tortoises in burnt and intact habitats. Conserv Physiol 2:cou019. https://doi.org/10.1093/conphys/cou019
Lee SY, Dunn RJK, Young RA et al (2006) Impact of urbanization on coastal wetland structure and function. Austral Ecol 31:149–163
Litzgus JD, Bolton F, Schulte-Hostedde AI (2008) Reproductive output depends on body condition in spotted turtles (Clemmys guttata). Copeia 2008:86–92
Marchand MN, Litvaitis JA (2004) Effects of habitat features and landscape composition on the population structure of a common aquatic turtle in a region undergoing rapid development. Conserv Biol 18:758–767
McCarthy MA (2007) Bayesian methods for ecology. Cambridge University Press, Cambridge
McDonald RI, Kareiva P, Forman RTT (2008) The implications of current and future urbanization for global protected areas and biodiversity conservation. Biol Conserv 141:1695–1703
McDonnell MJ, Holland K (2008) Biodiversity. In: Newton PW (ed) Transitions: transitioning to resilient cities. CSIRO Publishing, Melbourne, pp 253–266
McKinney ML (2002) Urbanization, biodiversity, and conservation. Bioscience 52:883–890
Mitchell JC, Klemens MW (2000) Primary and secondary effects of habitat alteration. In: Klemens MW (ed) Turtle conservation. Smithsonian Institution Press, Washington, DC, pp 5–32
Norton BA, Evans KL, Warren PH (2016) Urban biodiversity and landscape ecology: patterns, processes and planning. Curr Landsc Ecol Rep 1:178–192
Plummer M (2016) JAGS: Just Another Gibbs Sampler. Available from https://sourceforge.net/projects/mcmc-jags/. Accessed Jan 2017
Pollock KH, Alpizar-Jara R (2005) Classical open-population capture-recapture models. In: Arnstrup SC, McDonald TL, Manly BFJ (eds) Handbook of capture-recapture analysis. Princeton University Press, Princeton, pp 36–57
Quesnelle PE, Fahrig L, Lindsay KE (2013) Effects of habitat loss, habitat configuration and matrix composition on declining wetland species. Biol Conserv 160:200–208
Rees M, Roe JH, Georges A (2009) Life in the suburbs: behavior and survival of a freshwater turtle in response to drought and urbanization. Biol Conserv 142:3172–3181
Rizkalla CE, Swihart RK (2006) Community structure and differential responses of aquatic turtles to agriculturally induced habitat fragmentation. Landsc Ecol 21:1361–1375
Roe JH, Georges A (2007) Heterogeneous wetland complexes, buffer zones, and travel corridors: landscape management for freshwater reptiles. Biol Conserv 135:67–76
Roe JH, Georges A (2008a) Maintenance of variable responses for coping with wetland drying in freshwater turtles. Ecology 89:485–494
Roe JH, Georges A (2008b) Terrestrial activity, movements and spatial ecology of an Australian freshwater turtle, Chelodina longicollis, in a temporally dynamic wetland system. Austral Ecol 33:1045–1056
Roe JH, Georges A, Green B (2008) Energy and water flux during terrestrial estivation and overland movement in a freshwater turtle. Physiol Biochem Zool 81:570–583
Roe JH, Brinton AC, Georges A (2009) Temporal and spatial variation in landscape connectivity for a freshwater turtle in a temporally dynamic wetland system. Ecol Appl 19:1288–1299
Roe JH, Rees M, Georges A (2011) Suburbs: dangers or drought refugia for freshwater turtle populations? J Wildl Manag 75:1544–1552
Schulte-Hostedde AI, Zinner B, Millar JS, Hickling GJ (2005) Restitution of mass-size residuals: validating body condition indices. Ecology 86:155–163
Seto KC, Güneralp B, Hutyra LR (2012) Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc Natl Acad Sci U S A 109:16083–16088
Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D (2006) From patterns to emerging processes in mechanistic urban ecology. Trends Ecol Evol 21:186–191
Soga M, Yamaura Y, Koike S, Gaston KJ (2014) Land sharing vs. land sparing: does the compact city reconcile urban development and biodiversity conservation? J Appl Ecol 51:1378–1386
Souza FL, Abe AS (2000) Feeding ecology, density and biomass of the freshwater turtle, Phrynops geoffroanus, inhabiting a polluted urban river in south-eastern Brazil. J Zool 252:437–446
Spencer R-J, Van Dyke JU, Thompson MB (in press) Critically evaluating best management practices for preventing freshwater turtle extinctions. Conserv Biol
Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A (2002) Bayesian measures of model complexity and fit. J Roy Statist Soc B 64:583–639
Steen DA, Robinson OJ (in press) Estimating freshwater turtle mortality rates and population declines following hook ingestion. Conserv Biol
Steen DA, Aresco MJ, Beilke SG et al (2006) Relative vulnerability of female turtles to road mortality. Anim Conserv 9:269–273
Steen DA, Gibbs JP, Buhlmann KA et al (2012) Terrestrial habitat requirements of nesting freshwater turtles. Biol Conserv 150:121–128
Steen DA, Hopkins BC, Van Dyke JU, Hopkins WA (2014) Prevalence of ingested fish hooks in freshwater turtles from five rivers in the southeastern United States. PLoS One 9:e91368. https://doi.org/10.1371/journal.pone.0091368
Stern H (2005) Climate. In: Brown-May A, Swain S (eds) The encyclopedia of Melbourne. Cambridge University Press, Melbourne, Australia, pp 147–154
Stokeld D (2012) Impacts of urbanisation on freshwater turtles in Melbourne. MSc thesis, School of Botany, University of Melbourne
Stokeld D, Hamer AJ, van der Ree R, Pettigrove V, Gillespie G (2014) Factors influencing occurrence of a freshwater turtle in an urban landscape: a resilient species? Wildl Res 41:163–171
Stott P (1987) Terrestrial movements of the freshwater tortoise Chelodina longicollis Shaw as monitored with a spool tracking device. Aust Wildl Res 14:559–567
Su Y, Yajima M (2015) Package ‘R2jags’: using R to run ‘JAGS’. Version 0.5–7. http://CRAN.R-project.org/package=R2jags
Sushinsky JR, Rhodes JR, Possingham HP, Gill TK, Fuller RA (2013) How should we grow cities to minimize their biodiversity impacts? Glob Chang Biol 19:401–410
Tesche MR, Hodges KE (2015) Unreliable population inferences from common trapping practices for freshwater turtles. Global. Ecol Conserv 3:802–813
Tilman D, May RM, Lehman CL, Nowak MA (1994) Habitat destruction and the extinction debt. Nature 371:65–66
Victorian Stormwater Committee (1999) Urban stormwater: best practice environmental management guidelines. CSIRO Publishing, Collingwood
Zedler JB, Kercher S (2005) Wetland resources: status, trends, ecosystem services, and restorability. Annu Rev Environ Resour 30:39–74
Acknowledgements
We thank all the citizen scientists who assisted with fieldwork. Financial and logistic support was provided by the Earthwatch Institute. We thank Cassandra Nichols and other Earthwatch staff for their valued assistance throughout the project. This research was supported by grants from the William Buckland Foundation and the George Alexander Foundation. The Baker Foundation provided generous support. We thank the land managers who facilitated our access to wetland sites: Melbourne Water, Parks Victoria, Royal Botanic Gardens Cranbourne and local council staff. We thank Stefano Canessa, Geoff Heard and Matt West for assistance with modelling. This study was approved by the University of Melbourne Animal Ethics Committee (register no. 0811058, 1212608 and 1513683). Fieldwork was conducted under research permit no. 10005308, 10006610 and 10007715 issued by the Victorian Department of Environment, Land, Water and Planning.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 239 kb)
Rights and permissions
About this article
Cite this article
Hamer, A.J., Harrison, L.J. & Stokeld, D. Terrestrial habitat and individual fitness increase survival of a freshwater turtle in an urban landscape. Urban Ecosyst 21, 71–83 (2018). https://doi.org/10.1007/s11252-017-0708-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-017-0708-8